Pulmonary Vein Stenosis in Children: A Programmatic Approach Employing Primary and Anatomic Therapy
Abstract
:1. Introduction
2. PVS Program
3. Diagnosis and Surveillance
4. Therapy for Pulmonary Vein Stenosis
4.1. Primary Therapy
4.2. Anatomic Therapy
5. Team Approach
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pazos-López, P.; García-Rodríguez, C.; Guitián-González, A.; Paredes-Galán, E.; Álvarez-Moure, M.D.L.G.; Rodríguez-Álvarez, M.; Baz-Alonso, J.A.; Teijeira-Fernández, E.; Calvo-Iglesias, F.E.; Íñiguez-Romo, A. Pulmonary vein stenosis: Etiology, diagnosis and management. World J. Cardiol. 2016, 8, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Drossner, D.M.; Kim, D.W.; Maher, K.O.; Mahle, W.T. Pulmonary Vein Stenosis: Prematurity and Associated Conditions. Pediatrics 2008, 122, e656–e661. [Google Scholar] [CrossRef] [PubMed]
- Kanter, K.R.; Kirshbom, P.M.; Kogon, B.E. Surgical Repair of Pulmonary Venous Stenosis: A Word of Caution. Ann. Thorac. Surg. 2014, 98, 1687–1692; discussion 1691–1692. [Google Scholar] [CrossRef]
- Kalfa, D.; Belli, E.; Bacha, E.; Lambert, V.; di Carlo, D.; Kostolny, M.; Salminen, J.; Nosal, M.; Poncelet, A.; Horer, J.; et al. Primary Pulmonary Vein Stenosis: Outcomes, Risk Factors, and Severity Score in a Multicentric Study. Ann. Thorac. Surg. 2017, 104, 182–189. [Google Scholar] [CrossRef] [Green Version]
- DiLorenzo, M.P.; Santo, A.; Rome, J.J.; Zhang, H.; Faerber, J.A.; Mercer-Rosa, L.; Hopper, R.K. Pulmonary Vein Stenosis: Outcomes in Children with Congenital Heart Disease and Prematurity. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 266–273. [Google Scholar] [CrossRef]
- Benjamin, J.T.; Hamm, C.R.; Zayek, M.; Eyal, F.G.; Carlson, S.; Manci, E. Acquired left-sided pulmonary vein stenosis in an extremely premature infant: A new entity? J. Pediatr. 2009, 154, 459. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.B.; Levy, P.T.; Stiver, C.A.; Boe, B.A.; Baird, C.W.; Callahan, R.M.; Smith, C.V.; Vanderlaan, R.D.; Backes, C.H. Primary pulmonary vein stenosis during infancy: State of the art review. J. Perinatol. 2021, 41, 1528–1539. [Google Scholar] [CrossRef]
