Adverse Childhood Experiences Predict Common Neurodevelopmental and Behavioral Health Conditions among U.S. Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analyses
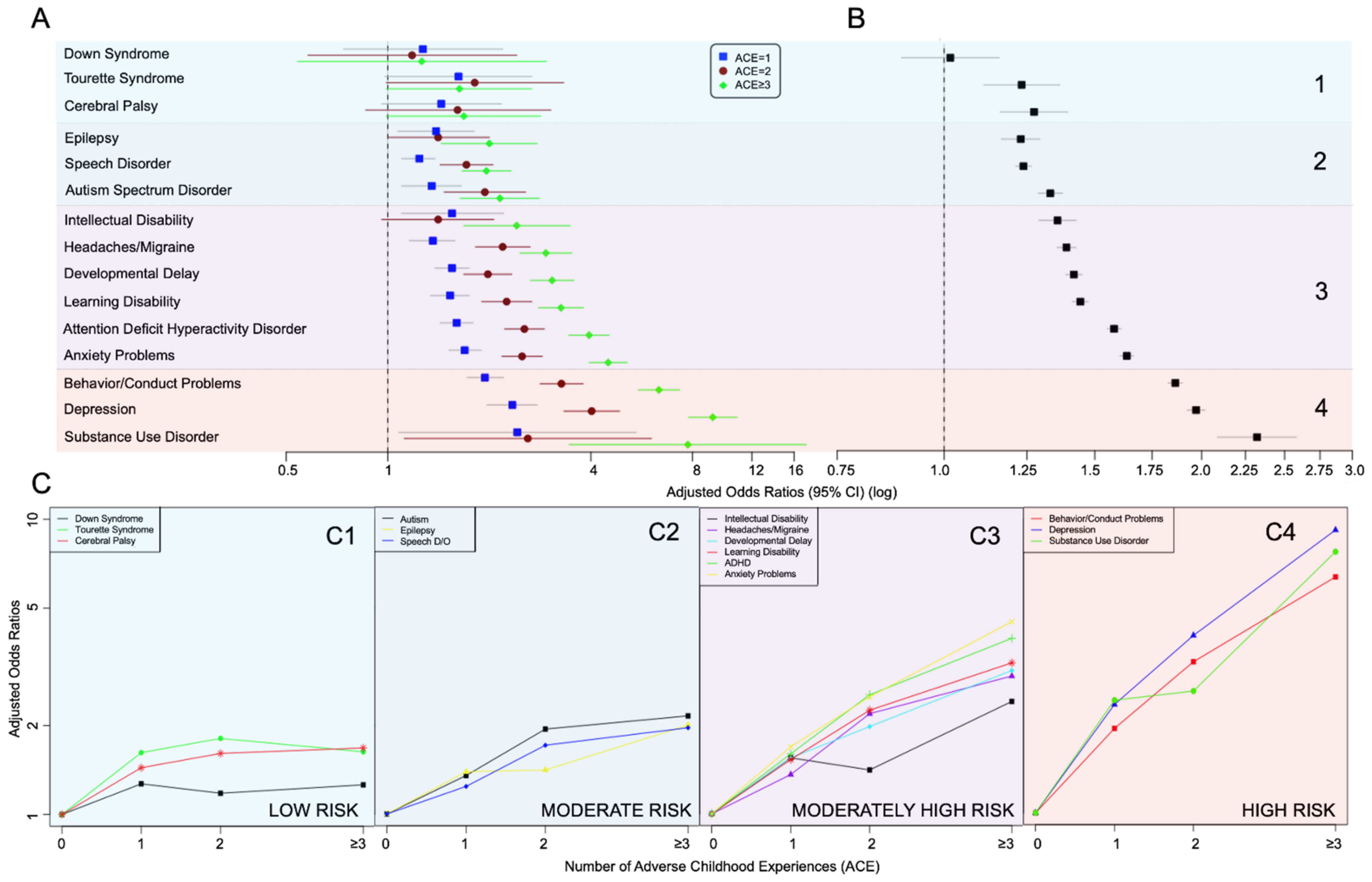
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults: The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Danese, A.; Moffitt, T.; Harrington, H.; Milne, B.J.; Polanczyk, G.; Pariante, C.; Poulton, R.; Caspi, A. Adverse childhood experiences predict adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Arch. Pediatr. Adolesc. Med. 2009, 163, 1135–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, M.; Giles, W.H.; Felitti, V.J.; Dube, S.R.; Williams, J.E.; Chapman, D.P.; Anda, R.F. Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation 2004, 110, 1761–1766. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.W.; Anda, R.F.; Tiemeier, H.; Felitti, V.J.; Edwards, V.J.; Croft, J.B.; Giles, W.H. Adverse Childhood Experiences and the Risk of Premature Mortality. Am. J. Prev. Med. 2009, 37, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Anda, R.F.; Dong, M.; Brown, D.W.; Felitti, V.J.; Giles, W.H.; Perry, G.S.; Valerie, E.J.; Dube, S.R. The relationship of adverse childhood experiences to a history of premature death of family members. BMC Public Health 2009, 9, 106. [Google Scholar] [CrossRef] [Green Version]
- Burke, N.J.; Hellman, J.L.; Scott, B.G.; Weems, C.; Carrion, V.G. The impact of adverse childhood experiences on an urban pediatric population. Child Abus. Negl. 2011, 35, 408–413. [Google Scholar] [CrossRef] [Green Version]
- Briggs, E.S.; Price, I.R. The relationship between adverse childhood experience and obsessive-compulsive symptoms and beliefs: The role of anxiety, depression, and experiential avoidance. J. Anxiety Disord. 2009, 23, 1037–1046. [Google Scholar] [CrossRef]
- Kessler, R.C.; McLaughlin, K.; Green, J.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Angermeyer, M.; et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry 2010, 197, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Mersky, J.; Topitzes, J.; Reynolds, A. Impacts of adverse childhood experiences on health, mental health, and substance use in early adulthood: A cohort study of an urban, minority sample in the U.S. Child Abus. Negl. 2013, 37, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Schilling, E.A.; Aseltine, R.H.; Gore, S. Adverse childhood experiences and mental health in young adults: A longitudinal survey. BMC Public Health 2007, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Pilowsky, D.J.; Keyes, K.M.; Hasin, D.S. Adverse Childhood Events and Lifetime Alcohol Dependence. Am. J. Public Health 2009, 99, 258–263. [Google Scholar] [CrossRef]
- Dube, S.R.; Anda, R.F.; Felitti, V.J.; Edwards, V.J.; Croft, J.B. Adverse childhood experiences and personal alcohol abuse as an adult. Addict. Behav. 2002, 27, 713–725. [Google Scholar] [CrossRef] [Green Version]
