Positive Associations between Body Mass Index and Hematological Parameters, Including RBCs, WBCs, and Platelet Counts, in Korean Children and Adolescents
Abstract
:1. Introduction
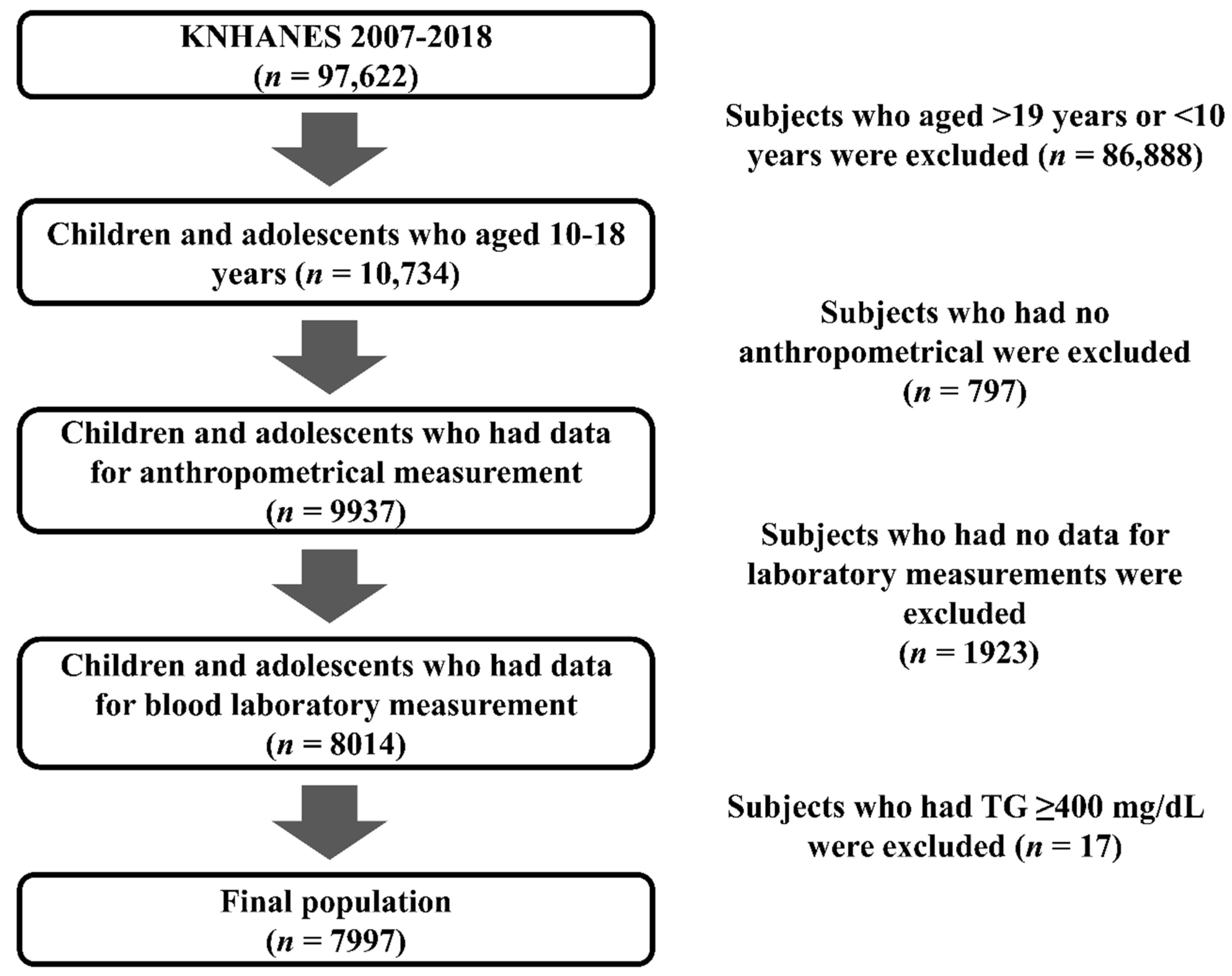
2. Materials and Methods
3. Statistical Analyses
4. Results
4.1. Clinical Characteristics According to BMI
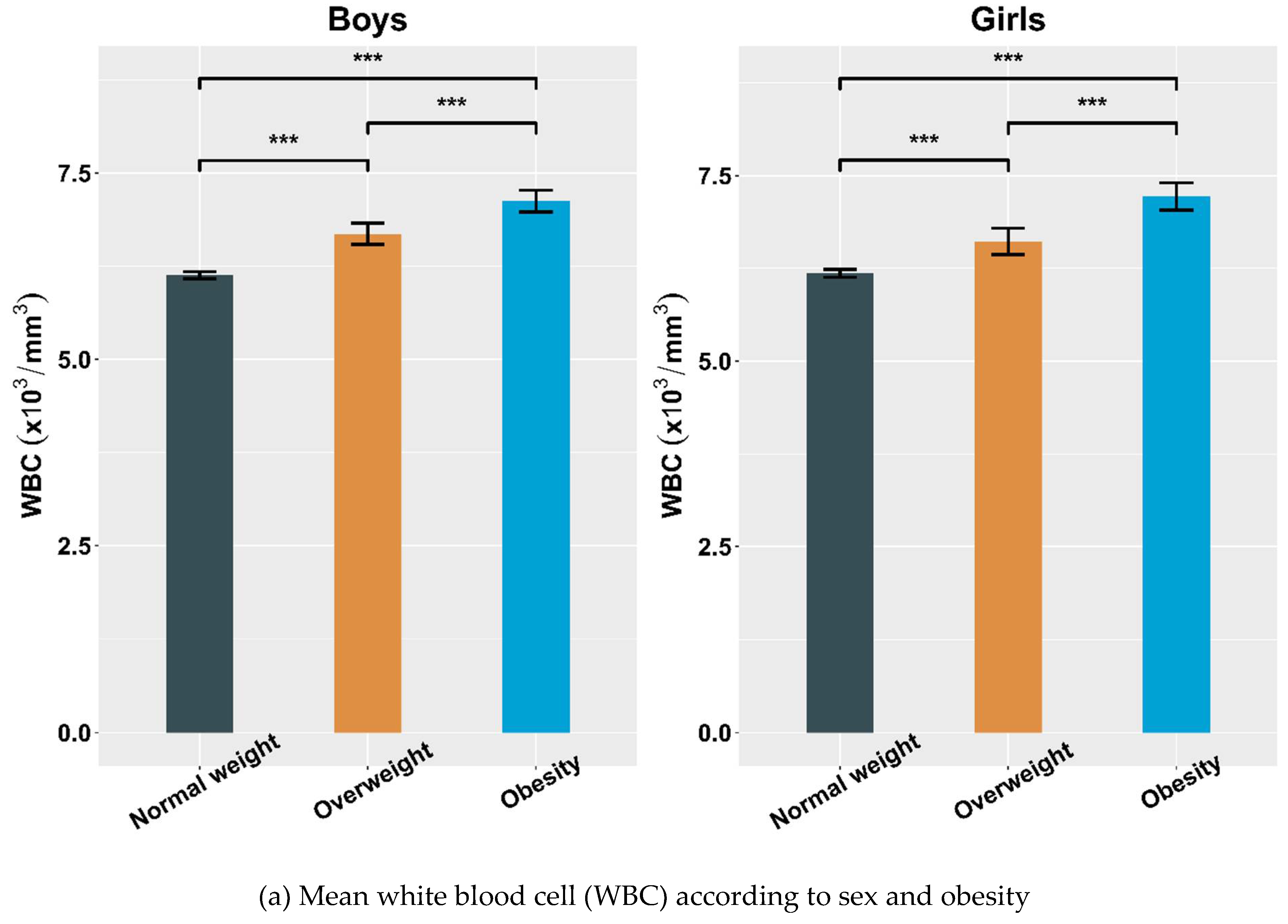
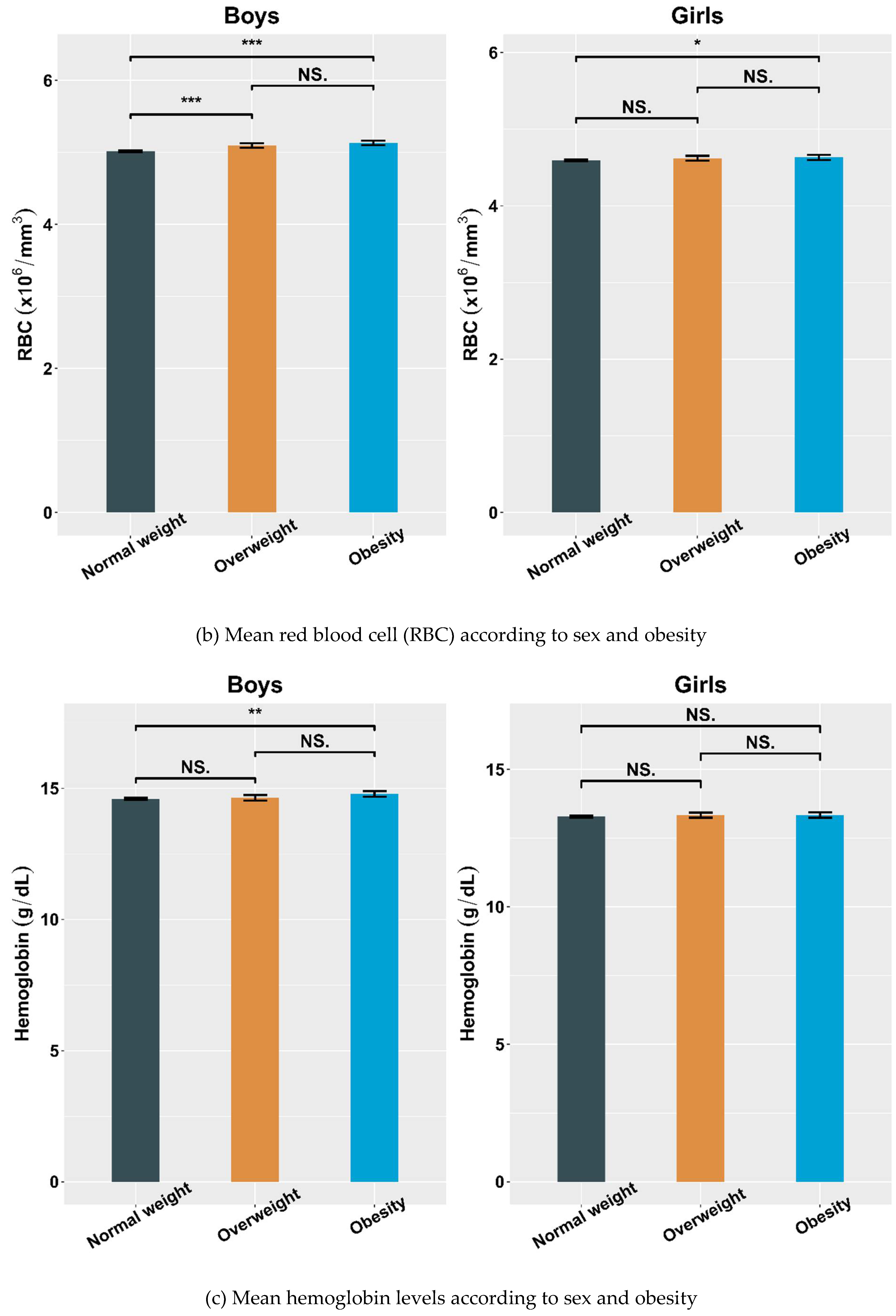
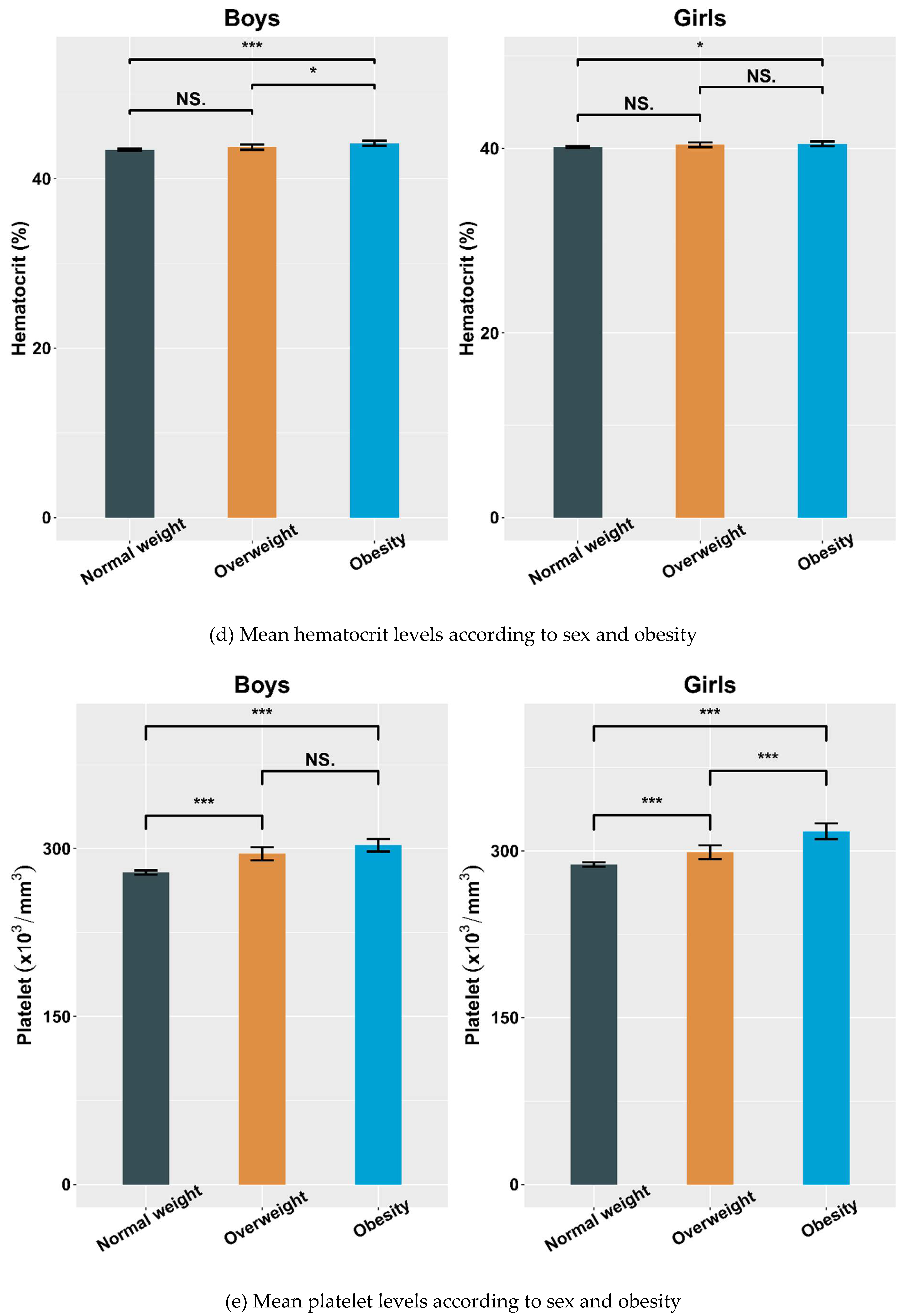
4.2. Hematological Parameters Adjusted by Sex and Obesity
4.3. Correlations between BMI SDS and Hematological Indices
4.4. Multiple Linear Regression Analyses of the BMI Groups with Hematological Parameters
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Chia, D.J.; Boston, B.A. Childhood obesity and the metabolic syndrome. Adv. Pediatr. 2006, 53, 23–53. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J. Role of obesity-induced inflammation in the development of insulin resistance and type 2 diabetes: History of the research and remaining questions. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stępień, M.; Stępień, A.; Wlazeł, R.N.; Paradowski, M.; Banach, M.; Rysz, J. Obesity indices and inflammatory markers in obese non-diabetic normo- and hypertensive patients: A comparative pilot study. Lipids Health Dis. 2014, 13, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.R.; Bao, W.; Wattigney, W.A.; Berenson, G.S. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: The Bogalusa heart study. Metabolism 1996, 45, 235–240. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA J. Am. Med Assoc. 2014, 311, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Twig, G.; Yaniv, G.; Levine, H.; Leiba, A.; Goldberger, N.; Derazne, E.; Ben-Ami Shor, D.; Tzur, D.; Afek, A.; Shamiss, A.; et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N. Eng. J. Med. 2016, 374, 2430–2440. [Google Scholar] [CrossRef]
