Physical Therapy to Prevent Osteopenia in Preterm Infants: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.2.1. Electronic Baseline Data
2.2.2. Other Sources of Information
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Study Risk of Bias Assessment
3. Results
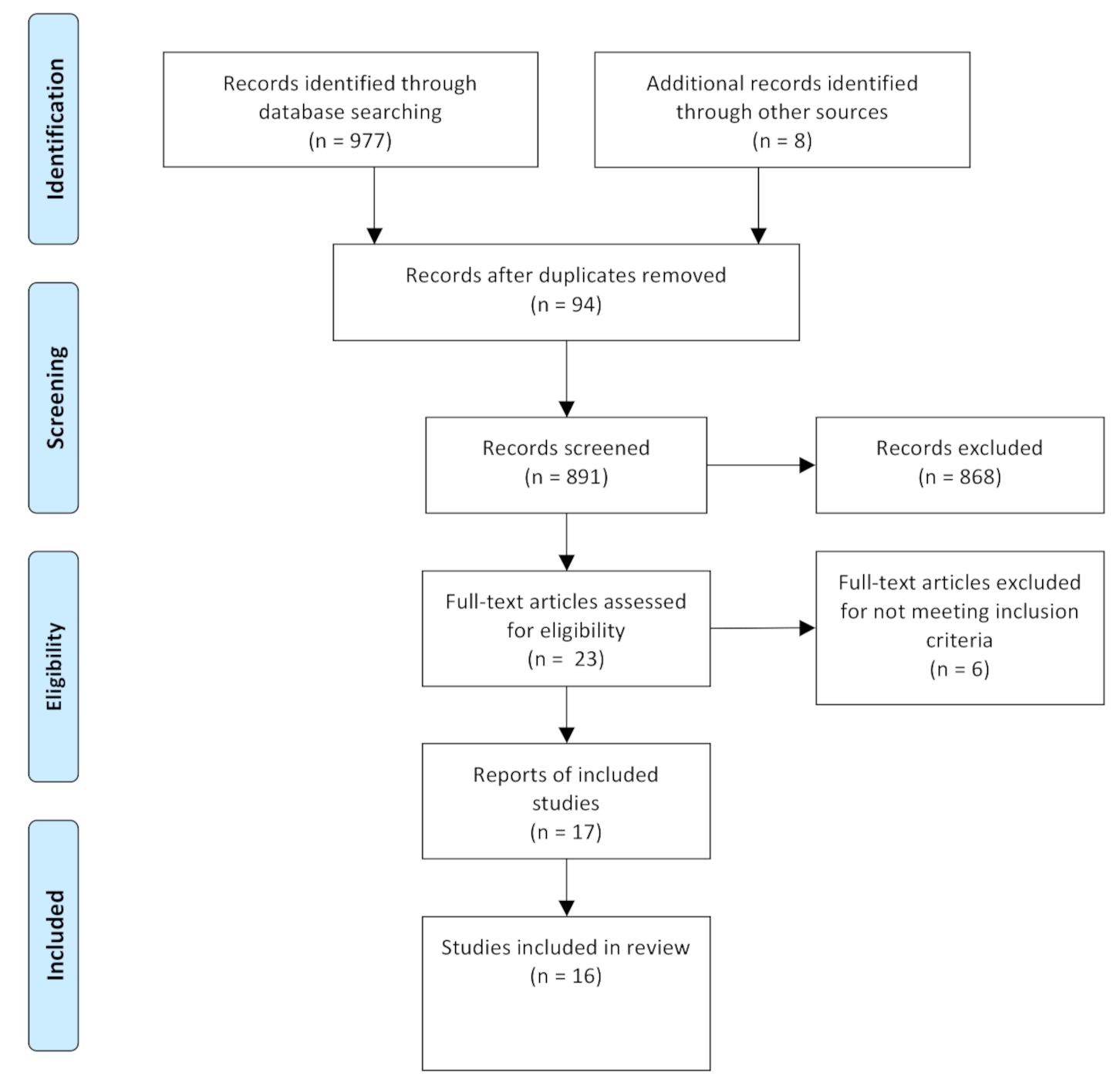
3.1. Study Selection
3.2. Study Characteristics
3.3. Participants
3.4. Characteristics of the Physical Therapy Treatment
3.5. Outcome Measures
3.6. Risk of Bias in Studies
3.7. Results of Individual Studies
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
4.3. Implications for Clinical Practice
4.4. Implications for Future Research
5. Conclusions
6. Other Information
Protocol and Registration
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manuck, T.A.; Rice, M.M.; Bailit, J.L.; Grobman, W.A.; Reddy, U.M.; Wapner, R.; Thorp, J.M.; Caritis, S.N.; Prasad, M.; Tita, A.; et al. Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am. J. Obstet. Gynecol. 2016, 215, 1–103. [Google Scholar] [CrossRef]
- Harrison, C.M.; Gibson, A.T. Osteopenia in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 2013, 98, F272–F275. [Google Scholar] [CrossRef]
- Angelika, D.; Ugrasena, I.D.G.; Etika, R.; Rahardjo, P.; Bos, A.F.; Sauer, P.J.J. The incidence of osteopenia of prematurity in preterm infants without phosphate supplementation. Medicine (Baltim.) 2021, 100, e25758. [Google Scholar] [CrossRef] [PubMed]
- Chinoy, A.; Mughal, M.Z.; Padidela, R. Metabolic bone disease of prematurity: Causes, recognition, prevention, treatment and long-term consequences. Arch. Dis. Child Fetal Neonatal Ed. 2019, 104, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; D–Amato, E.; Natale, M.P.; Grano, M.; Chiarito, M.; Brunetti, G.; D–Amato, G. Metabolic Bone Disease of Prematurity: Diagnosis and Management. Front Pediatr 2019, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Dokos, C.; Tsakalidis, C.; Tragiannidis, A.; Rallis, D. Inside the fragile infant: Pathophysiology, molecular background, risk factors and investigation of neonatal osteopenia. Bone Metab. 2013, 10, 86–90. [Google Scholar]
- Kovacs, C.S. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef]
- Goulding, A.; Jones, I.E.; Taylor, R.W.; Williams, S.M.; Manning, P.J. Bone mineral density and body composition in boys with distal forearm fractures: A dual-energy x-ray absorptiometry study. J. Pediatr 2001, 139, 509–515. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, L.; Ding, W.; Ma, J.; Zhang, Y. The influence of perinatal and maternal factors on physical growth at 12 months in prematurely born infants treated in the neonatal intensive care unit: A retrospective chart review and a prospective cohort study. Int. J. Nurs. Stud. 2020, 109, 103656. [Google Scholar] [CrossRef]
- Rehman, M.U. Metabolic bone disease in the preterm infant: Current state and future directions. World J. Methodol. 2015, 5, 115. [Google Scholar] [CrossRef]