- Loudermilk, E.; Loudermilk, K.; Obenauer, J.; Quinn, M.A. Impact of adverse childhood experiences (ACEs) on adult alcohol consumption behaviors. Child Abus. Negl. 2018, 86, 368–374. [Google Scholar] [CrossRef]
- Anda, R.F.; Croft, J.B.; Felitti, V.J.; Nordenberg, D.; Giles, W.H.; Williamson, D.F.; Giovino, G.A. Adverse Childhood Experiences and Smoking During Adolescence and Adulthood. JAMA 1999, 282, 1652–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anda, R.F.; Brown, D.W.; Felitti, V.J.; Dube, S.R.; Giles, W.H. Adverse childhood experiences and prescription drug use in a cohort study of adult HMO patients. BMC Public Health 2008, 8, 198. [Google Scholar] [CrossRef] [Green Version]
- Chapman, D.P.; Whitfield, C.L.; Felitti, V.J.; Dube, S.R.; Edwards, V.J.; Anda, R.F. Adverse childhood experiences and the risk of depressive disorders in adulthood. J. Affect. Disord. 2004, 82, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Frewen, P.; Zhu, J.; Lanius, R. Lifetime traumatic stressors and adverse childhood experiences uniquely predict concurrent PTSD, complex PTSD, and dissociative subtype of PTSD symptoms whereas recent adult non-traumatic stressors do not: Results from an online survey study. Eur. J. Psychotraumatol. 2019, 10, 1606625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swopes, R.M.; Simonet, D.V.; Jaffe, A.E.; Tett, R.P.; Davis, J.L. Adverse Childhood Experiences, Posttraumatic Stress Disorder Symptoms, and Emotional Intelligence in Partner Aggression. Violence Vict. 2013, 28, 513–530. [Google Scholar] [CrossRef]
- Hillis, S.D.; Anda, R.F.; Felitti, V.J.; Nordenberg, D.; Marchbanks, P.A. Adverse Childhood Experiences and Sexually Transmitted Diseases in Men and Women: A Retrospective Study. Pediatrics 2000, 106, e11. [Google Scholar] [CrossRef] [Green Version]
- Grey, H.R.; Ford, K.; Bellis, M.A.; Lowey, H.; Wood, S. Associations between childhood deaths and adverse childhood experiences: An audit of data from a child death overview panel. Child Abus. Negl. 2019, 90, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Dube, S.R.; Anda, R.F.; Felitti, V.J.; Chapman, D.P.; Williamson, D.F.; Giles, W.H. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: Findings from the Adverse Childhood Experiences Study. JAMA 2001, 286, 3089–3096. [Google Scholar] [CrossRef] [Green Version]
