Does a Split-Week Gestational Age Model Provide Valuable Information on Neurodevelopmental Outcomes in Extremely Preterm Infants?
Abstract
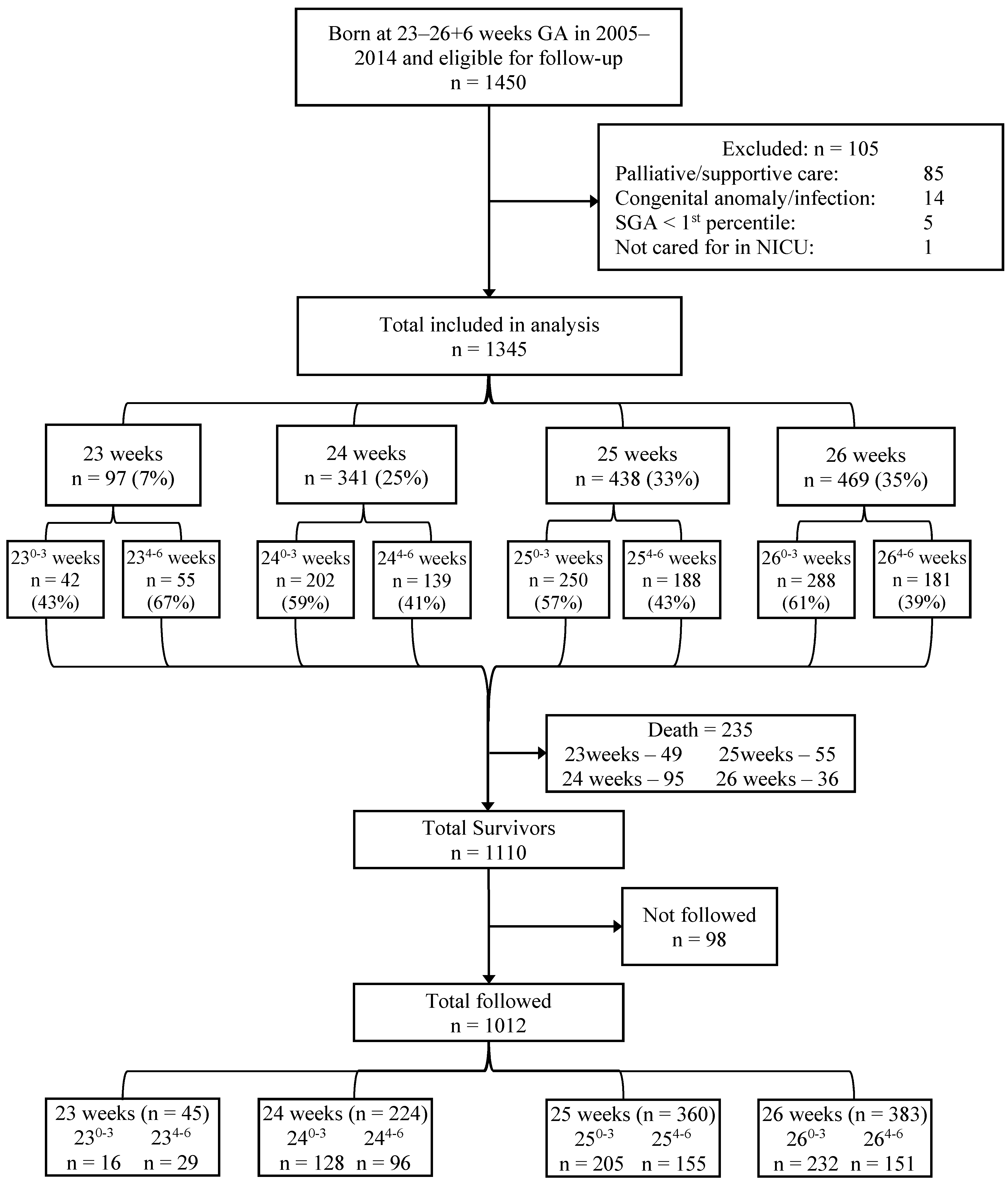
:1. Introduction
2. Methods
Statistical Analyses
3. Results
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pierrat, V.; Marchand-Martin, L.; Arnaud, C.; Kaminski, M.; Resche-Rigon, M.; Lebeaux, C.; Bodeau-Livinec, F.; Morgan, A.S.; Goffinet, F.; Marret, S.; et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ 2017, 358, j3448. [Google Scholar] [CrossRef] [Green Version]
- Younge, N.; Goldstein, R.F.; Bann, C.M.; Hintz, S.R.; Patel, R.M.; Smith, P.B.; Bell, E.F.; Rysavy, M.A.; Duncan, A.F.; Vohr, B.R.; et al. Survival and Neurodevelopmental Outcomes among Periviable Infants. N. Engl. J. Med. 2017, 376, 617–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serenius, F.; Källén, K.; Blennow, M.; Ewald, U.; Fellman, V.; Holmström, G.; Lindberg, E.; Lundqvist, P.; Maršál, K.; Norman, M.; et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA 2013, 309, 1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kono, Y.; Yonemoto, N.; Nakanishi, H.; Kusuda, S.; Fujimura, M. Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks’ gestation: A retrospective observational study in tertiary centres in Japan. BMJ Paediatr. Open 2018, 2, e000211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Söderström, F.; Normann, E.; Jonsson, M.; Ågren, J. Outcomes of a uniformly active approach to infants born at 22–24 weeks of gestation. Arch. Dis. Child Fetal Neonatal 2021, 106, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.L.; Dagle, J.M.; Bell, E.F.; Colaizy, T.T. Outcomes at 18 to 22 Months of Corrected Age for Infants Born at 22 to 25 Weeks of Gestation in a Center Practicing Active Management. J. Pediatr. 2020, 217, 52–58.e1. [Google Scholar] [CrossRef] [PubMed]
- Canadian Neonatal Network. Available online: http://www.canadianneonatalnetwork.org (accessed on 2 July 2021).
- Schmidt, B.; Asztalos, E.V.; Roberts, R.S.; Robertson, C.M.T.; Sauve, R.S.; Whitfield, M.F. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: Results from the trial of indomethacin prophylaxis in preterms. JAMA 2003, 289, 1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElrath, T.F.; Robinson, J.N.; Ecker, J.L.; Ringer, S.A.; Norwitz, E.R. Neonatal outcome of infants born at 23 weeks’ gestation. Obstet. Gynecol. 2001, 97, 49–52. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Amon, E.; Al-Hosni, M.; Gavard, J.A.; Gross, G.; Myles, T.D. “Early” versus “late” 23-week infant outcomes. Am. J. Obstet. Gynecol. 