- Castorani, V.; Polidori, N.; Giannini, C.; Blasetti, A.; Chiarelli, F. Insulin resistance and type 2 diabetes in children. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 217–226. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Diamond, D.; Baylin, A.; Mora-Plazas, M.; Villamor, E. Chronic inflammation is associated with overweight in Colombian school children. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 244–251. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Shin, Y.-H.; Kim, J.-K.; Shim, J.Y.; Kang, D.-R.; Lee, H.-R. Metabolic syndrome and its association with white blood cell count in children and adolescents in Korea: The 2005 korean national health and nutrition examination survey. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.J.; Szklo, M.; Folsom, A.R.; Rock, R.; Mercuri, M. Leukocyte count correlates in middle-aged adults: The atherosclerosis risk in communities (ARIC) study. Am. J. Epidemiol. 1992, 136, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Weiss, S.T. Host and environmental factors influencing the peripheral blood leukocyte count. Am. J. Epidemiol. 1991, 134, 1402–1409. [Google Scholar] [CrossRef]
- Samocha-Bonet, D.; Justo, D.; Rogowski, O.; Saar, N.; Abu-Abeid, S.; Shenkerman, G.; Shapira, I.; Berliner, S.; Tomer, A. Platelet counts and platelet activation markers in obese subjects. Mediat. Inflamm. 2008, 2008, 834153. [Google Scholar] [CrossRef] [Green Version]
- Santilli, F.; Vazzana, N.; Liani, R.; Guagnano, M.T.; Davì, G. Platelet activation in obesity and metabolic syndrome. Obes. Rev. 2012, 13, 27–42. [Google Scholar] [CrossRef]
- Hall, J.E.; Crook, E.D.; Jones, D.W.; Wofford, M.R.; Dubbert, P.M. Mechanisms of obesity-associated cardiovascular and renal disease. Am. J. Med. Sci. 2002, 324, 127–137. [Google Scholar] [CrossRef]
- Göbel, B.O.; Schulte-Göbel, A.; Weisser, B.; Glänzer, K.; Vetter, H.; Düsing, R. Arterial blood pressure. Correlation with erythrocyte count, hematocrit, and hemoglobin concentration. Am. J. Hypertens. 1991, 4, 14–19. [Google Scholar] [CrossRef]
- Atsma, F.; Veldhuizen, I.; de Kort, W.; van Kraaij, M.; Pasker-de Jong, P.; Deinum, J. Hemoglobin level is positively associated with blood pressure in a large cohort of healthy individuals. Hypertension 2012, 60, 936–941. [Google Scholar] [CrossRef] [Green Version]
