Perioperative Glial Fibrillary Acidic Protein Is Associated with Long-Term Neurodevelopment Outcome of Infants with Congenital Heart Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Perioperative Management
2.2. Sample Collection and GFAP Analysis
2.3. Neurodevelopmental Testing
2.4. Statistical Analysis
3. Results
3.1. Anatomical, Clinical, and Surgical Characteristics
3.2. GFAP and Neurodevelopmental Outcome
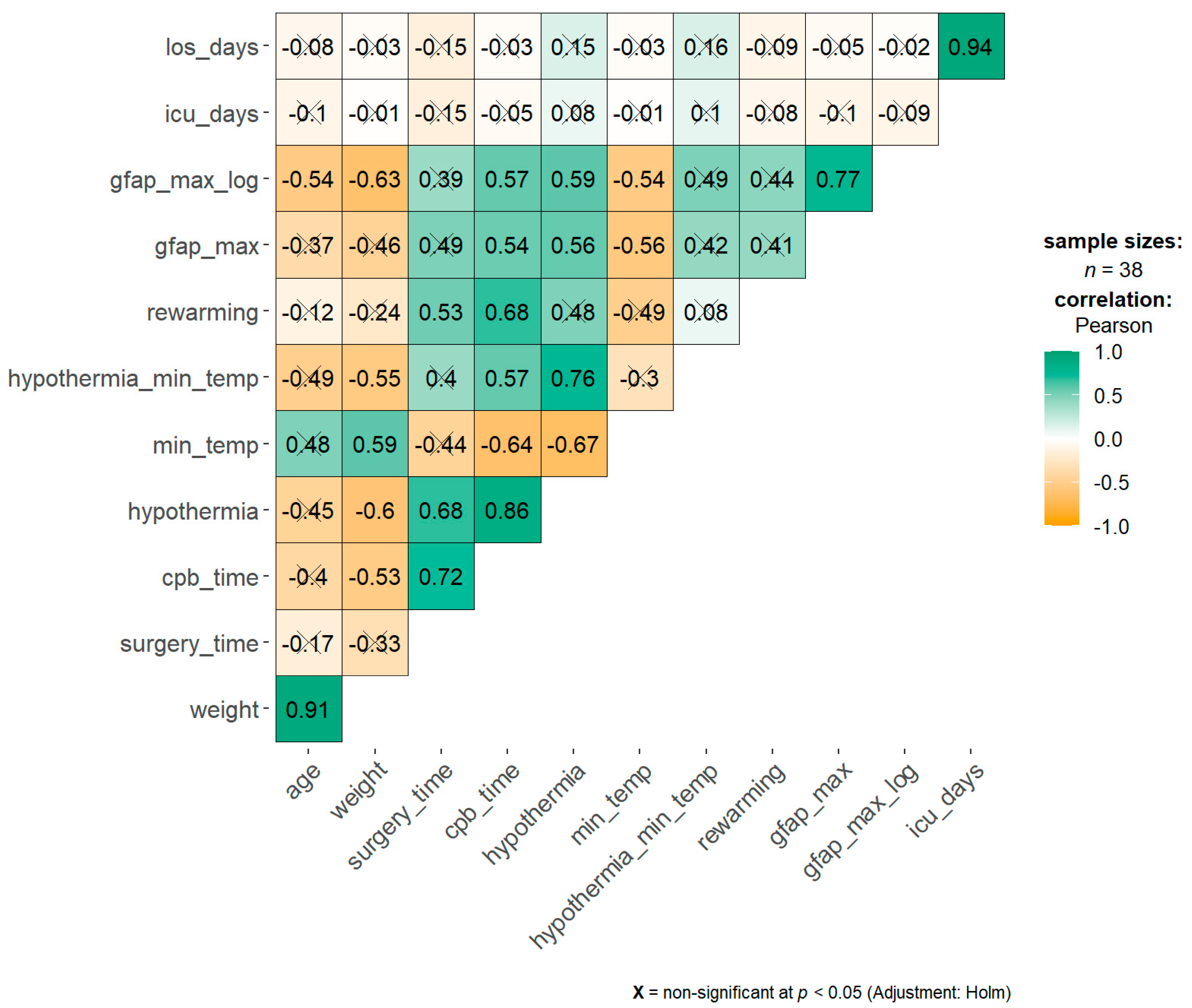
3.3. Correlation and Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, B.S.; Lipkin, P.H.; Newburger, J.W.; Peacock, G.; Gerdes, M.; Gaynor, J.W.; Mussatto, K.A.; Uzark, K.; Goldberg, C.S.; Johnson, W.H., Jr.; et al. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the American Heart Association. Circulation 2012, 126, 1143–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marelli, A.; Miller, S.P.; Marino, B.S.; Jefferson, A.L.; Newburger, J.W. Brain in Congenital Heart Disease Across the Lifespan: The Cumulative Burden of Injury. Circulation 2016, 133, 1951–1962. [Google Scholar] [CrossRef] [Green Version]
- Razzaghi, H.; Oster, M.; Reefhuis, J. Long-term outcomes in children with congenital heart disease: National Health Interview Survey. J. Pediatr. 2015, 166, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Beca, J.; Gunn, J.K.; Coleman, L.; Hope, A.; Reed, P.W.; Hunt, R.W.; Finucane, K.; Brizard, C.; Dance, B.; Shekerdemian, L.S. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation 2013, 127, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Claessens, N.H.P.; Kelly, C.J.; Counsell, S.J.; Benders, M. Neuroimaging, cardiovascular physiology, and functional outcomes in infants with congenital heart disease. Dev. Med. Child. Neurol. 2017, 59, 894–902. [Google Scholar] [CrossRef]
- Morton, P.D.; Ishibashi, N.; Jonas, R.A. Neurodevelopmental Abnormalities and Congenital Heart Disease: Insights Into Altered Brain Maturation. Circ. Res. 2017, 120, 960–977. [Google Scholar] [CrossRef] [Green Version]