2012, 207, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; van Dyk, J.; Zein, H.; Nettel Aguirre, A.; Hendson, L.; Church, P.; Banihani, R.; Asztalos, E. Split-week gestational age model provides valuable information on outcomes in extremely preterm infants. Acta Paediatr. 2020, 12, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayley, N. Bayley Scales of Infant and Toddler Development, 3rd ed.; Psychological Corporation: San Antonio, TX, USA, 2006. [Google Scholar]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child. Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, E.V.; Church, P.T.; Riley, P.; Fajardo, C.; Shah, P.S. Association between Primary Caregiver Education and Cognitive and Language Development of Preterm Neonates. Am. J. Perinatol. 2017, 34, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Lemyre, B.; Moore, G. Counselling and management for anticipated extremely preterm birth. Paediatr. Child Health 2017, 22, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janvier, A.; Barrington, K.J.; Payot, A. A time for hope: Guidelines for the perinatal management of extremely preterm birth. Arch. Dis. Child Fetal Neonatal 2020, 105, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, M.; Janvier, A.; Lefebvre, F.; Luu, T.M. Parental Perspectives Regarding Outcomes of Very Preterm Infants: Toward a Balanced Approach. J. Pediatr. 2018, 200, 58–63.e1. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Roberts, R.S.; Davis, P.G.; Doyle, L.W.; Asztalos, E.V.; Opie, G.; Bairam, A.; Solimano, A.; Arnon, S.; Sauve, R.S. Prediction of late death or disability at age 5 years using a count of 3 neonatal morbidities in very low birth weight infants. J. Pediatr. 2015, 167, 982–986.e2. [Google Scholar] [CrossRef] [PubMed]
| Diagnostic Criteria | Normal or Mild Impairment | Moderate Impairment | Severe Impairment |
|---|---|---|---|
| Cognitive | BSID-III cognitive composite score >85 | BSID-III cognitive composite score 70–85 | BSID-III cognitive composite score <70 |
| Motor | BSID-III motor composite score >85 or No diagnosis of CP or mild CP (GMFCS 0, 1) | BSID-III motor composite score 70–85 or Diagnosis of CP with GMFCS level 2 (walks with orthotics) | BSID-III motor composite score <70 or Diagnosis of CP with GMFCS level 3–5 (walks using a hand-held mobility device or self-mobility with a powered mobility device or transported in a manual wheelchair) |
| Language | BSID-III language composite score ≥85 | BSID-III language composite score 70–84 | BSID-III language composite score <70 |
| Vision | Mild visual impairment (visual acuity better than 20/200 in both eyes) | Bilateral blindness (visual acuity less than 20–200 in the strongest eye) | Bilateral blindness that cannot be corrected |
