Transnasal Sphenopalatine Ganglion Block for the Preventive Treatment of Chronic Daily Headache in Adolescents
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Demographics
3.2. Primary Endpoint
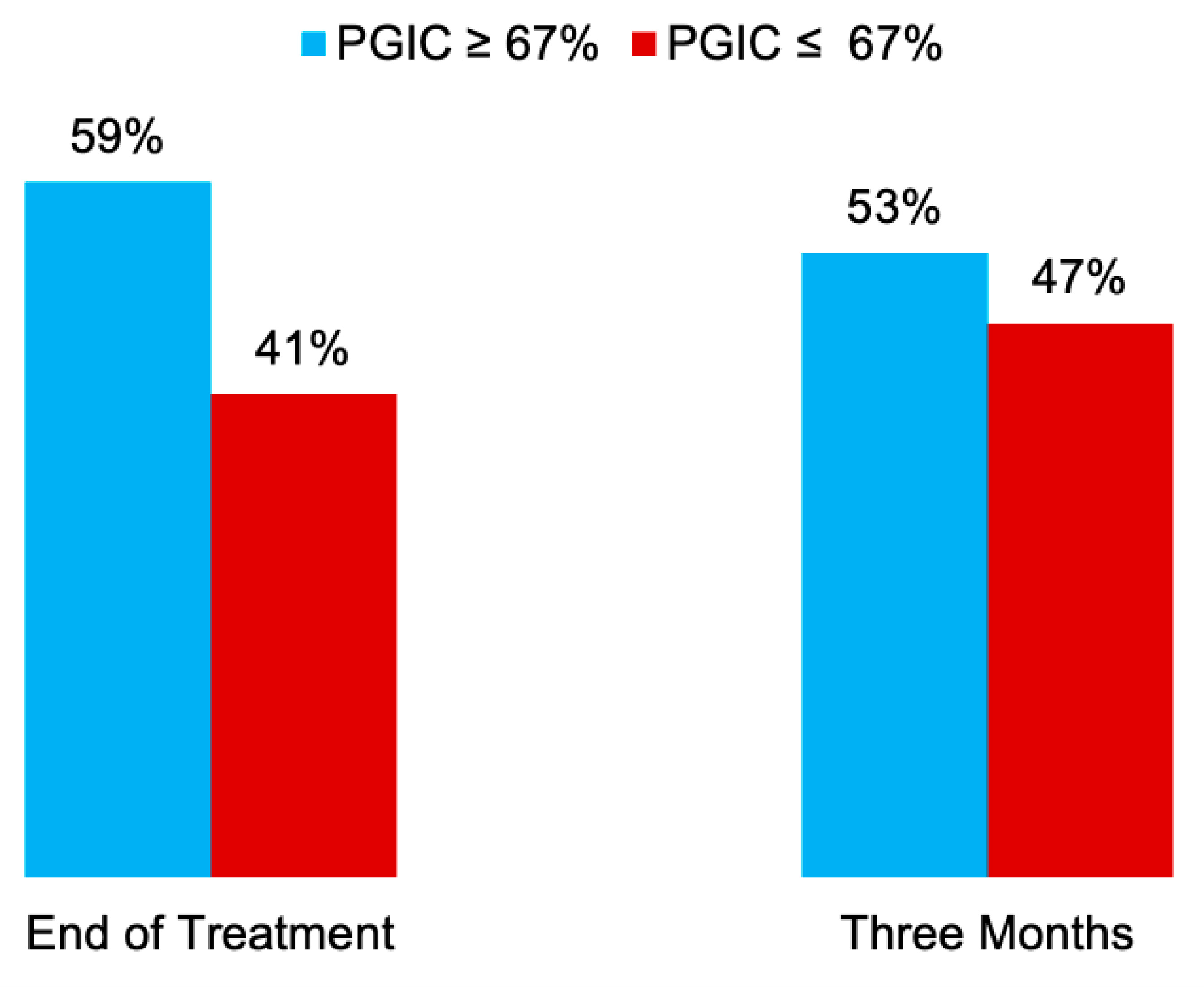
3.3. Secondary Endpoints
3.4. Side Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Langdon, R.; DiSabella, M.T. Pediatric Headache: An Overview. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 44–65. [Google Scholar] [CrossRef]
- Connelly, M.; Sekhon, S. Current perspectives on the development and treatment of chronic daily headache in children and adolescents. Pain Manag. 2019, 9, 175–189. [Google Scholar] [CrossRef]
- Mack, K.J. An approach to children with chronic daily headache. Dev. Med. Child Neurol. 2006, 48, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Koller, L.S.; Diesner, S.C.; Voitl, P. Quality of life in children and adolescents with migraine: An Austrian monocentric, cross-sectional questionnaire study. BMC Pediatr. 2019, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, M.; Pringsheim, T.; Billinghurst, L.; Potrebic, S.; Gersz, E.M.; Gloss, D.; Holler-Managan, Y.; Leininger, E.; Licking, N.; Mack, K.; et al. Practice guideline update summary: Pharmacologic treatment for pediatric migraine prevention: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2019, 93, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Vladacenco, O.; Teleanu, D.M.; Epure, D.A. Treatment of Pediatric Migraine: A Review. J. Clin. Med. 2016, 11, 136–143. [Google Scholar]
- Mojica, J.; Mo, B.; Ng, A. Sphenopalatine Ganglion Block in the Management of Chronic Headaches. Curr. Pain Headache Rep. 2017, 21, 196. [Google Scholar] [CrossRef]
- Cady, R.K.; Saper, J.R.; Dexter, K.; Cady, R.J.; Manley, H.R. Long-Term Efficacy of a Double-Blind, Placebo-Controlled, Randomized Study for Repetitive Sphenopalatine Blockade With Bupivacaine vs Saline With the Tx 360 ® Device for Treatment of Chronic Migraine. Headache: J. Head Face Pain 2015, 55, 529–542. [Google Scholar] [CrossRef]
- Candido, K.D.; Massey, S.T.; Sauer, R.; Darabad, R.R.; Knezevic, N.N. A novel revision to the classical transnasal topical sphenopalatine ganglion block for the treatment of headache and facial pain. Pain Physician 2013, 16, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Dance, L.; Aria, D.; Schaefer, C.; Kaye, R.; Yonker, M.; Towbin, R. Safety and efficacy of sphenopalatine ganglion blockade in children: Initial experience. J. Vasc. Interv. Radiol. 2017, 28, S8. [Google Scholar] [CrossRef]
- Tian Medical [Homepage on the Internet]. Available online: https://europe.tianmedical.com/ (accessed on 30 November 2020).
- Schaffer, J.T.; Hunter, B.R.; Ball, K.M.; Weaver, C.S. Noninvasive Sphenopalatine Ganglion Block for Acute Headache in the Emergency Department: A Randomized Placebo-Controlled Trial. Ann. Emerg. Med. 2015, 65, 503–510. [Google Scholar] [CrossRef]
- Hurst, H.; Bolton, J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J. Manip. Physiol. Ther. 2004, 27, 26–35. [Google Scholar] [CrossRef]
- Loncarić-Katušin, M.; Milošević, M.; Žilić, A.; Mišković, P.; Majerić-Kogler, V.; Žunić, J. Practical chronic pain assessment tools in clinical practice. Acta Clin. Croat. 2016, 55, 19–26. [Google Scholar] [CrossRef][Green Version]