- Barch, D.M.; Belden, A.C.; Tillman, R.; Whalen, D.; Luby, J.L. Early Childhood Adverse Experiences, Inferior Frontal Gyrus Connectivity, and the Trajectory of Externalizing Psychopathology. J. Am. Acad. Child Adolesc. Psychiatry 2018, 57, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Strathearn, L.; Giannotti, M.; Mills, R.; Kisely, S.; Najman, J.; Abajobir, A. Long-term Cognitive, Psychological, and Health Outcomes Associated With Child Abuse and Neglect. Pediatrics 2020, 146, e20200438. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.L.; Shiu, C.-S.; Acharya, K.; Stolbach, B.C.; Msall, M.E. Disparities in adversity among children with autism spectrum disorder: A population-based study. Dev. Med. Child Neurol. 2016, 58, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Folger, A.T.; Eismann, E.A.; Stephenson, N.B.; Shapiro, R.A.; Macaluso, M.; Brownrigg, M.E.; Gillespie, R.J. Parental Adverse Childhood Experiences and Offspring Development at 2 Years of Age. Pediatrics 2018, 141, e20172826. [Google Scholar] [CrossRef] [Green Version]
- Vervoort-Schel, J.; Mercera, G.; Wissink, I.; Mink, E.; Van Der Helm, P.; Lindauer, R.; Moonen, X. Adverse Childhood Experiences in Children with Intellectual Disabilities: An Exploratory Case-File Study in Dutch Residential Care. Int. J. Environ. Res. Public Health 2018, 15, 2136. [Google Scholar] [CrossRef]
- Anda, R.; Tietjen, G.; Schulman, E.; Felitti, V.; Croft, J. Adverse Childhood Experiences and Frequent Headaches in Adults. Headache J. Head Face Pain 2010, 50, 1473–1481. [Google Scholar] [CrossRef]
- Mansuri, F.; Nash, M.C.; Bakour, C.; Kip, K. Adverse Childhood Experiences (ACEs) and Headaches among Children: A Cross-Sectional Analysis. Headache J. Head Face Pain 2020, 60, 735–744. [Google Scholar] [CrossRef]
- Miklósi, M.; Máté, O.; Somogyi, K.; Szabó, M. Adult Attention Deficit Hyperactivity Disorder Symptoms, Perceived Stress, and Well-Being. J. Nerv. Ment. Dis. 2016, 204, 364–369. [Google Scholar] [CrossRef]
- Brown, N.; Brown, S.N.; Briggs, R.D.; Germán, M.; Belamarich, P.F.; Oyeku, S.O. Associations between Adverse Childhood Experiences and ADHD Diagnosis and Severity. Acad. Pediatr. 2017, 17, 349–355. [Google Scholar] [CrossRef]
- Jimenez, M.E.; Wade, R.; Schwartz-Soicher, O.; Lin, Y.; Reichman, N.E. Adverse Childhood Experiences and ADHD Diagnosis at Age 9 Years in a National Urban Sample. Acad. Pediatr. 2017, 17, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Bielas, H.; Barra, S.; Skrivanek, C.; Aebi, M.; Steinhausen, H.-C.; Bessler, C.; Plattner, B. The associations of cumulative adverse childhood experiences and irritability with mental disorders in detained male adolescent offenders. Child Adolesc. Psychiatry Ment. Health 2016, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Child and Adolescent Health Measurement Initiative. National Survey of Children’s Health (NSCH) 2016–2019. In Data Resource Center for Child and Adolescent Health Supported by the U.S. Department of Health and Human Services, Health Resources and Services Administration (HRSA), Maternal and Child Health Bureau (MCHB). Available online: https://www.childhealthdata.org/learn-about-the-nsch/NSCH (accessed on 28 August 2021).