- Moyer-Mileur, L.J.; Luetkemeier, M.; Boomer, L.; Chan, G.M. Effect of physical activity on bone mineralization in premature infants. J. Pediatr 1995, 127, 620–625. [Google Scholar] [CrossRef]
- Stalnaker, K.A.; Poskey, G.A. Osteopenia of Prematurity: Does Physical Activity Improve Bone Mineralization in Preterm Infants? Neonatal Netw. 2016, 35, 95–104. [Google Scholar] [CrossRef]
- Schulzke, S.; Kaempfen, S.; Trachsel, D.; Patole, S. Physical activity programs for promoting bone mineralization and growth in. Cochrane Database Syst. Rev. 2014, 38, 1685–1690. [Google Scholar] [CrossRef]
- Eliakim, A.; Litmanovitz, I.; Nemet, D. The Role of Exercise in Prevention and Treatment of Osteopenia of Prematurity: An Update. Pediatr Exerc. Sci. 2017, 29, 450–455. [Google Scholar] [CrossRef]
- Bhogal, S.K.; Teasell, R.W.; Foley, N.C.; Speechley, M.R. The PEDro scale provides a more comprehensive measure of methodological quality than the Jadad Scale in stroke rehabilitation literature. J. Clin. Epidemiol. 2005, 58, 668–673. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Available online: handbook.cochrane.org (accessed on 15 June 2021).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, 1–9. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; da Costa, B.R.; Cummings, G.; Ha, C.; Fuentes, J.; Saltaji, H.; Egger, M. PEDro or Cochrane to Assess the Quality of Clinical Trials? A Meta-Epidemiological Study. PLoS ONE 2015, 10, e0132634. [Google Scholar] [CrossRef]
- McQueen, D.; Lakes, K.D.; Rich, J.; Vaughan, J.; Hayes, G.R.; Cooper, D.M.; Olshansky, E. Feasibility of a caregiver-assisted exercise program for preterm infants. J. Perinat Neonatal Nurs. 2013, 27, 184–192. [Google Scholar] [CrossRef]
- Olshansky, E.; Vaughan, J.; Sando, K.; Rich, J.; Lakes, K.; Cooper, D. The Societal Importance of Embracing Counterintuitive Thought in Science: Assisted Exercise in Preterm Infants for Long-term Health Outcomes. Int. J. Sci. Soc. 2013, 4, 145–152. [Google Scholar] [CrossRef]
- Cooper, D.M.; Girolami, G.L.; Kepes, B.; Stehli, A.; Lucas, C.T.; Haddad, F.; Zalidvar, F.; Dror, N.; Ahmad, I.; Soliman, A.; et al. Body composition and neuromotor development in the year after NICU discharge in premature infants. Pediatr. Res. 2020, 88, 459–465. [Google Scholar] [CrossRef]
- Massaro, A.N.; Hammad, T.A.; Jazzo, B.; Aly, H. Massage with kinesthetic stimulation improves weight gain in preterm infants. J. Perinatol. 2009, 29, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.C. Daily exercise program in very low birth weight preterm infants. J. Pediatr Nurs. 2019, 108, 51. [Google Scholar] [CrossRef]
- Litmanovitz, I.; Dolfin, T.; Arnon, S.; Regev, R.H.; Nemet, D.; Eliakim, A. Assisted exercise and bone strength in preterm infants. Calcif. Tissue Int. 2007, 80, 39–43. [Google Scholar] [CrossRef]