- Honda, M.; Tsuruta, R.; Kaneko, T.; Kasaoka, S.; Yagi, T.; Todani, M.; Fujita, M.; Izumi, T.; Maekawa, T. Serum glial fibrillary acidic protein is a highly specific biomarker for traumatic brain injury in humans compared with S-100B and neuron-specific enolase. J. Trauma Acute Care Surg. 2010, 69, 104–109. [Google Scholar] [CrossRef]
- Brunetti, M.A.; Jennings, J.M.; Easley, R.B.; Bembea, M.; Brown, A.; Heitmiller, E.; Schwartz, J.M.; Brady, K.M.; Vricella, L.A.; Everett, A.D. Glial fibrillary acidic protein in children with congenital heart disease undergoing cardiopulmonary bypass. Cardiol. Young 2014, 24, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Vedovelli, L.; Padalino, M.; D’Aronco, S.; Stellin, G.; Ori, C.; Carnielli, V.P.; Simonato, M.; Cogo, P. Glial fibrillary acidic protein plasma levels are correlated with degree of hypothermia during cardiopulmonary bypass in congenital heart disease surgery. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Vedovelli, L.; Padalino, M.; Simonato, M.; D’Aronco, S.; Bertini, D.; Stellin, G.; Ori, C.; Carnielli, V.P.; Cogo, P.E. Cardiopulmonary Bypass Increases Plasma Glial Fibrillary Acidic Protein Only in First Stage Palliation of Hypoplastic Left Heart Syndrome. Can. J. Cardiol. 2016, 32, 355–361. [Google Scholar] [CrossRef]
- Graham, E.M.; Martin, R.H.; Atz, A.M.; Hamlin-Smith, K.; Kavarana, M.N.; Bradley, S.M.; Alsoufi, B.; Mahle, W.T.; Everett, A.D. Association of intraoperative circulating-brain injury biomarker and neurodevelopmental outcomes at 1 year among neonates who have undergone cardiac surgery. J. Thorac. Cardiovasc. Surg. 2019, 157, 1996–2002. [Google Scholar] [CrossRef] [Green Version]
- Vedovelli, L.; Padalino, M.; Suppiej, A.; Sartori, S.; Falasco, G.; Simonato, M.; Carnielli, V.P.; Stellin, G.; Cogo, P. Cardiopulmonary-Bypass Glial Fibrillary Acidic Protein Correlates With Neurocognitive Skills. Ann. Thorac. Surg. 2018, 106, 792–798. [Google Scholar] [CrossRef]
- Bembea, M.M.; Rizkalla, N.; Freedy, J.; Barasch, N.; Vaidya, D.; Pronovost, P.J.; Everett, A.D.; Mueller, G. Plasma Biomarkers of Brain Injury as Diagnostic Tools and Outcome Predictors After Extracorporeal Membrane Oxygenation. Crit. Care Med. 2015, 43, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.R.; McGaurn, S.A.; Wernovsky, G.; Spray, T.L.; Norwood, W.I.; Jacobs, M.L.; Murphy, J.D.; Gaynor, J.W.; Goin, J.E. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J. Thorac. Cardiovasc. Surg. 2000, 119, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Rigby, M.L.; Rosenthal, M. Cardiorespiratory Interactions in Paediatrics: ‘It’s (almost always) the circulation stupid!’. Paediatr. Respir. Rev. 2017, 22, 60–65. [Google Scholar] [CrossRef]
- Jacobs, J.P.; O’Brien, S.M.; Pasquali, S.K.; Gaynor, J.W.; Mayer, J.E., Jr.; Karamlou, T.; Welke, K.F.; Filardo, G.; Han, J.M.; Kim, S.; et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database Mortality Risk Model: Part 2-Clinical Application. Ann. Thorac. Surg. 2015, 100, 1063–1068. [Google Scholar] [CrossRef] [Green Version]
- Wechler, D. Wechsler Preschool and Primary Scale of Intelligence—Third Edition: Canadian (WPPSI-III CDN); Pearson Clinical Assessment: Toronto, ON, Canada, 2002. [Google Scholar]
- Grizzle, R. Wechsler Intelligence Scale for Children. In Encyclopedia of Child Behavior and Development, 4th ed.; Goldstein, S., Naglieri, J.A., Eds.; Springer: Boston, MA, USA, 2011; pp. 1553–1555. [Google Scholar] [CrossRef]
- Orsini, A.; Pezzuti, L.; Picone, L. WISC-IV: Contributo Alla Taratura Italiana; Giunti O.S. Organizzazioni Speciali: Firenze, Italy, 2012. [Google Scholar]
- Marini, A.; Marotta, L.; Bulgheroni, S.; Fabbro, F. Batteria per la Valutazione del Linguaggio in Bambini dai 4 ai 12 Anni (BVL_4-12); Giunti O.S.: Firenze, Italy, 2015. [Google Scholar]
- Davis, J.L.; Matthews, R.N. NEPSY-II Review: Korkman, M., Kirk, U., & Kemp, S. (2007). NEPSY—Second Edition (NEPSY-II). San Antonio, TX: Harcourt Assessment. J. Psychoeduc. Assess. 2010, 28, 175–182. [Google Scholar] [CrossRef]
- Achenbach, T.M.; Rescorla, L. Achenbach System of Empirically Based Assessment; Springer: Burlington, VT, USA, 2013; pp. 31–39. [Google Scholar] [CrossRef]
- Conners’ Rating Scales-Revised—Children, Adults, Used, Score, Health, Definition, Purpose, Precautions, Description. Available online: http://www.minddisorders.com/Br-Del/Conners-Rating-Scales-Revised.html (accessed on 15 July 2021).