- Kweon, S.; Kim, Y.; Jang, M.-J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.-H.; Oh, K. Data resource profile: The Korea national health and nutrition examination survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Park, H.K.; Shim, Y.S. Distribution of Tri-Ponderal Mass Index and its Relation to Body Mass Index in Children and Adolescents Aged 10 to 20 Years. J. Clin. Endocrinol. Metab. 2020, 105, e826–e834. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, S.; Hwang, S.-S.; Shim, J.O.; Chae, H.W.; Lee, Y.J.; Lee, J.-H.; Kim, S.C.; Lim, D.; Yang, S.W.; et al. The 2017 Korean national growth charts for children and adolescents: Development, improvement, and prospects. Korean J. Pediatr. 2018, 61, 135–149. [Google Scholar] [CrossRef]
- Sait, S.; Alqassas, M.; Othman, S.; Shihon, S.; Alqalayta, L.; Alqusair, S. Obesity correlates with neutrophilia. Hematol. Transfus. Int. J. 2016, 3, 159–162. [Google Scholar] [CrossRef]
- Gu, Y.; Hu, K.; Huang, Y.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Xia, Y.; Bao, X.; Shi, H.; et al. White blood cells count as an indicator to identify whether obesity leads to increased risk of type 2 diabetes. Diabetes Res. Clin. Pr. 2018, 141, 140–147. [Google Scholar] [CrossRef]
- Alrubaie, A.; Majid, S.; Alrubaie, R.; Al-Zahraa, B.; Kadhim, F. Effects of Body Mass Index (BMI) on complete blood count parameters. Prensa Med. Argent 2019, 105, 164–171. [Google Scholar]
- Veronelli, A.; Laneri, M.; Ranieri, R.; Koprivec, D.; Vardaro, D.; Paganelli, M.; Folli, F.; Pontiroli, A.E. White blood cells in obesity and diabetes: Effects of weight loss and normalization of glucose metabolism. Diabetes Care 2004, 27, 2501–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohshita, K.; Yamane, K.; Hanafusa, M.; Mori, H.; Mito, K.; Okubo, M.; Hara, H.; Kohno, N. Elevated white blood cell count in subjects with impaired glucose tolerance. Diabetes Care 2004, 27, 491–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, P.C.; Lee, K.-F.; So, W.-Y.; Ng, M.H.; Chan, W.-B.; Lo, M.K.; Chan, N.N.; Chan, J.C. White blood cell count is associated with macro-and microvascular complications in Chinese patients with type 2 diabetes. Diabetes care 2004, 27, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wärnberg, J.; Marcos, A. Low-grade inflammation and the metabolic syndrome in children and adolescents. Curr. Opin. Lipidol. 