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data. In U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Atlanta, GA. 2012. Available online: https://www.cdc.gov/brfss/index.html (accessed on 28 August 2021).
- Franz, A.P.; Bolat, G.U.; Bolat, H.; Matijasevich, A.; Santos, I.S.; Silveira, R.C.; Procianoy, R.S.; Rohde, L.A.; Moreira-Maia, C.R. Attention-Deficit/Hyperactivity Disorder and Very Preterm/Very Low Birth Weight: A Meta-analysis. Pediatrics 2017, 141, e20171645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NICHD Early Child Care Research Network. Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. Dev. Psychol. 1999, 35, 1297–1310. [Google Scholar] [CrossRef]
- Leonardo, B.; Yvonne, K.; Anja, H.; Naomi, P.; Rebecca, E.L. Adverse childhood experiences and trajectories of internalizing, externalizing, and prosocial behaviors from childhood to adolescence. Child Abuse Negl. 2021, 112, 104890. [Google Scholar]
- McLaughlin, K.A.; Sheridan, M.; Winter, W.; Fox, N.A.; Zeanah, C.H.; Nelson, C.A. Widespread Reductions in Cortical Thickness Following Severe Early-Life Deprivation: A Neurodevelopmental Pathway to Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2014, 76, 629–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, M.; Sims, T.L.; Bruce, M.A. Race, ethnicity, concentrated poverty, and low birth weight disparities. J. Natl. Black Nurses’ Assoc. JNBNA 2008, 19, 12. [Google Scholar]
- Sharpley, C.F.; Bitsika, V.; Andronicos, N.M.; Agnew, L.L. Further evidence of HPA-axis dysregulation and its correlation with depression in Autism Spectrum Disorders: Data from girls. Physiol. Behav. 2016, 167, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Corbett, B.A. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology 2014, 49, 207–228. [Google Scholar] [CrossRef] [Green Version]
- Mahar, I.; Bambico, F.R.; Mechawar, N.; Nobrega, J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014, 38, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Carrion, V.G.; Weems, C.F.; Reiss, A.L. Stress Predicts Brain Changes in Children: A Pilot Longitudinal Study on Youth Stress, Posttraumatic Stress Disorder, and the Hippocampus. Pediatrics 2007, 119, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Levitt, P. Toxic Stress and Its Impact on Early Learning and Health: Building a Formula for Human Capital Development. Paper presented at: The Science of Early Brain Development: A Foundation for the Success of Our Children and the State Economy. The Wisconsin Family Impact Seminars. Madison, Wisconsin, 2013. Available online: https://healthpolicy.usc.edu/wp-content/uploads/1970/01/Levitt_2017.pdf (accessed on 28 August 2021).
- Strathearn, L.; Gray, P.H.; Wood, D.O. Childhood Neglect and Cognitive Development in Extremely Low Birth Weight Infants: A Prospective Study. Pediatrics 2001, 108, 142–151. [Google Scholar] [CrossRef]
- Mills, R.; Alati, R.; O’Callaghan, M.; Najman, J.M.; Williams, G.M.; Bor, W.; Strathearn, L. Child Abuse and Neglect and Cognitive Function at 14 Years of Age: Findings from a Birth Cohort. Pediatrics 2010, 127, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.; Kisely, S.; Alati, R.; Strathearn, L.; Najman, J.M. Cognitive and educational outcomes of maltreated and non-maltreated youth: A birth cohort study. Aust. N. Z. J. Psychiatry 2019, 53, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zeegers, M.A.J.; Colonnesi, C.; Stams, G.-J.J.M.; Meins, E. Mind matters: A meta-analysis on parental mentalization and sensitivity as predictors of infant–parent attachment. Psychol. Bull. 2017, 143, 1245–1272. [Google Scholar] [CrossRef] [PubMed]