- Martin, S.R.; Zeltzer, L.K.; Seidman, L.C.; E Allyn, K.; A Payne, L. Caregiver–Child Discrepancies in Reports of Child Emotional Symptoms in Pediatric Chronic Pain. J. Pediatr. Psychol. 2019, 45, 359–369. [Google Scholar] [CrossRef]
- Kashikar-Zuck, S.; Flowers, S.R.; Claar, R.L.; Guite, J.; Logan, D.E.; Lynch-Jordan, A.M.; Palermo, T.M.; Wilson, A.C. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain 2011, 152, 1600–1607. [Google Scholar] [CrossRef]
- Raniti, M.B.; Waloszek, J.M.; Schwartz, O.; Allen, N.B.; Trinder, J. Factor structure and psychometric properties of the Pittsburgh Sleep Quality Index in community-based adolescents. Sleep 2018, 41. [Google Scholar] [CrossRef]
- Erwin, A.M.; Bashore, L. Subjective Sleep Measures in Children: Self-Report. Front. Pediatr. 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Piagkou, M.; Demesticha, T.; Troupis, T.; Vlasis, K.; Skandalakis, P.; Makri, A.; Mazarakis, A.; Lappas, D.; Piagkos, G.; O Johnson, E. The Pterygopalatine Ganglion and its Role in Various Pain Syndromes: From Anatomy to Clinical Practice. Pain Pract. 2011, 12, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.W.D.; Przkora, R.; Kumar, S. Sphenopalatine ganglion: Block, radiofrequency ablation and neurostimulation—A systematic review. J. Headache Pain 2017, 18, 118. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, D.L.; Townsend, T.; Walter, C.; Clark, L.; Alli, A.; Fahrbach, T.; Madarang, E.J.; Lemons, S.; Reeves, A.; Collins, Z. Lidocaine Versus Bupivacaine in the Treatment of Headache with Intranasal Sphenopalatine Nerve Block. Pain Physician 2020, 23, 423–428. [Google Scholar]
- Fares, H.E.; Mohamed, S.A.; Badawy, F.A.; Abdelfattah, K.A.M. Comparative study between lidocaine 2%, lidocaine 5% and bupivacaine 0.5% in transnasal sphenopalatine ganglion block for the treatment of postdural puncture headache. Indian J. Appl. Res. 2020, 10. [Google Scholar] [CrossRef]
- Eccleston, C.; Fisher, E.; Cooper, T.E.; Grégoire, M.-C.; Heathcote, L.; Krane, E.; Lord, S.M.; Sethna, N.F.; Anderson, A.-K.; Anderson, B.; et al. Pharmacological interventions for chronic pain in children: An overview of systematic reviews. Pain 2019, 160, 1698–1707. [Google Scholar] [CrossRef]
- Kacperski, J.; Kabbouche, M.A.; O’Brien, H.L.; Weberding, J.L. The optimal management of headaches in children and adolescents. Ther. Adv. Neurol. Disord. 2016, 9, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Choinière, M.; Peng, P.; Gilron, I.; Buckley, N.; Williamson, O.; Janelle-Montcalm, A.; Baerg, K.; Boulanger, A.; Di Renna, T.; Finley, G.A.; et al. Accessing care in multidisciplinary pain treatment facilities continues to be a challenge in Canada. Reg. Anesthesia Pain Med. 2020, 45, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.; Law, E.; Dudeney, J.; Palermo, T.M.; Stewart, G.; Eccleston, C. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst. Rev. 2018, 9, CD003968. [Google Scholar] [CrossRef]