- Aly, H.; Moustafa, M.F.; Hassanein, S.M.; Massaro, A.N.; Amer, H.A.; Patel, K. Physical activity combined with massage improves bone mineralization in premature infants: A randomized trial. J. Perinatol. 2004, 24, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Moyer-Mileur, L.J.; Ball, S.D.; Brunstetter, V.L.; Chan, G.M. Maternal-administered physical activity enhances bone mineral acquisition in premature very low birth weight infants. J. Perinatol. 2008, 28, 432–437. [Google Scholar] [CrossRef]
- Nemet, D.; Dolfin, T.; Litmanowitz, I.; Shainkin-Kestenbaum, R.; Lis, M.; Eliakim, A. Evidence for exercise-induced bone formation in premature infants. Int. J. Sports Med. 2002, 23, 82–85. [Google Scholar] [CrossRef]
- Efe, S.Y.; Erdem, E.; Güneş, T. The effect of daily exercise program on bone mineral density and cortisol level in preterm infants with very low birth weight: A randomized controlled trial. J. Pediatr. Nurs. 2019, 51, 6–12. [Google Scholar] [CrossRef]
- Shaw, S.C.; Sankar, M.J.; Thukral, A.; Natarajan, C.K.; Deorari, A.K.; Paul, V.K.; Agarwal, R. Assisted Physical Exercise for Improving Bone Strength in Preterm Infants Less than 35 Weeks Gestation: A Randomized Controlled Trial. Indian Pediatr. 2017, 55, 111–112. [Google Scholar] [CrossRef]
- Tosun, Ö.; Bayat, M.; Güneş, T.; Erdem, E. Daily physical activity in low-risk preterm infants: Positive impact on bone strength and mid-upper arm circumference. Ann. Hum. Biol. 2011, 38, 635–639. [Google Scholar] [CrossRef]
- Vignochi, C.M.; Miura, E.; Canani, L.H.S. Effects of motor physical therapy on bone mineralization in premature infants: A randomized controlled study. J. Perinatol. 2008, 28, 624–631. [Google Scholar] [CrossRef]
- Vignochi, C.M.; Silveira, R.C.; Miura, E.; Canani, L.H.S.; Procianoy, R.S. Physical therapy reduces bone resorption and increases bone formation in preterm infants. Am. J. Perinatol. 2012, 29, 573–578. [Google Scholar] [CrossRef]
- Chen, H.-L.; Lee, C.-L.; Tseng, H.-I.; Yang, S.-N.; Yang, R.-C.; Jao, H.-C. Assisted exercise improves bone strength in very low birthweight infants by bone quantitative ultrasound. J. Paediatr. Child Health 2010, 46, 653–659. [Google Scholar] [CrossRef]
- El-Farrash, R.; Abo-Seif, I.; El-Zohiery, A.; Hamed, G.; Abulfadl, R. Passive Range-of-Motion Exercise and Bone Mineralization in Preterm Infants: A Randomized Controlled Trial. Am. J. Perinatol. 2019, 37, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, A.; Dolfin, T.; Weiss, E.; Shainkin-Kestenbaum, R.; Lis, M.; Nemet, D. The effects of exercise on body weight and circulating leptin in premature infants. J. Perinatol. 2002, 22, 550–554. [Google Scholar] [CrossRef][Green Version]
- Erdem, E.; Tosun, Ö.; Bayat, M.; Korkmaz, Z.; Halis, H.; Güneş, T. Daily physical activity in low-risk extremely low birth weight preterm infants: Positive impact on bone mineral density and anthropometric measurements. J. Bone Miner Metab. 2015, 33, 329–334. [Google Scholar] [CrossRef]