2008, 19, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Di Bonito, P.; Pacifico, L.; Chiesa, C.; Invitti, C.; Del Giudice, E.M.; Baroni, M.G.; Moio, N.; Pellegrin, M.; Tomat, M.; Licenziati, M.; et al. White blood cell count may identify abnormal cardiometabolic phenotype and preclinical organ damage in overweight/obese children. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 502–509. [Google Scholar] [CrossRef]
- Park, J.-M.; Lee, D.-C.; Lee, Y.-J. Relationship between high white blood cell count and insulin resistance (HOMA-IR) in Korean children and adolescents: Korean national health and nutrition examination survey 2008–2010. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 456–461. [Google Scholar] [CrossRef]
- Mărginean, C.O.; Meliţ, L.E.; Ghiga, D.V.; Mărginean, M.O. Early inflammatory status related to pediatric obesity. Front. Pediatr. 2019, 7, 241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Meng, Z.; Liu, M.; Ren, X.; Zhu, M.; He, Q.; Zhang, Q.; Liu, L.; Song, K.; Jia, Q.; et al. The associations between leukocyte, erythrocyte or platelet, and metabolic syndrome in different genders of Chinese. Medicine 2016, 95, e5189. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Ragno, E.; Benvenuti, E.; Zito, G.A.; Corsi, A.; Ferrucci, L.; Paolisso, G. New aspects of the insulin resistance syndrome: Impact on haematological parameters. Diabetologia 2001, 44, 1232–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.T.; Yang, S.; Yang, Y.M.; Zhao, H.L.; Chen, Y.C.; Zhao, X.H.; Wen, J.B.; Tian, Y.R.; Yan, W.L.; Shen, C. Exploring the relationship of peripheral total bilirubin, red blood cell, and hemoglobin with blood pressure during childhood and adolescence. J. Pediatr. 2018, 94, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, B.J.; Stults, H.B.; Mayer, J. Hypoferræmia in obese adolescents. Lancet 1962, 2, 327–328. [Google Scholar] [CrossRef]
- Bekri, S.; Gual, P.; Anty, R.; Luciani, N.; Dahman, M.; Ramesh, B.; Iannelli, A.; Staccini-Myx, A.; Casanova, D.; Ben Amor, I.; et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology 2006, 131, 788–796. [Google Scholar] [CrossRef]