| Hearing | Mild hearing impairment (not requiring amplification or requiring amplification in just one ear) | Bilateral hearing loss (requiring amplification) | Severe to profound hearing impairment (no functional hearing with amplification) |
| Gestational Weeks | ||||
|---|---|---|---|---|
| Maternal Characteristics and Morbidity among Mothers for Surviving Infants | 23 Weeks (n = 48) | 24 Weeks (n = 246) | 25 Weeks (n = 383) | 26 Weeks (n = 433) |
| Maternal age, mean (SD) | 29.94 (5.46) | 30.62 (5.51) | 30.68 (6.07) | 30.46 6.08) |
| Gravidity, mean (SD) | 2.79 (1.95) | 2.52 (1.66) | 2.61 (1.92) | 2.49 (1.72) |
| Maternal Education, n (%) | ||||
| Less than grade 12 equivalent | 9 (20.0) | 23 (10.6) | 27 (7.8) | 37 (9.9) |
| High school graduate | 13 (28.9) | 53 (24.4) | 66 (19.2) | 91 (24.3) |
| Some post-secondary degree | 12 (26.7) | 41 (18.9) | 66 (19.2) | 78 (20.8) |
| University/graduate degree | 11 (24.4) | 100 (46.1) | 185 (53.8) | 169 (45.1) |
| Unknown | 3 (6.0) | 29 (12.0) | 39 (10.0) | 58 (13.0) |
| Single parent family, n (%) | 6 (13.6) | 20 (9.4) | 32 (9.3) | 35 (9.4) |
| Obesity (weight > 91 kg), n (%) | 0 (0.0) | 10 (4.1) | 9 (2.4) | 15 (3.5) |
| Pre-pregnancy hypertension, n (%) | 0 (0.0) | 2 (0.8) | 10 (2.6) | 14 (3.2) |
| PIH, Gestational hypertension, Pre-eclampsia, n (%) | 0 (0.0) | 8 (3.3) | 27 (7.1) | 57 (13.2) |
| Assisted conception, n (%) | 8 (17.0) | 53 (21.7) | 55 (14.4) | 68 (15.7) |
| Smoking during pregnancy, n (%) | 7 (14.6) | 25 (10.2) | 43 (11.3) | 54 (12.5) |
| Alcohol use during pregnancy, n (%) | 1 (2.1) | 13 (5.3) | 12 (3.1) | 11 (2.5) |
| Multiple births, n (%) | 13 (27.1) | 66 (26.9) | 97 (25.3) | 112 (25.9) |
| Antenatal corticosteroids, n (%) | 36 (76.6) | 222 (90.2) | 353 (92.4) | 391 (90.9) |
| PPROM > 24 h, n (%) | 16 (34.0) | 61 (24.9) | 110 (28.9) | 117 (27.0) |
| Chorioamnionitis, n (%) | 15 (31.2) | 84 (34.1) | 130 (34.1) | 114 (26.3) |
| Antepartum hemorrhage, n (%) | 14 (29.2) | 74 (30.1) | 93 (24.5) | 116 (26.8) |
| Outborn, n (%) | 14 (29.2) | 45 (18.3) | 64 (16.70 | 89 (20.6) |
| Caesarean birth, n (%) | 11 (22.9) | 126 (51.2) | 204 (53.3) | 256 (59.1) |
| Neonatal Characteristics and Morbidity of Surviving Infants | 23 Weeks (n = 48) | 24 Weeks (n = 246) | 25 Weeks (n = 383) | 26 Weeks (n = 433) |
| Birth weight, mean (SD) | 591.77 (65.14) | 677.00 (90.22) | 766.97 (104.00) | 866.25 (152.73) |
| Small for gestational age, n (%) | 3 (6.2) | 14 (5.7) | 13 (3.4 | 43 9.9 |
| Female, n (%) | 23 (47.9) | 122 (49.6) | 202 (52.7) | 203 (46.9) |
| Necrotizing enterocolitis (≥Bell stage 2), n (%) | 7 (14.6) | 28 (11.4) | 48 (12.5) | 37 (8.5) |
| Retinopathy of prematurity, n (%) | ||||