- Haley, S.; Beachy, J.; Ivaska, K.K.; Slater, H.; Smith, S.; Moyer-Mileur, L.J. Tactile/kinesthetic stimulation (TKS) increases tibial speed of sound and urinary osteocalcin (U-MidOC and unOC) in premature infants (29–32weeks PMA). Bone 2012, 51, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Litmanovitz, I.; Dolfin, T.; Friedland, O.; Arnon, S.; Regev, R.; Shainkin-Kestenbaum, R.; Lis, M.; Eliakim, A. Early physical activity intervention prevents decrease of bone strength in very low birth weight infants. Pediatrics 2003, 112, 15–19. [Google Scholar] [CrossRef]
- Litmanovitz, I.; Erez, H.; Eliakim, A.; Bauer-Rusek, S.; Arnon, S.; Regev, R.H.; Sirota, G.; Nemet, D. The Effect of Assisted Exercise Frequency on Bone Strength in Very Low Birth Weight Preterm Infants: A Randomized Control Trial. Calcif. Tissue Int. 2016, 99, 237–242. [Google Scholar] [CrossRef]
- Moyer-Mileur, L.J.; Brunstetter, V.; McNaught, T.P.; Gill, G.; Chan, G.M. Daily physical activity program increases bone mineralization and growth in preterm very low birth weight infants. Pediatrics 2000, 106, 1088–1092. [Google Scholar] [CrossRef]
- Eliakim, A.; Raisz, L. Evidence for Increased Bone Formation Following a Brief Endurance-Type Training Intervention in Adolescent Males. J. Bone Miner Res. 1997, 12, 1708–1713. [Google Scholar] [CrossRef]
- Slemenda, C.; Miller, J. Role of physical activity in the development of skeletal mass in children. J. Bone Miner Res. 1991, 6, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Rayannavar, A.; Calabria, A.C. Screening for Metabolic Bone Disease of prematurity. Semin. Fetal Neonatal Med. 2020, 25, 101086. [Google Scholar] [CrossRef] [PubMed]
- Barco, C.M.R.; Arija, S.M.; Pérez, M.R. Biochemical markers in osteoporosis: Usefulness in clinical practice. Reumatol. Clin. 2012, 8, 149–152. [Google Scholar] [CrossRef]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Beyers, N.; Alheit, B.; Taljaard, J.F.; Hall, J.M.; Hough, S.F. High turnover osteopenia in preterm babies. Bone. 1994, 15, 5–13. [Google Scholar] [CrossRef]
- Anand, K.J. International, Evidence-Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Arch. Pediatr. Adolesc. Med. 2001, 155, 173–180. [Google Scholar] [CrossRef] [PubMed]
- McPherson, C.; Miller, S.P.; El-Dib, M.; Massaro, A.N.; Inder, T.E. The influence of pain, agitation, and their management on the immature brain. Pediatr. Res. 2020, 88, 168–175. [Google Scholar] [CrossRef]
- Nikander, R.; Sievänen, H.; Heinonen, A.; Daly, R.M.; Uusi-Rasi, K.; Kannus, P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010, 8, 47. [Google Scholar] [CrossRef]
- Hartman, C.; Shamir, R.; Eshach-Adiv, O.; Iosilevsky, G.; Brik, R. Assessment of Osteoporosis by Quantitative Ultrasound Versus Dual Energy X-ray Absorptiometry in Children With Chronic Rheumatic Diseases. J. Rheumatol. 2004, 31, 981–985. [Google Scholar]
