Maternal Sedentary Behavior and Physical Activity across Pregnancy and Early Childhood Motor Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maternal–Child Characteristics
2.2. Objective Measurement of SED and MVPA
2.3. Motor Development
2.4. Statistical Analysis
3. Results
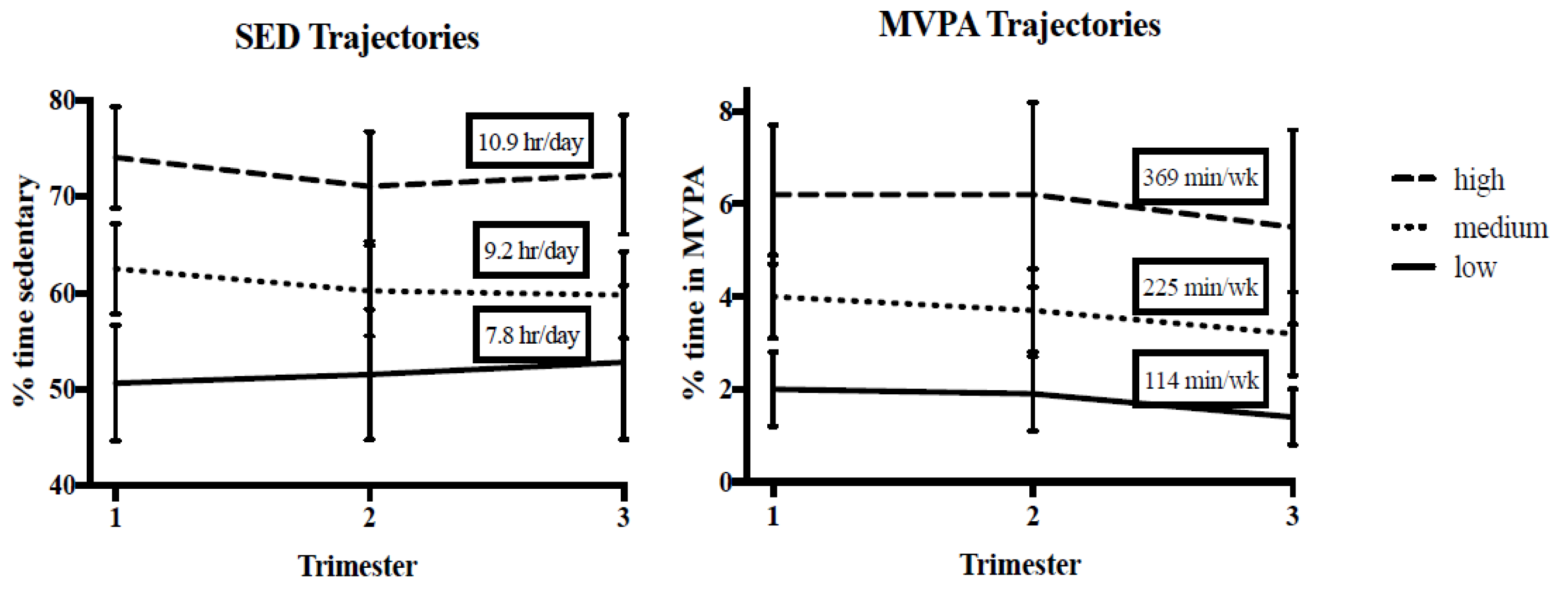
3.1. Early Motor Questionnaire and Maternal SED and MVPA Trajectories
3.2. Early Motor Questionnaire and Trimester-Specific Maternal SED and MVPA
3.3. Early Motor Questionnaire and Maternal Activity with and without Additional Adjustments
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Singh, A.S.; Mulder, C.; Twisk, J.W.; Van Mechelen, W.; Chinapaw, M.J. Tracking of childhood overweight into adulthood: A systematic review of the literature. Obes. Rev. 2008, 9, 474–488. [Google Scholar] [CrossRef]
- Kelly, R.K.; Thomson, R.; Smith, K.J.; Dwyer, T.; Venn, A.; Magnussen, C.G. Factors affecting tracking of blood pressure from childhood to adulthood: The childhood determinants of adult health study. J. Pediatrics 2015, 167, 1422–1428.e2. [Google Scholar] [CrossRef] [Green Version]
- Bames, J.; Behrens, T.K.; Benden, M.E.; Biddle, S.; Bond, D.; Brassard, P.; Brown, H.; Carr, L.; Carson, V.; Chaput, J.; et al. Letter to the Editor: Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2012, 37, 540–542. [Google Scholar]
- Barakat, R.; Cordero, Y.; Coteron, J.; Luaces, M.; Montejo, R. Exercise during pregnancy improves maternal glucose screen at 24–28 weeks: A randomised controlled trial. Br. J. Sports Med. 2012, 46, 656–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brett, K.; Ferraro, Z.; Holcik, M.; Adamo, K. Prenatal physical activity and diet composition affect the expression of nutrient transporters and mTOR signaling molecules in the human placenta. Placenta 2015, 36, 204–212. [Google Scholar] [CrossRef]
- Gibbs, B.B.; Jones, M.A.; Jakicic, J.M.; Jeyabalan, A.; Whitaker, K.M.; Catov, J.M. Objectively Measured Sedentary Behavior and Physical Activity Across 3 Trimesters of Pregnancy: The Monitoring Movement and Health Study. J. Phys. Act. Health 2021, 1, 1–8. [Google Scholar]
- Jones, M.A.; Catov, J.M.; Jeyabalan, A.; Whitaker, K.M.; Barone Gibbs, B. Sedentary behaviour and physical activity across pregnancy and birth outcomes. Paediatr. Perinat. Epidemiol. 2021, 35, 341–349. [Google Scholar] [CrossRef]
- Birsner, M.L.; Gyamfi-Bannerman, C. Physical Activity and Exercise During Pregnancy and the Postpartum Period. ACOG Committee Opinion No. 804. Obstet. Gynecol. 2020, 135, e178–e188. [Google Scholar]
- Pastorino, S.; Bishop, T.; Crozier, S.; Granström, C.; Kordas, K.; Küpers, L.; O’Brien, E.; Polanska, K.; Sauder, K.; Zafarmand, M.; et al. Associations between maternal physical activity in early and late pregnancy and offspring birth size: Remote federated individual level meta-analysis from eight cohort studies. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Gallahue, D.; Ozmun, J. Understanding Motor Development: Infants, Children, Adolescents, Adults; Chapter 13; McGraw-Hill: New York, NY, USA, 2006; pp. 267–274. [Google Scholar]
- Barnett, L.M.; Van Beurden, E.; Morgan, P.J.; Brooks, L.O.; Beard, J.R. Childhood motor skill proficiency as a predictor of adolescent physical activity. J. Adolesc. Health 2009, 44, 252–259. [Google Scholar] [CrossRef]