| Stage 3 | 28 (58.3) | 92 (37.7) | 75 (19.6) | 40 (9.3) |
| Stage 4, 5 | 0 (0) | 1 (0.4) | 1 (0.3) | 0 (0) |
| Sepsis (culture-proven), n (%) | 26 (54.2) | 96 (39.3) | 113 (29.6) | 102 (23.6) |
| Intraventricular hemorrhage, n (%) | ||||
| Grade 1 | 13 (27.1) | 54 (22.0) | 77 (20.1) | 70 (16.2) |
| Grade 2 | 6 (12.5) | 29 (11.8) | 47 (12.3) | 29 (6.7) |
| Grade 3 | 4 (8.3) | 11 (4.5) | 16 (4.2) | 16 (3.7) |
| Grade 4/periventricular venous infarct | 7 (14.6) | 32 (13.1) | 34 (8.9) | 21 (4.8) |
| Periventricular leukomalacia, n (%) | 2 (4.2) | 5 (2.0) | 17 (4.4) | 14 (3.2) |
| Hypotension requiring inotropes, n (%) | 31 (64.6) | 87 (35.5) | 92 (24.0) | 82 (18.9) |
| Bronchopulmonary dysplasia, n (%) | 38 (79.2) | 158 (65.0) | 183 (48.3) | 154 (36.7) |
| Days of respiratory support, median (IQR) | 78.50 (62.00, 89.50) | 66.00 (54.00, 84.00) | 56.00 (43.00, 69.00) | 43.00 (32.00, 55.00 |
| Postnatal corticosteroids, n (%) | 17 (35.4) | 58 (23.6) | 53 (13.8) | 30 (6.9) |
| Length of stay (days) for initial hospitalization, median (IQR) | 127.00 (116.50, 139.50) | 118.00 (106.00, 134.00) | 104.00 (91.00, 123.00) | 90.00 (78.00, 103.00) |
| Gestational Weeks | ||||
|---|---|---|---|---|
| Neurodevelopmental Characteristics | 23 Weeks (n = 45) | 24 Weeks (n = 224) | 25 Weeks (n = 360) | 26 Weeks (n = 383) |
| Growth | ||||
| Head circumference, mean (SD) | 46.26 (1.67) | 46.53 (1.70) | 46.93 (1.82) | 47.24 (0.72) |
| Weight, mean (SD) | 10.17 (1.50) | 10.19 (1.52) | 10.56 (1.52) | 10.76 (1.57) |
| Height, mean (SD) | 80.19 (3.77) | 80.07 (3.40) | 80.27 (2.99) | 80.84 (3.46) |
| Post-discharge morbidity, n (%) | ||||
| Discharged home on oxygen | 26 (57.8) | 92 (41.1) | 87 (24.2) | 77 (20.1) |
| Gastrostomy tube feedings | 7 (15.9) | 18 (8.1) | 18 (5.0) | 12 (3.1) |
| Hospitalization | 18 (40.0) | 90 (40.2) | 126 (35.0) | 103 (27.0) |
| Need for recurrent medications | 16 (37.2) | 64 (29.1) | 84 (23.7) | 74 (19.7) |
| Seizure disorder | 1 (2.4) | 7 (3.2) | 8 (2.3) | 2 (0.5) |
| Ventriculoperitoneal shunt | 1 (2.2) | 11 (4.9) | 13 (3.6) | 6 (1.6) |
| BSID-III Measures | ||||
| BSID-III completion, n (%) | 33 (73.3) | 175 (78.10) | 303 (84.2) | 330 (86.2) |
| Cognition composite score, mean (SD) | 85.38 (15.06) | 88.63 (14.45) | 92.51 (13.95) | 94.08 (12.35) |
| median (IQR) | 90.00 (80.00, 95.00) | 90.00 (80.00, 100.00) | 95.00 (85.00, 100.00) | 95.00 (85.00, 105.00) |
| Language composite score, mean (SD) | 77.43 (16.16) | 83.09 (16.97) | 87.72 (15.46) | 87.79 (14.99) |
| median (IQR) | 74.00 (66.50, 92.50) | 84.50 (71.00, 94.00) | 89.00 (77.00, 100.00) | 89.00 (77.00, 100.00) |
| Motor composite score, mean (SD) | 82.69 (16.75) | 88.50 (14.13) | 91.68 (13.15) | 92.77 (12.41) |