- Rack, B.; Lochmüller, E.-M.; Janni, W.; Lipowsky, G.; Engelsberger, I.; Friese, K.; Kuster, H. Ultrasound for the assessment of bone quality in preterm and term infants. J. Perinatol. 2012, 32, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.J.; Fernández, D.; Gómez-Salgado, J.; Rodríguez-González, D.; Rosón, M.; Lapeña, S. The effects of massage therapy in hospitalized preterm neonates: A systematic review. Int. J. Nurs. Stud. 2017, 69, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Niemi, A.-K. Review of Randomized Controlled Trials of Massage in Preterm Infants. Children 2017, 4, 21. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.D.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1–e34. [Google Scholar] [CrossRef] [PubMed]
| Study | Participant Characteristics | Design | Study Characteristics | |||||
|---|---|---|---|---|---|---|---|---|
| N | Details | |||||||
| EG | CG | wGA | Weight (gr) | Feeding | PEDro | Rob-2 Cochrane | ||
| Aly, et al. 2004 [25] | 15 | 15 | <35 | NS | CEN | RCT | 3/10 | Some concerns |
| Chen, et al. 2010 [33] | 8 | 8 | <37 | <1500 | SN | RCT | 5/10 | Some concerns |
| El-Farrash, et al. 2019 [34] | 18 | 18 | ≤32 | ≤1500 | SN | RCT | 6/10 | Some concerns |
| Eliakim, et al. 2002 [35] | 10 | 10 | <37 | <1500 | CEN | RCT | 4/10 | Some concerns |
| Erdem, et al. 2015 [36] | 14 | 14 | ≤32 | <1000 | CEN | RCT | 7/10 | Some concerns |
| Haley, et al. 2012 [37] | 20 | 20 | <33 | NS | CEN | RCT | 7/10 | Some concerns |
| Litmanovitz, et al. 2003 [38] | 12 | 12 | <37 | <1500 | SN | RCT | 6/10 | Some concerns |
| Litmanovitz, et al. 2016 [39] | EG1: 14EG2: 11 | 10 | <37 | <1500 | SN | RCT | 6/10 | Some concerns |
| Moyer-Mileur, et al. 1995 [11] | 13 | 13 | <34 | NS | CEN | RCT | 4/10 | Some concerns |
| Moyer-Mileur, et al. 2000 [40] | 16 | 16 | <32 | <1600 | CEN | RCT | 8/10 | Some concerns |
| Moyer-Mileur, et al. 2008 [26] | EG1: 11EG2: 11 | 11 | <32 | <1600 | CEN | RCT | 6/10 | Some concerns |
| Nemet, et al. 2002 [27] | 12 | 12 | <37 | NS | CEN | RCT | 6/10 | Some concerns |
| Sezer Efe, et al. 2019 [28] | 12 | 12 | <32 | <1500 | NS | RCT | 8/10 | Some concerns |
| Shaw, et al. 2017 [29] | 26 | 24 | <35 | NS | SN | RCT | 7/10 | Some concerns |
| Tosun, et al. 2011 [30] | 20 | 20 | <32 | <1600 | CEN | RCT | 6/10 | Some concerns |
| Vignochi, et al. 2008 [31,32] | 15 | 14 | <32 | <1600 | CEN | RCT | 7/10 | Some concerns |
| Study | Treatment | Desc. | Age S–I | Period of Aplication | Frequency | Intensity | Who? | Assessments | Outcome Measures | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | C | Inicial | During | Final | Follow up | Statistical Significance (p) | Group Contrast | ||||||||
| Aly, et al. 2004 [25] | PMC + Mas | NA | ++ | 2 postnatal weeks | Until 1.8 kg weight | 5 W/S | NS | Mo | Beginning of treatment | NS | 1.8 kg weight | NS | ALP | p > 0.050 | E = C |
| PICP | p < 0.001 | E > C | |||||||||||||
| Pyd | p = 0.984 | E = C | |||||||||||||
| PTH | p < 0.001 | E > C | |||||||||||||
| Ca | p = 0.002 | E > C | |||||||||||||