- Ausk, K.J.; Ioannou, G.N. Is obesity associated with anemia of chronic disease? A population-based study. Obesity 2008, 16, 2356–2361. [Google Scholar] [CrossRef]
- Kim, J.A.; Choi, Y.S.; Hong, J.I.; Kim, S.H.; Jung, H.H.; Kim, S.M. Association of metabolic syndrome with white blood cell subtype and red blood cells. Endocr. J. 2006, 53, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Farhangi, M.A.; Keshavarz, S.A.; Eshraghian, M.; Ostadrahimi, A.; Saboor-Yaraghi, A.-A. White blood cell count in women: Relation to inflammatory biomarkers, haematological profiles, visceral adiposity, and other cardiovascular risk factors. J. Health Popul. Nutr. 2013, 31, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.-J.; Seo, M.-S.; Shim, J.-Y.; Kim, K.-E.; Shin, Y.H.; Lee, Y.J. The association between platelet count and metabolic syndrome in children and adolescents. Platelets 2015, 26, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Charles, L.E.; Fekedulegn, D.; McCall, T.; Burchfiel, C.M.; Andrew, M.E.; Violanti, J.M. Obesity, white blood cell counts, and platelet counts among police officers. Obesity 2007, 15, 2846–2854. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, F.Q. Body mass index: Obesity, BMI, and health: A critical review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Nead, K.G.; Halterman, J.S.; Kaczorowski, J.M.; Auinger, P.; Weitzman, M. Overweight children and adolescents: A risk group for iron deficiency. Pediatrics 2004, 114, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Pinhas-Hamiel, O.; Newfield, R.S.; Koren, I.; Agmon, A.; Lilos, P.; Phillip, M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int. J. Obes. 2003, 27, 416–418. [Google Scholar] [CrossRef] [PubMed]
| Normal Weight | Overweight | Obesity | p | |
|---|---|---|---|---|
| Total, n (%) | 6421 (80.29%) | 782 (9.78%) | 794 (9.93%) | |
| Boys, n (%) | 3350 (52.17%) | 443(56.65%) | 466 (58.69%) | <0.001 |
| Age (year) | 14.33 ± 2.51 | 14.24 ± 2.51 | 14.78 ± 2.54 | <0.001 |
| Height SDS | 0.17 ± 1.03 | 0.47 ± 1.04 | 0.53 ± 1.11 | <0.001 |
| Weight SDS | −0.31 ± 0.89 | 1.29 ± 0.49 | 2.20 ± 0.74 | <0.001 |
| BMI SDS (kg/m2) | −0.59 ± 0.88 | 0.86 ± 0.45 | 1.64 ± 0.75 | <0.001 |