- Graf, C.; Koch, B.; Kretschmann-Kandel, E.; Falkowski, G.; Christ, H.; Coburger, S.; Lehmacher, W.; Bjarnason-Wehrens, B.; Platen, P.; Tokarski, W.; et al. Correlation between BMI, leisure habits and motor abilities in childhood (CHILT-project). Int. J. Obes. 2004, 28, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubans, D.R.; Morgan, P.J.; Cliff, D.P.; Barnett, L.M.; Okely, A.D. Fundamental Movement Skills in Children and Adolescents. Sports Med. 2010, 40, 1019–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hediger, M.L.; Overpeck, M.D.; Ruan, W.J.; Troendle, J.F. Birthweight and gestational age effects on motor and social development. Paediatr. Perinat. Epidemiol. 2002, 16, 33–46. [Google Scholar] [CrossRef]
- Álvarez-Bueno, C.; Cavero-Redondo, I.; Sánchez-López, M.; Garrido-Miguel, M.; Martínez-Hortelano, J.A.; Martínez-Vizcaíno, V. Pregnancy leisure physical activity and children’s neurodevelopment: A narrative review. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1235–1242. [Google Scholar] [CrossRef]
- Hellenes, O.M.; Vik, T.; Løhaugen, G.C.; Salvesen, K.Å.; Stafne, S.N.; Mørkved, S.; Evensen, K.A.I. Regular moderate exercise during pregnancy does not have an adverse effect on the neurodevelopment of the child. Acta Paediatr. 2015, 104, 285–291. [Google Scholar] [CrossRef]
- Ellingsen, M.S.; Pettersen, A.; Stafne, S.N.; Mørkved, S.; Salvesen, K.Å.; Evensen, K.A.I. Neurodevelopmental outcome in 7-year-old children is not affected by exercise during pregnancy: Follow up of a multicentre randomised controlled trial. BJOG: BJOG Int. J. Obstet. Gynaecol. 2020, 127, 508–517. [Google Scholar] [CrossRef] [Green Version]
- McMillan, A.G.; May, L.E.; Gaines, G.G.; Isler, C.; Kuehn, D. Effects of Aerobic Exercise during Pregnancy on 1-Month Infant Neuromotor Skills. Med. Sci. Sports Exerc. 2019, 51, 1671–1676. [Google Scholar] [CrossRef]
- Leroy, J. ZSCORE06: Stata Module to Calculate Anthropometric Z-Scores Using the 2006 WHO Child Growth Standards; Statistical Software Components; Boston College Department of Economics: Chestnut Hill, MA, USA, 2011. [Google Scholar]
- Barone Gibbs, B.; Kline, C.E. When does sedentary behavior become sleep? A proposed framework for classifying activity during sleep-wake transitions. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 81. [Google Scholar] [CrossRef]
- Edwardson, C.L.; Winkler, E.A.; Bodicoat, D.H.; Yates, T.; Davies, M.; Dunstan, D.W.; Healy, G.N. Considerations when using the activPAL monitor in field-based research with adult populations. J. Sport Health Sci. 2017, 6, 162–178. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, J.E.; John, D.; Freedson, P.S. Validation and comparison of ActiGraph activity monitors. J. Sci. Med. Sport 2011, 14, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.E.; Hagstromer, M.; Pober, D.M.; Bowles, H.R. Best practices for using physical activity monitors in population-based research. Med. Sci. Sports Exerc. 2012, 44 (Suppl. 1), S68–S76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, B.L.; Nagin, D.S. A note on a Stata plugin for estimating group-based trajectory models. Sociol. Methods Res. 2013, 42, 608–613. [Google Scholar] [CrossRef]
- Libertus, K.; Landa, R.J. The Early Motor Questionnaire (EMQ): A parental report measure of early motor development. Infant Behav. Dev. 2013, 36, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Department of Health and Human Services. Effective Practice Guide: Perceptual, Motor, and Physical Development. Early Childhood Learning and Knowledge Center. Available online: https://eclkc.ohs.acf.hhs.gov/school-readiness/effective-practice-guides/perceptual-motor-physical-development (accessed on 15 June 2021).