- Riedlinger, W.F.; Juraszek, A.L.; Jenkins, K.J.; Nugent, A.W.; Balasubramanian, S.; Calicchio, M.L.; Kieran, M.; Collins, T. Pulmonary vein stenosis: Expression of receptor tyrosine kinases by lesional cells. Cardiovasc. Pathol. 2006, 15, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Cory, M.J.; Ooi, Y.K.; Kelleman, M.S.; Vincent, R.N.; Kim, D.W.; Petit, C.J. Reintervention is associated with improved survival in pediatric patients with pulmonary vein stenosis. JACC Cardiovasc. Interv. 2017, 10, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.D.; Briones, M.; Jones, S.; Suthar, D.; Gray, R.; Petit, C.J. Medical and Invasive Management of Congenital and Acquired Pulmonary Vein Stenosis. Curr. Treat. Options Pediatr. 2020, 6, 170–181. [Google Scholar] [CrossRef]
- Roman, K.S.; Kellenberger, C.J.; Macgowan, C.; Coles, J.; Redington, A.N.; Benson, L.N.; Yoo, S.-J. How is pulmonary arterial blood flow affected by pulmonary venous obstruction in children? A phase-contrast magnetic resonance study. Pediatr. Radiol. 2005, 35, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, G.; Troughton, R.W.; Xu, X.-F.; Salazar, H.P.; Wazni, O.M.; Grimm, R.A.; White, R.D.; Natale, A.; Klein, A.L. Detection of pulmonary vein stenosis by transesophageal echocardiography: Comparison with multidetector computed tomography. Am. Heart J. 2007, 153, 800–806. [Google Scholar] [CrossRef]
- Vyas, H.V.; Greenberg, S.B.; Krishnamurthy, R. MR Imaging and CT Evaluation of Congenital Pulmonary Vein Abnormalities in Neonates and Infants. Radiographics 2012, 32, 87–98. [Google Scholar] [CrossRef]
- Grosse-Wortmann, L.; Al-Otay, A.; Goo, H.W.; Macgowan, C.; Coles, J.G.; Benson, L.N.; Redington, A.N.; Yoo, S.-J. Anatomical and Functional Evaluation of Pulmonary Veins in Children by Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2007, 49, 993–1002. [Google Scholar] [CrossRef] [Green Version]
- Ghoshhajra, B.B.; Lee, A.M.; Engel, L.-C.; Celeng, C.; Kalra, M.K.; Brady, T.J.; Hoffmann, U.; Westra, S.; Abbara, S. Radiation Dose Reduction in Pediatric Cardiac Computed Tomography: Experience from a Tertiary Medical Center. Pediatr. Cardiol. 2013, 35, 171–179. [Google Scholar] [CrossRef]
- Gorlin, R.; Gorlin, S. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts. I. Am. Heart J. 1951, 41, 1–29. [Google Scholar] [CrossRef]