- Lindberg, M.D. Finally, Actual Data for the FDA-Approved Biomarkers of Traumatic Brain Injury. NEJM J. Watch 2018. [Google Scholar]
- Stewart, A.; Tekes, A.; Huisman, T.A.; Jennings, J.M.; Allen, M.C.; Northington, F.J.; Everett, A.D.; Graham, E.M. Glial fibrillary acidic protein as a biomarker for periventricular white matter injury. Am. J. Obstet. Gynecol. 2013, 209, 27.e1–27.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ennen, C.S.; Huisman, T.A.; Savage, W.J.; Northington, F.J.; Jennings, J.M.; Everett, A.D.; Graham, E.M. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am. J. Obstet. Gynecol. 2011, 205, 251.e1–251.e7. [Google Scholar] [CrossRef] [Green Version]
- Petzold, A. Glial fibrillary acidic protein is a body fluid biomarker for glial pathology in human disease. Brain Res. 2015, 1600, 17–31. [Google Scholar] [CrossRef]
- Cainelli, E.; Arrigoni, F.; Vedovelli, L. White matter injury and neurodevelopmental disabilities: A cross-disease (dis)connection. Prog. Neurobiol. 2020, 193, 101845. [Google Scholar] [CrossRef]
- Alonso-Alconada, D.; Broad, K.D.; Bainbridge, A.; Chandrasekaran, M.; Faulkner, S.D.; Kerenyi, A.; Hassell, J.; Rocha-Ferreira, E.; Hristova, M.; Fleiss, B.; et al. Brain cell death is reduced with cooling by 3.5 degrees C to 5 degrees C but increased with cooling by 8.5 degrees C in a piglet asphyxia model. Stroke 2015, 46, 275–278. [Google Scholar] [CrossRef] [Green Version]
- Bhalala, U.S.; Appachi, E.; Mumtaz, M.A. Neurologic Injury Associated with Rewarming from Hypothermia: Is Mild Hypothermia on Bypass Better than Deep Hypothermic Circulatory Arrest? Front. Pediatr. 2016, 4, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huisenga, D.; La Bastide-Van Gemert, S.; Van Bergen, A.; Sweeney, J.; Hadders-Algra, M. Developmental outcomes after early surgery for complex congenital heart disease: A systematic review and meta-analysis. Dev. Med. Child. Neurol. 2020. [Google Scholar] [CrossRef]
- Bembea, M.M.; Savage, W.; Strouse, J.J.; Schwartz, J.M.; Graham, E.; Thompson, C.B.; Everett, A. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr. Crit. Care Med. 2011, 12, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Lavinio, A.; Timofeev, I.; Nortje, J.; Outtrim, J.; Smielewski, P.; Gupta, A.; Hutchinson, P.J.; Matta, B.F.; Pickard, J.D.; Menon, D.; et al. Cerebrovascular reactivity during hypothermia and rewarming. Br. J. Anaesth. 2007, 99, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Armstrong, J.S.; Lee, J.H.; Bhalala, U.; Kulikowicz, E.; Zhang, H.; Reyes, M.; Moy, N.; Spicer, D.; Zhu, J.; et al. Rewarming from therapeutic hypothermia induces cortical neuron apoptosis in a swine model of neonatal hypoxic-ischemic encephalopathy. J. Cereb. Blood Flow Metab. 2015, 35, 781–793. [Google Scholar] [CrossRef]
- Visconti, K.J.; Saudino, K.J.; Rappaport, L.A.; Newburger, J.W.; Bellinger, D.C. Influence of parental stress and social support on the behavioral adjustment of children with transposition of the great arteries. J. Dev. Behav. Pediatr. 2002, 23, 314–321. [Google Scholar] [CrossRef]


| Characteristic | Number | Mean ± SD | Median (IQR) |
|---|---|---|---|
| Sex (F/M) | 16/22 | ||
| Gestational Age (weeks) | 37.9 ± 3.2 | 38.0 (38.0–40.0) | |