| WC SDS | −0.49 ± 0.85 | 1.32 ± 0.18 | 2.40 ± 0.67 | <0.001 |
| SBP (mmHg) | 105.65 ± 9.77 | 109.94 ± 10.28 | 113.68 ± 10.95 | <0.001 |
| DBP (mmHg) | 65.59 ± 8.91 | 66.88 ± 8.88 | 69.07 ± 9.19 | <0.001 |
| WBC (×103/mm3) | 6.16 ± 1.49 | 6.65 ± 1.58 | 7.16 ± 1.65 | <0.001 |
| RBC (×106/mm3) | 4.81 ± 0.39 | 4.89 ± 0.40 | 4.93 ± 0.41 | <0.001 |
| Hemoglobin (g/dL) | 13.97 ± 1.23 | 14.07 ± 1.22 | 14.19 ± 1.30 | <0.001 |
| Hematocrit (%) | 41.86 ± 3.40 | 42.28 ± 3.42 | 42.65 ± 3.53 | <0.001 |
| Platelets (×103/mm3) | 282.96 ± 58.58 | 296.81 ± 59.92 | 309.03 ± 63.53 | <0.001 |
| Glucose (mg/dL) | 90.01 ± 7.39 | 91.83 ± 11.05 | 92.45 ± 12.16 | <0.001 |
| T-C (mg/dL) | 158.25 ± 26.27 | 163.00 ± 28.46 | 169.35 ± 29.50 | <0.001 |
| HDL-C (mg/dL) | 52.16 ± 9.94 | 47.68 ± 8.63 | 44.99 ± 8.37 | <0.001 |
| TG (mg/dL) | 79.29 ± 41.79 | 99.34 ± 54.29 | 112.75 ± 58.76 | <0.001 |
| LDL-C (mg/dL) | 90.24 ± 22.42 | 95.46 ± 25.07 | 101.81 ± 25.63 | <0.001 |
| Alcohol consumption | 1564 (24.36%) | 188 (24.04%) | 237 (29.85%) | 0.003 |
| Smoker | 718 (11.18%) | 88 (11.25%) | 106 (13.35%) | 0.191 |
| Household income ≤ 1st quartile | 687 (10.70%) | 82 (10.49%) | 95 (11.96%) | 0.531 |
| Rural residence | 985 (15.34%) | 113 (14.45%) | 125 (15.74%) | 0.757 |
| Physical activity | 2368 (36.88%) | 279 (35.68%) | 316 (39.80%) | 0.194 |
| Hypertension | 1 (0.02%) | 1 (0.13%) | 1 (0.13%) | 0.124 |
| T2DM | 0 (0%) | 0 (0%) | 0 (0%) | >0.999 |
| Dyslipidemia | 0 (0%) | 0 (0%) | 0 (0%) | >0.999 |
| Boys | Girls | |||||
|---|---|---|---|---|---|---|
| NW | OW | OB | NW | OW | OB | |
| n = 3350 | n = 443 | n = 466 | n = 3071 | n = 339 | n = 328 | |
| WBC (×103/mm3) | 6.13 ± 1.45 | 6.68 ± 1.53 a | 7.13 ± 1.60 b,c | 6.18 ± 1.53 | 6.61 ± 1.64 a | 7.22 ± 1.71 b,c |
| RBC (×106/mm3) | 5.02 ± 0.34 | 5.10 ± 0.34 a | 5.13 ± 0.33 c | 4.59 ± 0.31 | 4.62 ± 0.30 | 4.63 ± 0.31 c |
| Hemoglobin (g/dL) | 14.60 ± 1.11 | 14.64 ± 1.15 | 14.79 ± 1.20 c | 13.28 ± 0.96 | 13.33 ± 0.85 | 13.34 ± 0.92 |
| Hematocrit (%) | 43.43 ± 3.30 | 43.73 ± 3.38 | 44.18 ± 3.34 c | 40.15 ± 2.60 | 40.38 ± 2.42 | 40.49 ± 2.50 c |
| Platelet (×103/mm3) | 278.59 ± 58.48 | 295.33 ± 61.62 a | 302.91 ± 61.66 c | 287.73 ± 58.32 | 298.74 ± 57.65 a | 317.73 ± 65.21 b,c |