- Storebo, O.J.; Rasmussen, P.D.; Simonsen, E. Association between Insecure Attachment and ADHD: Environmental Mediating Factors. J. Atten. Disord. 2016, 20, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Pauli-Pott, U.; Schloß, S.; Becker, K. Maternal Responsiveness as a Predictor of Self-Regulation Development and Attention-Deficit/Hyperactivity Symptoms across Preschool Ages. Child Psychiatry Hum. Dev. 2017, 49, 42–52. [Google Scholar] [CrossRef] [PubMed]
- BakerDaniel, J.K.; Messinger, D.S.; Lyons, K.K.; Grantz, C.J. A Pilot Study of Maternal Sensitivity in the Context of Emergent Autism. J. Autism Dev. Disord. 2010, 40, 988–999. [Google Scholar] [CrossRef] [Green Version]

| Household Challenge ACEs |
|---|
| Child has ever experienced: |
| • Parental incarceration |
| • Family violence |
| • Household mental illness |
| • Household alcohol/drug problems |
| • Parental divorce/separation |
| • Parental death |
| • Household poverty |
| Variable | Number of Household Challenge ACEs | Chi-Sq. p-Value | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | ≥3 | ||
| No. of participants (N) | 80,399 | 27,603 | 10,961 | 10,938 | |
| Age of child (N, %) | |||||
| 0–5 years | 27,296 (33.95) | 6471 (23.44) | 1734 (15.82) | 1313 (12.00) | <0.0001 |
| 6–11 years | 23,970 (29.81) | 8437 (30.57) | 3462 (31.58) | 3520 (32.18) | |
| ≥12 years | 29,133 (36.24) | 12,695 (45.99) | 5765 (52.60) | 6105 (55.81) | |
| Sex (N, %) | |||||
| Male | 41,609 (51.75) | 14,332 (51.92) | 5596 (51.05) | 5566 (50.89) | 0.15 |
| Female | 38,790 (48.25) | 13,271 (48.08) | 5365 (48.95) | 5372 (49.11) | |
| Child’s Race/Ethnicity (N, %) | |||||
| Hispanic | 8208 (10.21) | 3620 (13.11) | 1540 (14.05) | 1494 (13.66) | <0.0001 |
| Non-Hispanic White | 57,846 (71.95) | 18,447 (66.83) | 7156 (65.29) | 7161 (65.47) | |
| Non-Hispanic Black | 3668 (4.56) | 2317 (8.39) | 1026 (9.36) | 857 (7.84) | |
| Non-Hispanic Other | 10,677 (13.28) | 3219 (11.66) | 1239 (11.30) | 1426 (13.04) | |
| Parental Education (N, %) | |||||
| Less than high school | 1413 (1.76) | 799 (2.89) | 340 (3.10) | 425 (3.89) | <0.0001 |
| High school | 6898 (8.58) | 4551 (16.49) | 2342 (21.37) | 2500 (22.86) | |
| College or higher | 71,751 (89.24) | 22,094 (80.04) | 8221 (75.00) | 7938 (72.57) | |
| Missing | 337 (0.42) | 159 (0.58) | 58 (0.53) | 75 (0.69) | |
| Family income to poverty ratio (N, %) | |||||
| <1.0 | 5314 (6.61) | 3951 (14.31) | 2231 (20.35) | 2630 (24.04) | |
| 1.0–1.9 | 9107 (11.33) | 5909 (21.41) | 2692 (24.56) | 3011 (27.53) | <0.0001 |
| 2.0–3.9 | 23,898 (29.72) | 9419 (34.12) | 3559 (32.47) | 3359 (30.71) | |
| ≥4.0 | 42,080 (52.34) | 8324 (30.16) | 2479 (22.62) | 1938 (17.72) | |
| Low birth weight child (N, %) | |||||
| Yes | 5812 (7.23) | 2242 (8.12) | 1002 (9.14) | 1037 (9.48) | <0.0001 |
| No | 71,289 (88.67) | 23,992 (86.92) | 9322 (85.05) | 9065 (82.88) | |
| Missing | 3298 (4.10) | 1369 (4.96) | 637 (5.81) | 836 (7.64) | |