- Tang, W.-X.; Zhang, L.-F.; Ai, Y.-Q.; Li, Z.-S. Efficacy of Internet-delivered cognitive-behavioral therapy for the management of chronic pain in children and adolescents: A systematic review and meta-analysis. Medicine 2018, 97, e12061. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.W.; Hershey, A.D.; Coffey, C.S. The Childhood and Adolescent Migraine Prevention (CHAMP) Study: “What Do We Do Now?”. Headache J. Head Face Pain 2017, 57, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.W.; McCabe, E.J.; MacGregor, D.L. Botox Treatment for Migraine and Chronic Daily Headache in Adolescents. J. Neurosci. Nurs. 2009, 41, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Hershey, A.D.; Powers, S.W.; Vockell, A.-L.B.; LeCates, S.; Kabbouche, M.; Maynard, M.K. PedMIDAS: Development of a questionnaire to assess disability of migraines in children. Neurology 2001, 57, 2034–2039. [Google Scholar] [CrossRef]
| PGIC | ||
|---|---|---|
| 1 | No change (or condition has got worse) | 0% |
| 2 | Almost the same, hardly any change at all | 17% |
| 3 | A little better, but no noticeable change | 34% |
| 4 | Somewhat better, but the change has not made any real difference | 50% |
| 5 | Moderately better, and a slight but noticeable change | 67% |
| 6 | Better, and a definite improvement that has made a real and worthwhile difference | 84% |
| 7 | A great deal better, and a considerable improvement that has made all the difference | 100% |
| PGIC | End of Treatment (N = 17) | 3 Months (N = 17) |
|---|---|---|
| 0% | 2 | 1 |
| 17% | 3 | 1 |
| 34% | 0 | 3 |
| 50% | 2 | 3 |
| 67% | 5 | 3 |
| 84% | 3 | 4 |
| 100% | 2 | 2 |
| Baseline | End of Treatment | 3 Months | |
|---|---|---|---|
| RCADS | |||
| Separation Anxiety | 50 (42.5–65) | 50 (43.5–63) | 50 (46–63) |
| General Anxiety | 41 (34–50) | 41 (31.5–44.5) | 39 (33–46) |
| Panic | 47 (39–61) | 47 (41–57.5) | 47 (40–55.5) |
| Social Phobia | 31 (27–39) | 28 (26–35) | 26 (24–34) |
| Obsessions/Compulsions | 49 (43.5–53.5) | 46 (37–54) | 49 (40.5–52) |
| Depression | 58 (40–60) | 42 (37–52.5) | 45 (34.5–47.5) |
| FDI | 17 (7.5–29.5) | 12 (3.5–20.5) | 17 (5–24) |
| PSQI | 8 (6–9.5) | 6.5 (4–10.25) | 9 (3–12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouri, M.; Somaini, M.; Cárdenas, V.H.G.; Niburski, K.; Vigouroux, M.; Ingelmo, P. Transnasal Sphenopalatine Ganglion Block for the Preventive Treatment of Chronic Daily Headache in Adolescents. Children 2021, 8, 606. https://doi.org/10.3390/children8070606
Kouri M, Somaini M, Cárdenas VHG, Niburski K, Vigouroux M, Ingelmo P. Transnasal Sphenopalatine Ganglion Block for the Preventive Treatment of Chronic Daily Headache in Adolescents. Children. 2021; 8(7):606. https://doi.org/10.3390/children8070606
Chicago/Turabian StyleKouri, Megan, Marta Somaini, Victor Hugo González Cárdenas, Kacper Niburski, Marie Vigouroux, and Pablo Ingelmo. 2021. "Transnasal Sphenopalatine Ganglion Block for the Preventive Treatment of Chronic Daily Headache in Adolescents" Children 8, no. 7: 606. https://doi.org/10.3390/children8070606
APA StyleKouri, M., Somaini, M., Cárdenas, V. H. G., Niburski, K., Vigouroux, M., & Ingelmo, P. (2021). Transnasal Sphenopalatine Ganglion Block for the Preventive Treatment of Chronic Daily Headache in Adolescents. Children, 8(7), 606. https://doi.org/10.3390/children8070606