| median (IQR) | 88.00 (70.00, 95.50) | 94.00 (82.00, 97.00) | 94.00 (85.00, 100.00) | 94.00 (85.00, 100.00) |
| Neurosensory/Neuromotor | ||||
| Hearing Impairment, n (%) | ||||
| Mild | 2 (4.4) | 13 (5.8) | 8 (2.2) | 5 (1.3) |
| Severe | 1 (2.2) | 14 (6.3) | 14 (3.9) | 8 (2.1) |
| Vision Impairment, n (%) | ||||
| Mild | 15 (33.33) | 36 (16.1) | 19 (5.3) | 16 (4.2) |
| Severe | 1 (2.2) | 6 (2.7) | 9 (2.5) | 5 (1.3) |
| Motor Impairment (Cerebral palsy), n (%) | ||||
| None | 39 (86.7) | 190 (87.6) | 318 (90.3) | 356 (93.9) |
| Mild (GMFCS 1, 2) | 2 (4.4) | 8 (3.7) | 23 (6.6) | 15 (4.0) |
| Moderate/severe (GMFCS 3, 4, 5) | 4 (8.8) | 19 (9.13) | 11 (3.1) | 8 (2.2) |
| Gestational Age (Weeks) | Outcome n (%) | p-Values | |||
|---|---|---|---|---|---|
| No Impairment | NDI β | SNI * | |||
| 23 | Full Early split Late split | 14 (37.8) 2 (13.3) 12 (54.5) | 10 (27.0) 5 (33.3) 5 (22.7) | 13 (35.1) 8 (53.3) 5 (22.7) | 0.034 |
| 24 | Full Early split Late split | 94 (49.0) 51 (45.9) 43 (53.1) | 55 (28.6) 34 (30.6) 21 (25.9) | 43 (22.4) 26 (23.4) 17 (21.0) | 0.615 |
| 25 | Full Early split Late split | 202 (65.0) 111 (63.8) 91 (66.4) | 66 (21.2) 39 (22.4) 27 (19.7) | 43 (13.8) 24 (13.8) 19 (13.9) | 0.841 |
| 26 | Full Early split Late split | 227 (69.0) 127 (65.1) 100 (74.6) | 77 (23.4) 53 (27.2) 24 (17.9) | 25 (7.6) 15 (7.7) 10 (7.5) | 0.138 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asztalos, E.; Aguirre, A.N.; Hendson, L.; Church, P.; Banihani, R.; van Dyk, J.; Zein, H.; Thomas, S. Does a Split-Week Gestational Age Model Provide Valuable Information on Neurodevelopmental Outcomes in Extremely Preterm Infants? Children 2021, 8, 731. https://doi.org/10.3390/children8090731
Asztalos E, Aguirre AN, Hendson L, Church P, Banihani R, van Dyk J, Zein H, Thomas S. Does a Split-Week Gestational Age Model Provide Valuable Information on Neurodevelopmental Outcomes in Extremely Preterm Infants? Children. 2021; 8(9):731. https://doi.org/10.3390/children8090731
Chicago/Turabian StyleAsztalos, Elizabeth, Alberto Nettel Aguirre, Leonora Hendson, Paige Church, Rudaina Banihani, Jessie van Dyk, Hussein Zein, and Sumesh Thomas. 2021. "Does a Split-Week Gestational Age Model Provide Valuable Information on Neurodevelopmental Outcomes in Extremely Preterm Infants?" Children 8, no. 9: 731. https://doi.org/10.3390/children8090731
APA StyleAsztalos, E., Aguirre, A. N., Hendson, L., Church, P., Banihani, R., van Dyk, J., Zein, H., & Thomas, S. (2021). Does a Split-Week Gestational Age Model Provide Valuable Information on Neurodevelopmental Outcomes in Extremely Preterm Infants? Children, 8(9), 731. https://doi.org/10.3390/children8090731