- Domingo, L.; Magdo, H.S.; Day, R.W. Acute pulmonary vasodilator testing and long-term clinical course in segmental pulmonary vascular disease. Pediatr. Cardiol. 2018, 39, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Fu, Y.Y.; Zhu, J.; Wang, L.; Aafaqi, S.; Rahkonen, O.; Slorach, C.; Traister, A.; Leung, C.H.; Chiasson, D.; et al. Pulmonary vein stenosis and the pathophysiology of “upstream” pulmonary veins. J. Thorac. Cardiovasc. Surg. 2014, 148, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Masaki, N.; Adachi, O.; Katahira, S.; Saiki, Y.; Horii, A.; Kawamoto, S.; Saiki, Y. Progression of vascular remodeling in pulmonary vein obstruction. J. Thorac. Cardiovasc. Surg. 2020, 160, 777–790.e5. [Google Scholar] [CrossRef]
- Rehman, M.; Jenkins, K.J.; Juraszek, A.L. A prospective phase II trial of vinblastine and methotrexate in multivessel intraluminal pulmonary vein stenosis in infants and children. Congenit. Heart Dis. 2011, 6, 608–623. [Google Scholar] [CrossRef] [PubMed]
- Callahan, R.; Kieran, M.W.; Baird, C.W. Adjunct targeted biologic inhibition agents to treat aggressive multivessel intraluminal pediatric pulmonary vein stenosis. J. Pediatr. 2018, 198, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bromberg-Marin, G.; Tsimikas, S.; Mahmud, E. Treatment of recurrent pulmonary vein stenoses with endovascular stenting and adjuvant oral sirolimus. Catheter. Cardiovasc. Interv. 2007, 69, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Callahan, R.; Esch, J.J.; Wang, G.; Ireland, C.M.; Gauvreau, K.; Jenkins, K.J. Systemic Sirolimus to Prevent In-Stent Stenosis in Pediatric Pulmonary Vein Stenosis. Pediatr. Cardiol. 2019, 41, 282–289. [Google Scholar] [CrossRef]
- Zhu, J.; Ide, H.; Fu, Y.Y.; Colan, S.D.; Gauvreau, K.; Ireland, C.M.; Marshall, A.C.; Sena, L.M.; Vargas, S.; Jenkins, K.J.; et al. Losartan ameliorates “upstream” pulmonary vein vasculopathy in a piglet model of pulmonary vein stenosis. J. Thorac. Cardiovasc. Surg. 2014, 148, 2550–2557. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.D.; Briones, M.; Mandhani, M.; Jones, S.; Suthar, D.; Gray, R.; Pettus, J.; McCracken, C.; Thomas, A.; Petit, C.J. Sirolimus as primary medical therapy in pulmonary vein stenosis. J. Am. Coll. Cardiol. 2021, 77, 2807–2818. [Google Scholar] [CrossRef]