| Term/Preterm (GA < 37 weeks) | 32/6 | ||
| Neonatal weight (grams) | 3058 ± 648 | 3228 (2798–3468) | |
| Head circumference (cm) | 33.7 ± 1.7 | 34.0 (34.0–34.5) | |
| Percentile of HC (%) | 47 ± 25 | 43 (34–61) | |
| Apgar 1′ | 8.4 ± 0.7 | 9.0 (8.0–9.0) | |
| Apgar 5′ | 9.2 ± 0.7 | 9.0 (9.0–10.0) | |
| SpO2 (%) | 89.2 ± 8.6 | 88.5 (85.0–98.0) | |
| STAT category: | |||
| 1 | 5 (13.2%) | ||
| 2 | 15 (39.5%) | ||
| 3 | 7 (18.4%) | ||
| 4 | 8 (21%) | ||
| 5 | 3 (7.9%) | ||
| Age at surgery (months) | 14.9 ± 20.2 | 4.0 (0.0–23.8) | |
| <1 month of age (n, %) | 12 (31.6%) | ||
| 1–12 months of age (n, %) | 14 (36.8%) | ||
| >12 months of age (n, %) | 12 (31.6%) | ||
| Weight at surgery (kg) | 7.6 ± 4.9 | 5.8 (3.5–11.9) | |
| Time (minutes) | |||
| Surgery | 249 ± 71 | 248 (196–281) | |
| CPB | 123 ± 49 | 120 (87–152) | |
| Aortic clamp (n = 30) | 62 ± 26 | 63 (41–79) | |
| Circulatory arrest (n = 4) | 26 ± 27 | 24 (3–46) | |
| Duration of Hypothermia | 75 ± 34 | 70 (50–90) | |
| Duration of Rewarming | 33 ± 21 | 30 (20–40) | |
| Minimal body temperature (°C) | 30.2 ± 3.9 | 32.0 (28.0–32.7) | |
| rO2 during CPB (%) | 55.9 ± 10.4 | 56 (48–60) | |
| rO2 <45% during CPB (%) | 19.6 ± 27.5 | 10 (0–20) | |
| Intubation (hours) | 13 ± 267 | 50 (26–90) | |
| Admission time (days) | Data are given as mean ± SD, median (IQR), or n (%). CPB, cardiopulmonary bypass; ICU, intensive care unit; NA, not available. | ||
| ICU | 7 ± 13 | 4 (2–5) | |
| Total | 15 ± 18 | 12 (7–14) |
| Neuropsychological Assessment | |
|---|---|
| NDI abnormal: n (%) | 22 (57.9%) |
| Total IQ | 99.7 ± 16.7 |
| Pathological cases: (n, %) | |
| IQ | 6 (15.8%) |
| Language | 1 (2.6%) |
| Attention | 14 (36.8%) |
| Executive function | 2 (5.3%) |
| Social abilities | 13 (34.2%) |
| Psychopathology | 17 (44.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergine, M.; Vedovelli, L.; Simonato, M.; Tonazzo, V.; Correani, A.; Cainelli, E.; Gregori, D.; Padalino, M.A.; Cogo, P. Perioperative Glial Fibrillary Acidic Protein Is Associated with Long-Term Neurodevelopment Outcome of Infants with Congenital Heart Disease. Children 2021, 8, 655. https://doi.org/10.3390/children8080655
Vergine M, Vedovelli L, Simonato M, Tonazzo V, Correani A, Cainelli E, Gregori D, Padalino MA, Cogo P. Perioperative Glial Fibrillary Acidic Protein Is Associated with Long-Term Neurodevelopment Outcome of Infants with Congenital Heart Disease. Children. 2021; 8(8):655. https://doi.org/10.3390/children8080655
Chicago/Turabian StyleVergine, Michela, Luca Vedovelli, Manuela Simonato, Valentina Tonazzo, Alessio Correani, Elisa Cainelli, Dario Gregori, Massimo A. Padalino, and Paola Cogo. 2021. "Perioperative Glial Fibrillary Acidic Protein Is Associated with Long-Term Neurodevelopment Outcome of Infants with Congenital Heart Disease" Children 8, no. 8: 655. https://doi.org/10.3390/children8080655
APA StyleVergine, M., Vedovelli, L., Simonato, M., Tonazzo, V., Correani, A., Cainelli, E., Gregori, D., Padalino, M. A., & Cogo, P. (2021). Perioperative Glial Fibrillary Acidic Protein Is Associated with Long-Term Neurodevelopment Outcome of Infants with Congenital Heart Disease. Children, 8(8), 655. https://doi.org/10.3390/children8080655