| Chen, et al. 2010 [33] | PMC | TS | ++ | 1 postnatal week | 4 weeks | 5 W/S | 10 MIN | N | Birth | 2nd and 4th postnatal week | 6th postnatal week | 8th postnatal week | US 2 weeks | p = 0.156 | E = C |
| US 4 weeks | p = 0.636 | E = C | |||||||||||||
| US 6 weeks | p = 0.031 | E > C | |||||||||||||
| US 8 weeks | p = 0.020 | E > C | |||||||||||||
| PICP | p > 0.050 | E = C | |||||||||||||
| ALP | p > 0.050 | E = C | |||||||||||||
| Weight | p > 0.050 | E = C | |||||||||||||
| El-Farrash, et al. 2019 [34] | PMC | TS | ++ | 1 postnatal week | 4 weeks | 5 W/S | 10 MIN | NS | Beginning of treatment | NS | End of treatment | NS | DEXA | p < 0.001 | E > C |
| ALP | p = 0.005 | E < C | |||||||||||||
| Ca/PO4 | p = 0.040 | E < C | |||||||||||||
| PO4 | p = 0.001 | E > C | |||||||||||||
| CTX | p = 0.254 | E = C | |||||||||||||
| Weight | p < 0.001 | E > C | |||||||||||||
| Height | p > 0.050 | E = C | |||||||||||||
| HC | p > 0.050 | E = C | |||||||||||||
| Eliakim, et al. 2002 [35] | PMC | TS | ++ | 1 Month | 4 weeks | 5 W/S | 5–10 MIN | NS | 1 M | NS | 2 M | NS | Leptin | p < 0.050 | E > C |
| IGF-I | p < 0.050 | E > C | |||||||||||||
| Weight | p < 0.050 | E > C | |||||||||||||
| Erdem, et al. 2015 [36] | PMC | UC | ++ | ≤3 postnatal days | 4 weeks | 5 W/S | 5–8 MIN | NS | Beginning of treatment | NS | End of treatment | NS | US | p = 0.001 | E > C |
| Weight | p = 0.002 | E > C | |||||||||||||
| Height | p = 0.015 | E > C | |||||||||||||
| HC | p > 0.050 | E = C | |||||||||||||
| Haley, et al. 2012 [37] | TKs | UC | + | 32–34 wGA | 2 weeks | 12 W/S | 20 MIN | NS | Beginning of treatment | NS | End of treatment | NS | US | p < 0.050 | E > C |
| Pyd | p > 0.050 | E = C | |||||||||||||
| Dpd | p > 0.050 | E = C | |||||||||||||
| U-MidOC | p < 0.001 | E > C | |||||||||||||
| Litmanovitz, et al. 2003 [38] | PMC | TS | ++ | 5–6 days | 4 weeks | 5 W/S | 5 MIN | NS | Beginning of treatment | NS | End of treatment | NS | US | p < 0.006 | E > C |
| BSAP | p < 0.050 | E > C | |||||||||||||
| ICTP | p < 0.050 | E > C | |||||||||||||
| Weight | p < 0.050 | E < C | |||||||||||||
| Height | p < 0.050 | E > C | |||||||||||||
| HC | p < 0.050 | E > C | |||||||||||||
| Litmanovitz, et al. 2016 [39] | PMC | TS | ++ | <2 postnatal weeks | 4 weeks | E1: 10 W/SE2: 5 W/S | 10 MIN | NS | Beginning of treatment | 2 weeks | End of treatment | NS | US at 2 weeks | p < 0.040 | E1 > E2 > C |
| US at 4 weeks | p < 0.030 | E1 > C | |||||||||||||
| Weight | p > 0.050 | E = C | |||||||||||||
| Height | p > 0.050 | E = C | |||||||||||||
| HC | p > 0.050 | E = C | |||||||||||||
| Moyer-Mileur, et al. 1995 [11] | PMC | TS | ++ | NS | 4 weeks | 6 W/S | 5–10 MIN | TO | Beginning of treatment | NS | End of treatment | NS | DEXA | p < 0.050 | E > C |
| ALP | p < 0.050 | E < C | |||||||||||||
| PTH | p > 0.050 | E = C | |||||||||||||
| Ca (urine) | p > 0.050 | E = C | |||||||||||||
| Weight | p < 0.050 | E > C | |||||||||||||