- Peng, L.F.; Lock, J.E.; Nugent, A.W.; Jenkins, K.J.; McElhinney, D.B. Comparison of conventional and cutting balloon angioplasty for congenital and postoperative pulmonary vein stenosis in infants and young children. Catheter. Cardiovasc. Interv. 2010, 75, 1084–1090. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Marshall, A.C.; Gauvreau, K.; Peng, L.F.; Nugent, A.W.; Lock, J.E.; McElhinney, D.B. Outcomes After Stent Implantation for the Treatment of Congenital and Postoperative Pulmonary Vein Stenosis in Children. Circ. Cardiovasc. Interv. 2012, 5, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Qureshi, A.M.; Justino, H. Comparison of drug eluting versus bare metal stents for pulmonary vein stenosis in childhood. Catheter. Cardiovasc. Interv. 2019, 94, 233–242. [Google Scholar] [CrossRef]
- McMahon, C.J.; Mullins, C.E.; El Said, H.G. Intrastent sonotherapy in pulmonary vein restenosis: A new treatment for a recalcitrant problem. Heart 2003, 89, E6. [Google Scholar] [CrossRef]
- Petit, C.J.; Fraser, C.D.; Mattamal, R.; Slesnick, T.C.; Cephus, C.E.; Ocampo, E.C. The impact of a dedicated single-ventricle home-monitoring program on interstage somatic growth, interstage attrition, and 1-year survival. J. Thorac. Cardiovasc. Surg. 2011, 142, 1358–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderlaan, R.D.; Rome, J.; Hirsch, R.; Ivy, D.; Caldarone, C.A. Pulmonary vein stenosis: Treatment and challenges. J. Thorac. Cardiovasc. Surg. 2021, 161, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
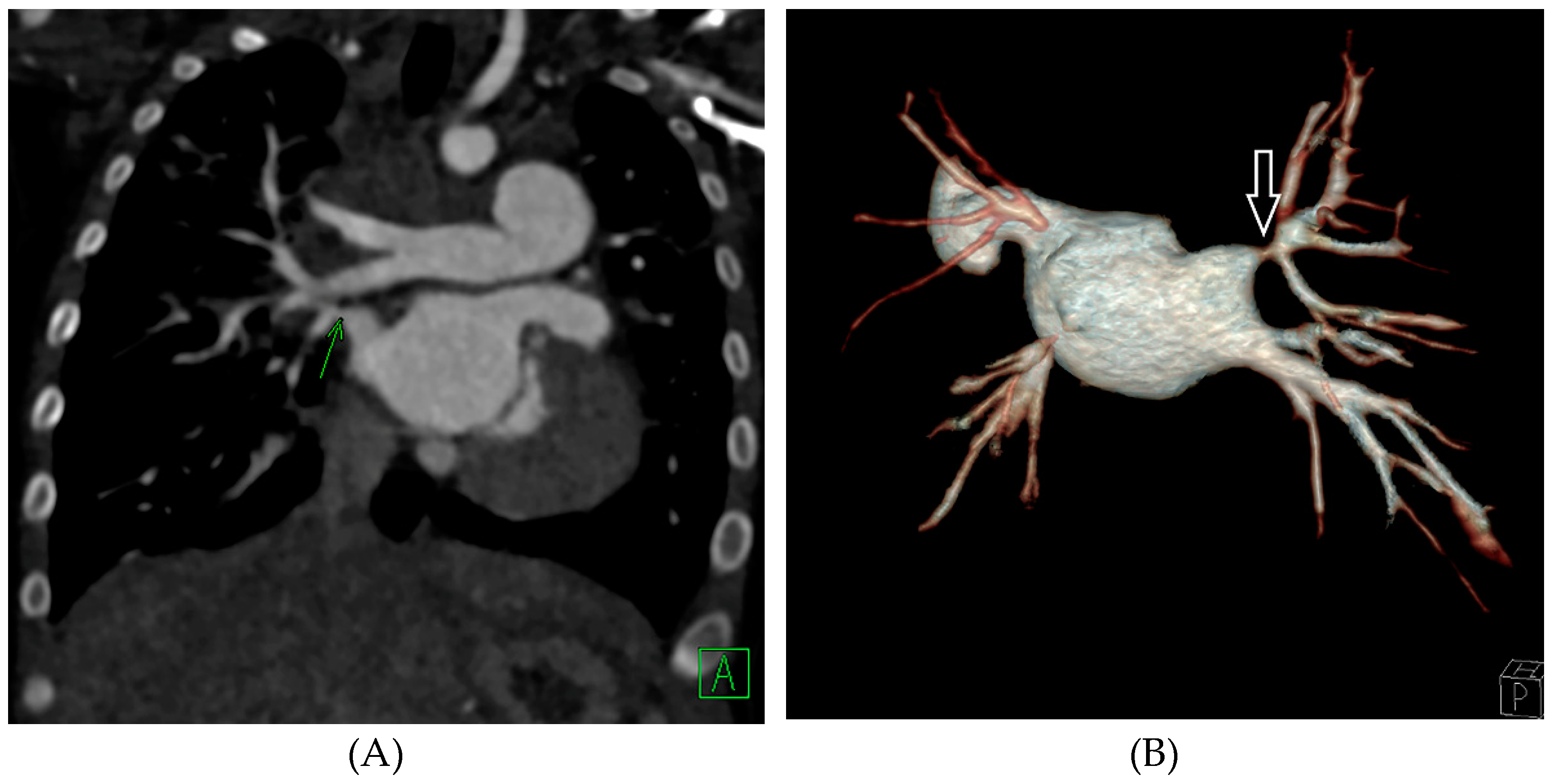
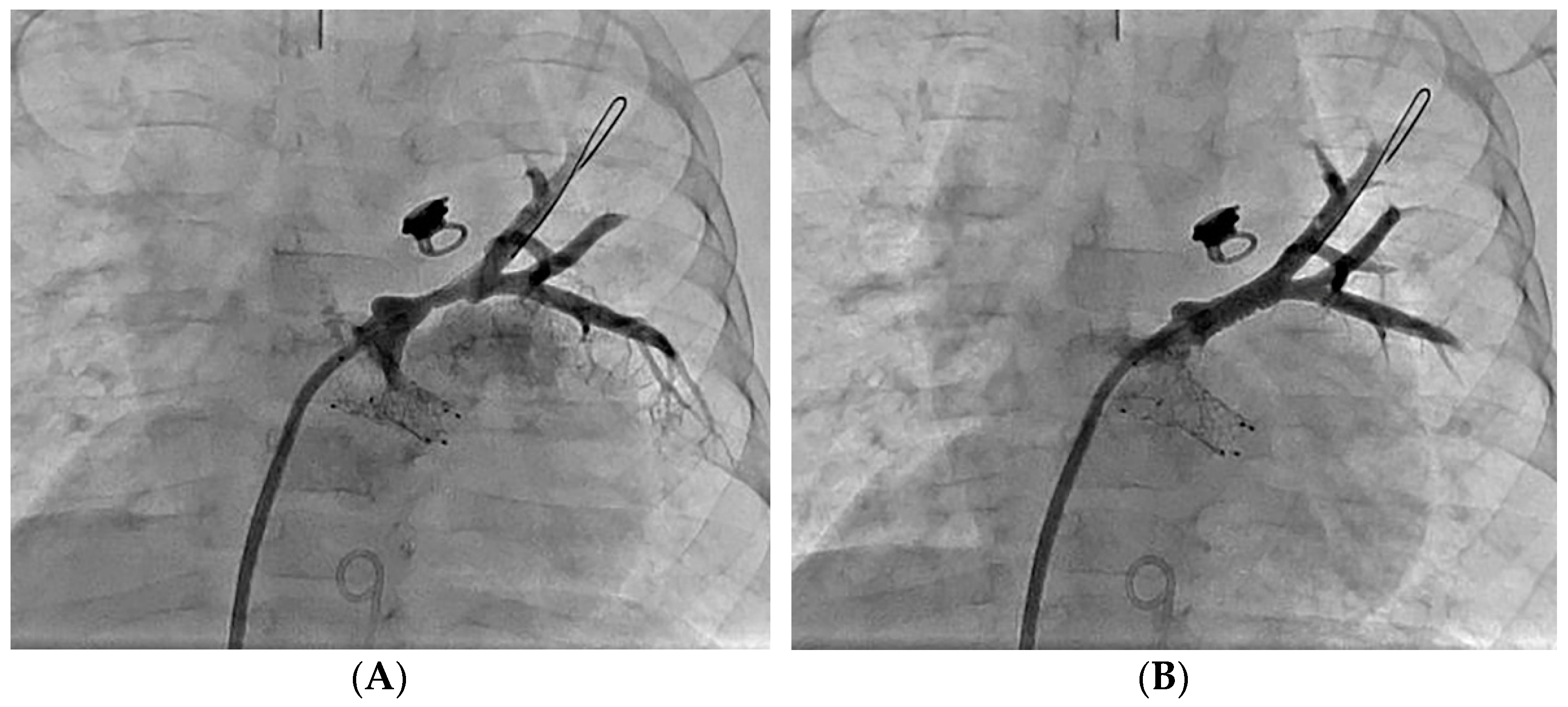
| Grade | A Normal Upstream Vein Caliber | B Hypoplastic Upstream Vein Caliber (<2 mm or <10 mm/m2) |
|---|---|---|
| 0 Normal Ostium | 0 | 0 |
| 1 <50% Stenosis | 1A | 1B |
| 2 >50% Stenosis | 2A | 2B |
| 3 Atretic | 3A | 3B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, J.A.; Petit, C.J. Pulmonary Vein Stenosis in Children: A Programmatic Approach Employing Primary and Anatomic Therapy. Children 2021, 8, 663. https://doi.org/10.3390/children8080663
Kuo JA, Petit CJ. Pulmonary Vein Stenosis in Children: A Programmatic Approach Employing Primary and Anatomic Therapy. Children. 2021; 8(8):663. https://doi.org/10.3390/children8080663
Chicago/Turabian StyleKuo, James A., and Christopher J. Petit. 2021. "Pulmonary Vein Stenosis in Children: A Programmatic Approach Employing Primary and Anatomic Therapy" Children 8, no. 8: 663. https://doi.org/10.3390/children8080663