| All Participants 1 (n = 7997) | Normal Weight | Overweight | Obesity | p for Trend |
|---|---|---|---|---|
| n = 6421 | n = 782 | n = 794 | ||
| WBC (×103/mm3) | 6.16 ± 0.02 | 6.66 ± 0.05 a | 7.16 ± 0.05 b,c | <0.001 |
| RBC (×106/mm3) | 4.82 ± 0.01 | 4.88 ± 0.01 a | 4.90 ± 0.01 c | <0.001 |
| Hemoglobin (g/dL) | 13.99 ± 0.01 | 14.04 ± 0.04 | 14.07 ± 0.04 | 0.016 |
| Hematocrit (%) | 41.91 ± 0.04 | 42.21 ± 0.10 a | 42.31 ± 0.10 c | <0.001 |
| Platelets (×103/mm3) | 282.66 ± 0.72 | 296.38 ± 2.05 a | 311.87 ± 2.04 b,c | <0.001 |
| Boys 2 | ||||
| (n = 4259) | n = 3350 | n = 443 | n = 466 | |
| WBC (×103/mm3) | 6.13 ± 0.03 | 6.69 ± 0.07 a | 7.12 ± 0.07 b,c | <0.001 |
| RBC (×106/mm3) | 5.02 ± 0.01 | 5.11 ± 0.01 a | 5.12 ± 0.01 c | <0.001 |
| Hemoglobin (g/dL) | 14.60 ± 0.01 | 14.72 ± 0.04 a | 14.72 ± 0.04 c | <0.001 |
| Hematocrit (%) | 43.43 ± 0.04 | 43.96 ± 0.12 a | 44.00 ± 0.12 c | <0.001 |
| Platelets (×103/mm3) | 278.64 ± 0.97 | 293.21 ± 2.66 a | 304.58 ± 2.59 b,c | <0.001 |
| Girls 3 | ||||
| (n = 3738) | n = 3071 | n = 339 | n = 328 | |
| WBC (×103/mm3) | 6.18 ± 0.03 | 6.61 ± 0.08 a | 7.20 ± 0.09 b,c | <0.001 |
| RBC (×106/mm3) | 4.59 ± 0.01 | 4.62 ± 0.02 | 4.66 ± 0.02 c | <0.001 |
| Hemoglobin (g/dL) | 13.27 ± 0.02 | 13.34 ± 0.05 | 13.39 ± 0.05 | 0.015 |
| Hematocrit (%) | 40.14 ± 0.05 | 40.39 ± 0.14 | 40.59 ± 0.14 c | <0.001 |
| Platelets (×103/mm3) | 287.47 ± 1.05 | 298.90 ± 3.17 a | 319.95 ± 3.23 b,c | <0.001 |
| All Participants (n = 7997) | r 1 | p | r 2 | p |
|---|---|---|---|---|
| WBC (×103/mm3) | 0.223 | <0.001 | 0.222 | <0.001 |
| RBC (×106/mm3) | 0.096 | <0.001 | 0.109 | <0.001 |
| Hemoglobin (g/dL) | 0.044 | <0.001 | 0.042 | <0.001 |
| Hematocrit (%) | 0.067 | <0.001 | 0.067 | <0.001 |
| Platelets (×103/mm3) | 0.169 | <0.001 | 0.180 | <0.001 |
| Boys (n = 4259) | r3 | p | r4 | p |
| WBC (×103/mm3) | 0.239 | <0.001 | 0.240 | <0.001 |
| RBC (×106/mm3) | 0.157 | <0.001 | 0.180 | <0.001 |
| Hemoglobin (g/dL) | 0.070 | <0.001 | 0.111 | <0.001 |
| Hematocrit (%) | 0.090 | <0.001 | 0.137 | <0.001 |
| Platelets (×103/mm3) | 0.175 | <0.001 | 0.177 | <0.001 |
| Girls (n = 3738) | r5 | p | r6 | p |
| WBC (×103/mm3) | 0.206 | <0.001 | 0.202 | <0.001 |