| Height | p > 0.050 | E = C | |||||||||||||
| HC | p > 0.050 | E = C | |||||||||||||
| Moyer-Mileur, et al. 2000 [40] | PMC | TS | ++ | At the beginning of CEN | Until 2 kg weight | 6 W/S | 5–10 MIN | TO | Beginning of treatment | - | 2 kg weight | - | DEXA | p < 0.050 | E > C |
| PICP | p = 0.030 | E > C | |||||||||||||
| Pyd | p > 0.050 | E = C | |||||||||||||
| Weight | p = 0.020 | E > C | |||||||||||||
| Height | p > 0.050 | E = C | |||||||||||||
| HC | p > 0.050 | E = C | |||||||||||||
| Moyer-Mileur, et al. 2008 [26] | PMC | TS | + | 31–33 wGA | Until 2 kg weight | 6 W/S | 5–10 MIN | OT/Mo | Beginning of treatment | - | 2 kg weight | - | DEXA | p < 0.050 | E > C |
| BSAP | p = 0.040 | E > C | |||||||||||||
| Pyd | p > 0.050 | E = C | |||||||||||||
| Weight | p = 0.020 | E > C | |||||||||||||
| Height | p > 0.050 | E = C | |||||||||||||
| HC | p > 0.050 | E = C | |||||||||||||
| Nemet, et al. 2002 [27] | PMC | TS | ++ | 32–33 wGA | 4 weeks | 5 W/S | 5–10 MIN | - | Beginning of treatment | - | 36–37wGA | - | BSAP | p < 0.050 | E > C |
| PICP | p > 0.050 | E = C | |||||||||||||
| ICTP | p < 0.050 | E < C | |||||||||||||
| Weight | p < 0.050 | E > C | |||||||||||||
| Sezer Efe, et al. 2019 [28] | PMC | UC | ++ | - | 30 days | 7W/S | 7-10 MIN | I | Beginning of treatment | - | End of treatment | - | US | p = 0.009 | E > C |
| Cortisol levels | p > 0.050 | E = C | |||||||||||||
| Weight | p > 0.050 | E = C | |||||||||||||
| Height | p > 0.050 | E = C | |||||||||||||
| HC | p > 0.050 | E = C | |||||||||||||
| Shaw, et al. 2017 [29] | PMC | UC | ++ | 1 postnatal week | Until 40 wGA | 5 W/S | 10 MIN | Mo | Beginning of treatment | - | 40 wGA | - | US | p > 0.050 | E = C |
| ALP | p > 0.050 | E = C | |||||||||||||
| Ca (serum) | p > 0.050 | E = C | |||||||||||||
| PO4 | p > 0.050 | E = C | |||||||||||||
| Weight | p > 0.050 | E = C | |||||||||||||
| Height | p > 0.050 | E = C | |||||||||||||
| HC | p > 0.050 | E = C | |||||||||||||
| Tosun, et al. 2011 [30] | PMC | UC | ++ | NS | 4 weeks | 5 W/S | 5–10 MIN | NS | Beginning of treatment | NS | End of treatment | NS | US | p < 0.050 | E > C |
| Weight | p > 0.050 | E = C | |||||||||||||
| Height | p > 0.050 | E = C | |||||||||||||
| HC | p > 0.050 | E = C | |||||||||||||
| Vignochi, et al. 2008 [31,32] | PMC | UC | ++ | 32–33 wGA | Until HD/2 kg weight | 5 W/S | 15 MIN | NS | Beginning of treatment | NS | HD | NS | DEXA | p < 0.050 | E > C |
| Weight | p < 0.001 | E > C | |||||||||||||
| Height | p > 0.050 | E = C | |||||||||||||
| HC | p > 0.050 | E = C | |||||||||||||
| BSAP | p < 0.001 | E > C | |||||||||||||
| Dpd | p < 0.003 | E < C | |||||||||||||
| Ca (serum) | p > 0.050 | E = C | |||||||||||||
| PO4 | p > 0.050 | E = C | |||||||||||||
| PTH | p > 0.050 | E = C | |||||||||||||
| PEDro Scale | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | T |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aly, et al. 2004 [25] | X | X | X | X | 3 | |||||||