| Preterm birth (N, %) | |||||
| Yes | 7912 (9.84) | 3212 (11.64) | 1381 (12.60) | 1367 (12.50) | <0.0001 |
| No | 71,511 (88.95) | 23,942 (86.74) | 9367 (85.46) | 9314 (85.15) | |
| Missing | 976 (1.21) | 449 (1.63) | 213 (1.94) | 257 (2.35) | |
| Household Smoking (N, %) | |||||
| Yes | 7033 (8.75) | 4947 (17.92) | 2825 (25.77) | 3840 (35.11) | <0.0001 |
| No | 72,903 (90.68) | 22,507 (81.54) | 8077 (73.69) | 7032 (64.29) | |
| Missing | 463 (0.58) | 149 (0.54) | 59 (0.54) | 66 (0.60) | |
| Health Condition | Number of Household Challenge ACEs | |||
|---|---|---|---|---|
| 0 | 1 | 2 | ≥3 | |
| Down Syndrome, (N = 129,533) | ||||
| Cases/total participants | 126/80160 | 74/27539 | 26/10927 | 20/10907 |
| Unadjusted Model | (reference) | 1.42 (0.82–2.46) | 1.36 (0.65–2.85) | 1.58 (0.71–3.50) |
| Adjusted Model | (reference) | 1.27 (0.74–2.19) | 1.18 (0.58–2.41) | 1.26 (0.54–2.95) |
| Tourette Syndrome, (N = 129,506) | ||||
| Cases/total participants | 164/80187 | 85/27504 | 42/10925 | 52/10890 |
| Unadjusted Model | (reference) | 1.74 (1.11–2.74) * | 2.09 (1.15–3.81) * | 2.20 (1.37–3.55) ** |
| Adjusted Model | (reference) | 1.62 (0.98–2.67) | 1.81 (0.99–3.32) | 1.63 (0.99–2.67) |
| Cerebral Palsy, (N =129,400) | ||||
| Cases/total participants | 188/80113 | 130/27492 | 51/10914 | 65/10881 |
| Unadjusted Model | (reference) | 1.63 (1.10–2.42) * | 2.05 (1.14–3.71) * | 2.30 (1.41–3.74) *** |
| Adjusted Model | (reference) | 1.44 (0.96–2.17) | 1.61 (0.86–3.04) | 1.68 (0.99–2.84) |
| Epilepsy or Seizure Disorder, (N = 129,481) | ||||
| Cases/total participants | 669/80143 | 357/27519 | 167/10920 | 200/10899 |
| Unadjusted Model | (reference) | 1.47 (1.16–1.86) ** | 1.61 (1.16–2.24) ** | 2.39 (1.79–3.17) *** |
| Adjusted Model | (reference) | 1.39 (1.07–1.80) * | 1.41 (1.00–2.00) | 2.00 (1.44–2.77) *** |
| Speech Disorder, (N = 129,499) | ||||
| Cases/total participants | 5340/80168 | 2370/27509 | 1132/10922 | 1303/10900 |
| Unadjusted Model | (reference) | 1.25 (1.13–1.39) *** | 1.76 (1.49–2.08) *** | 2.05 (1.76–2.38) *** |
| Adjusted Model | (reference) | 1.24 (1.10–1.38) *** | 1.71 (1.43–2.05) *** | 1.96 (1.66–2.32) *** |
| Autism Spectrum Disorder, (N = 129,309) | ||||
| Cases/total participants | 1522/80040 | 939/27483 | 502/10897 | 552/10889 |
| Unadjusted Model | (reference) | 1.57 (1.29–1.91) *** | 2.46 (1.88–3.23) *** | 2.98 (2.35–3.77) *** |
| Adjusted Model | (reference) | 1.35 (1.10–1.65) ** | 1.94 (1.47–2.56) *** | 2.15 (1.64–2.81) *** |
| Intellectual Disability, (N = 129,427) | ||||
| Cases/total participants | 528/80106 | 360/27506 | 172/10918 | 269/10897 |
| Unadjusted Model | (reference) | 1.91 (1.39–2.64) *** | 1.96 (1.37–2.80) *** | 3.58 (2.59–4.96) *** |
| Adjusted Model | (reference) | 1.55 (1.10–2.20) * | 1.41 (0.96–2.06) | 2.41 (1.68–3.47) *** |
| Severe or Frequent Headaches, (N = 129,504) | ||||
| Cases/total participants | 2412/80164 | 1372/27509 | 874/10923 | 1189/10908 |
| Unadjusted Model | (reference) | 1.69 (1.45–1.96) *** | 3.14 (2.62–3.77) *** | 4.65 (3.93–5.49) *** |
| Adjusted Model | (reference) | 1.36 (1.16–1.58) *** | 2.19 (1.82–2.64) *** | 2.94 (2.47–3.51) *** |
| Developmental Delay, (N = 129,413) | ||||
| Cases/total participants | 3898/80123 | 2248/27496 | 1109/10906 | 1503/10888 |