| RBC (×106/mm3) | 0.047 | 0.004 | 0.078 | <0.001 |
| Hemoglobin (g/dL) | 0.014 | 0.383 | 0.033 | 0.043 |
| Hematocrit (%) | 0.042 | 0.009 | 0.054 | <0.001 |
| Platelets (×103/mm3) | 0.164 | <0.001 | 0.176 | <0.001 |
| All Participants 1 (n = 7997) | Β | SE | p |
|---|---|---|---|
| WBC (×103/mm3) | 0.275 | 0.013 | <0.001 |
| RBC (×106/mm3) | 0.028 | 0.003 | <0.001 |
| Hemoglobin (g/dL) | 0.034 | 0.009 | <0.001 |
| Hematocrit (%) | 0.152 | 0.025 | <0.001 |
| Platelet (×103/mm3) | 8.372 | 0.510 | <0.001 |
| Boys 2 (n = 4259) | |||
| WBC (×103/mm3) | 0.279 | 0.017 | <0.001 |
| RBC (×106/mm3) | 0.043 | 0.004 | <0.001 |
| Hemoglobin (g/dL) | 0.073 | 0.010 | <0.001 |
| Hematocrit (%) | 0.267 | 0.030 | <0.001 |
| Platelet (×103/mm3) | 7.658 | 0.657 | <0.001 |
| Girls 3 (n = 3738) | |||
| WBC (×103/mm3) | 0.270 | 0.021 | <0.001 |
| RBC (×106/mm3) | 0.019 | 0.004 | <0.001 |
| Hemoglobin (g/dL) | 0.027 | 0.013 | 0.038 |
| Hematocrit (%) | 0.118 | 0.035 | <0.001 |
| Platelet (×103/mm3) | 8.715 | 0.798 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.R.; Lee, H.S.; Shim, Y.S.; Hwang, J.S. Positive Associations between Body Mass Index and Hematological Parameters, Including RBCs, WBCs, and Platelet Counts, in Korean Children and Adolescents. Children 2022, 9, 109. https://doi.org/10.3390/children9010109
Jeong HR, Lee HS, Shim YS, Hwang JS. Positive Associations between Body Mass Index and Hematological Parameters, Including RBCs, WBCs, and Platelet Counts, in Korean Children and Adolescents. Children. 2022; 9(1):109. https://doi.org/10.3390/children9010109
Chicago/Turabian StyleJeong, Hwal Rim, Hae Sang Lee, Young Suk Shim, and Jin Soon Hwang. 2022. "Positive Associations between Body Mass Index and Hematological Parameters, Including RBCs, WBCs, and Platelet Counts, in Korean Children and Adolescents" Children 9, no. 1: 109. https://doi.org/10.3390/children9010109
APA StyleJeong, H. R., Lee, H. S., Shim, Y. S., & Hwang, J. S. (2022). Positive Associations between Body Mass Index and Hematological Parameters, Including RBCs, WBCs, and Platelet Counts, in Korean Children and Adolescents. Children, 9(1), 109. https://doi.org/10.3390/children9010109