| Chen, et al. 2010 [33] | X | X | X | X | X | X | 5 | |||||
| El-Farrash, et al. 2019 [34] | X | X | X | X | X | X | X | 6 | ||||
| Eliakim, et al. 2002 [35] | X | X | X | X | 4 | |||||||
| Erdem, et al. 2015 [36] | X | X | X | X | X | X | X | X | 7 | |||
| Haley, et al. 2012 [37] | X | X | X | X | X | X | X | X | 7 | |||
| Litmanovitz, et al. 2003 [38] | X | X | X | X | X | X | X | 6 | ||||
| Litmanovitz, et al. 2016 [39] | X | X | X | X | X | X | X | 6 | ||||
| Moyer-Mileur, et al. 1995 [11] | X | X | X | X | X | 4 | ||||||
| Moyer-Mileur, et al. 2000 [40] | X | X | X | X | X | X | X | X | X | 8 | ||
| Moyer-Mileur, et al. 2008 [26] | X | X | X | X | X | X | X | 6 | ||||
| Nemet, et al. 2002 [27] | X | X | X | X | X | X | X | 6 | ||||
| Sezer Efe, et al. 2019 [28] | X | X | X | X | X | X | X | X | X | 8 | ||
| Shaw, et al. 2017 [29] | X | X | X | X | X | X | X | X | 7 | |||
| Tosun, et al. 2011 [30] | X | X | X | X | X | X | X | 6 | ||||
| Vignochi, et al. 2008 [31,32] | X | X | X | X | X | X | X | X | 7 |
| Study | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall |
|---|---|---|---|---|---|---|
| Aly, et al. 2004 [25] |  |  |  |  |  |  |
| Chen, et al. 2010 [33] |  |  |  |  |  |  |
| El-Farrash, et al. 2019 [34] |  |  |  |  |  |  |
| Eliakim, et al. 2002 [35] |  |  |  |  |  |  |
| Erdem, et al. 2015 [36] |  |  |  |  |  |  |
| Haley, et al. 2012 [37] |  |  |  |  |  |  |
| Litmanovitz, et al. 2003 [38] |  |  |  |  |  |  |
| Litmanovitz, et al. 2016 [39] |  |  |  |  |  |  |
| Moyer-Mileur, et al. 1995 [11] |  |  |  |  |  |  |
| Moyer-Mileur, et al. 2000 [40] |  |  |  |  |  |  |
| Moyer-Mileur, et al. 2008 [26] |  |  |  |  |  |  |
| Nemet, et al. 2002 [27] |  |  |  |  |  |  |
| Sezer Efe, et al. 2019 [28] |  |  |  |  |  |  |
| Shaw, et al. 2017 [29] |  |  |  |  |  |  |
| Tosun, et al. 2011 [30] |  |  |  |  |  |  |
| Vignochi, et al. 2008 [31,32] |  |  |  |  |  |  |
 : Some concerns;
: Some concerns;  : Some concerns;
: Some concerns;  : High Risk.
: High Risk.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torró-Ferrero, G.; Fernández-Rego, F.J.; Gómez-Conesa, A. Physical Therapy to Prevent Osteopenia in Preterm Infants: A Systematic Review. Children 2021, 8, 664. https://doi.org/10.3390/children8080664
Torró-Ferrero G, Fernández-Rego FJ, Gómez-Conesa A. Physical Therapy to Prevent Osteopenia in Preterm Infants: A Systematic Review. Children. 2021; 8(8):664. https://doi.org/10.3390/children8080664
Chicago/Turabian StyleTorró-Ferrero, Galaad, Francisco Javier Fernández-Rego, and Antonia Gómez-Conesa. 2021. "Physical Therapy to Prevent Osteopenia in Preterm Infants: A Systematic Review" Children 8, no. 8: 664. https://doi.org/10.3390/children8080664
APA StyleTorró-Ferrero, G., Fernández-Rego, F. J., & Gómez-Conesa, A. (2021). Physical Therapy to Prevent Osteopenia in Preterm Infants: A Systematic Review. Children, 8(8), 664. https://doi.org/10.3390/children8080664