| Unadjusted Model | (reference) | 1.64 (1.47–1.84) *** | 2.17 (1.86–2.52) *** | 3.42 (2.99–3.91) *** |
| Adjusted Model | (reference) | 1.55 (1.38–1.74) *** | 1.98 (1.68–2.33) *** | 3.07 (2.65–3.56) *** |
| Learning Disability, (N = 112,531) | ||||
| Cases/total participants | 3439/66996 | 2191/24675 | 1247/10318 | 1824/10542 |
| Unadjusted Model | (reference) | 1.76 (1.56–2.00) *** | 2.83 (2.40–3.32) *** | 4.35 (3.79–4.99) *** |
| Adjusted Model | (reference) | 1.53 (1.34–1.74) *** | 2.25 (1.90–2.67) *** | 3.26 (2.80–3.80) *** |
| Attention Deficit Hyperactivity Disorder, (N = 128,897) | ||||
| Cases/total participants | 5029/79840 | 3098/27365 | 1859/10855 | 2677/10837 |
| Unadjusted Model | (reference) | 1.81 (1.64–2.00) *** | 3.16 (2.81–3.57) *** | 5.36 (4.78–6.00) *** |
| Adjusted Model | (reference) | 1.60 (1.43–1.79) *** | 2.54 (2.22–2.91) *** | 3.95 (3.44–4.53) *** |
| Anxiety Problems, (N = 129,488) | ||||
| Cases/total participants | 5166/80183 | 3101/27498 | 1878/10920 | 2948/10887 |
| Unadjusted Model | (reference) | 1.82 (1.64–2.01) *** | 2.92 (2.57–3.32) *** | 5.63 (5.03–6.29) *** |
| Adjusted Model | (reference) | 1.69 (1.52–1.89) *** | 2.50 (2.18–2.87) *** | 4.50 (3.96–5.12) *** |
| Behavior/Conduct Problems, (N = 129,509) | ||||
| Cases/total participants | 3623/80185 | 2508/27505 | 1660/10918 | 2830/10901 |
| Unadjusted Model | (reference) | 2.14 (1.91–2.40) *** | 3.91 (3.42–4.46) *** | 7.98 (7.08–9.00) *** |
| Adjusted Model | (reference) | 1.94 (1.72–2.20) *** | 3.27 (2.83–3.79) *** | 6.36 (5.53–7.32) *** |
| Depression, (N = 129,504) | ||||
| Cases/total participants | 1704/80187 | 1477/27508 | 1065/10919 | 2058/10890 |
| Unadjusted Model | (reference) | 2.76 (2.34–3.25) *** | 5.29 (4.42–6.34) *** | 12.82 (11.06–14.87) *** |
| Adjusted Model | (reference) | 2.34 (1.97–2.77) *** | 4.02 (3.33–4.86) *** | 9.19 (7.79–10.84) *** |
| Substance Use Disorder, (N = 92,132) | ||||
| Cases/total participants | 53/52588 | 51/20900 | 46/9135 | 132/9509 |
| Unadjusted Model | (reference) | 2.85 (1.30–6.26) ** | 3.39 (1.61–7.15) ** | 12.13 (6.18–23.84) *** |
| Adjusted Model | (reference) | 2.42 (1.08–5.44) * | 2.60 (1.12–6.04) * | 7.75 (3.45–17.39) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarei, K.; Xu, G.; Zimmerman, B.; Giannotti, M.; Strathearn, L. Adverse Childhood Experiences Predict Common Neurodevelopmental and Behavioral Health Conditions among U.S. Children. Children 2021, 8, 761. https://doi.org/10.3390/children8090761
Zarei K, Xu G, Zimmerman B, Giannotti M, Strathearn L. Adverse Childhood Experiences Predict Common Neurodevelopmental and Behavioral Health Conditions among U.S. Children. Children. 2021; 8(9):761. https://doi.org/10.3390/children8090761
Chicago/Turabian StyleZarei, Kasra, Guifeng Xu, Bridget Zimmerman, Michele Giannotti, and Lane Strathearn. 2021. "Adverse Childhood Experiences Predict Common Neurodevelopmental and Behavioral Health Conditions among U.S. Children" Children 8, no. 9: 761. https://doi.org/10.3390/children8090761
APA StyleZarei, K., Xu, G., Zimmerman, B., Giannotti, M., & Strathearn, L. (2021). Adverse Childhood Experiences Predict Common Neurodevelopmental and Behavioral Health Conditions among U.S. Children. Children, 8(9), 761. https://doi.org/10.3390/children8090761