- Acock, A.C. A Gentle Introduction to Stata; Stata Press: College Station, TX, USA, 2008. [Google Scholar]
- Polańska, K.; Muszyński, P.; Sobala, W.; Dziewirska, E.; Merecz-Kot, D.; Hanke, W. Maternal lifestyle during pregnancy and child psychomotor development—Polish Mother and Child Cohort study. Early Hum. Dev. 2015, 91, 317–325. [Google Scholar] [CrossRef]
- Borodulin, K.; Evenson, K.R.; Herring, A.H. Physical activity patterns during pregnancy through postpartum. BMC Women’s Health 2009, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinkley, T.; Crawford, D.; Salmon, J.; Okely, A.D.; Hesketh, K. Preschool children and physical activity: A review of correlates. Am. J. Prev. Med. 2008, 34, 435–441.e7. [Google Scholar] [CrossRef] [PubMed]
- Cools, W.; De Martelaer, K.; Samaey, C.; Andries, C. Fundamental movement skill performance of preschool children in relation to family context. J. Sports Sci. 2011, 29, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Barnett, L.M.; Hnatiuk, J.A.; Salmon, J.; Hesketh, K.D. Modifiable factors which predict children’s gross motor competence: A prospective cohort study. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 129. [Google Scholar] [CrossRef] [Green Version]

| Sedentary Trajectory (n) | MVPA Trajectory (n) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (70) | Low (12) | Med (29) | High (29) | p | Low (18) | Med (38) | High (14) | p | |
| Median (IQR 25%, 75%) | |||||||||
| Child age, months | 22.7 (16.7, 26.5) | 25.4 (21.9, 28.0) | 20.7 (16.8, 26.5) | 22.8 (16.6, 26.0) | 0.327 | 23.6 (15.1, 26.56) | 22.7 (16.8, 26.2) | 22.8 (16.8, 26.09) | 0.964 |
| Gestational age at birth, weeks | 38.3 (38.4, 40.0) | 39.6 (39.14, 39.71) | 39.0 (38.1, 40.0) | 39.4 (38.3, 40.0) | 0.679 | 39.1 (38.6, 39.6) | 39.7 (38.3, 40.0) | 38.9 (37.0, 39.7) | 0.285 |
| Maternal pre-pregnancy BMI, kg/m2 | 21.5 (21.5, 29.5) | 27.5 (23.3, 21.9) | 24.3 (19.9, 27.4) | 23.9 (21.9, 25.7) | 0.199 | 25.2 (21.0, 31.6) | 24.0 (21.7, 30.8) | 24.0 (21.7, 27.5) | 0.845 |
| Maternal EPDS score * | 2.0 (1.0, 5.0) | 3.5 (0.0, 8.0) | 1.0 (0.0, 4.0) | 3.0 (2.0, 5.0) | 0.153 | 4.0 (2.0, 5.0) | 2.0 (0.0, 5.0) | 2.0 (0.5, 5.5) | 0.469 |
| n (%) | |||||||||
| Sex | 0.059 | 0.470 | |||||||
| Male | 37 (53) | 4 (33) | 13 (45) | 20 (69) | 12 (66) | 18 (47) | 7 (50) | ||
| Female | 33 (47) | 8 (67) | 16 (55) | 9 (31) | 6 (33) | 20 (53) | 7 (50) | ||
| Feeding type | 0.926 | 0.757 | |||||||
| Exclusively breastfed | 36 (51) | 7 (58) | 15 (52) | 14 (48) | 9 (50) | 20 (53) | 7 (50) | ||
| Partial breastfeeding | 31 (44) | 5 (42) | 12 (41) | 14 (48) | 9 (50) | 15 (39) | 7 (50) | ||
| Exclusively formula-fed | 3 (4) | 0 | 2 (7) | 1 (4) | 0 | 3 (8) | 0 | ||
| Maternal Education | 0.214 | 0.157 | |||||||
| High school or less | 1 (1) | 0 | 0 | 1 (2) | 1 (5) | 0 | 0 | ||
| Some college or training | 11 (15) | 4 (33) | 4 (14) | 3 (10) | 5 (28) | 3 (8) | 3 (21) | ||
| College graduate | 18 (27) | 4 (33) | 5 (17) | 9 (31) | 5 (28) | 11 (29) | 2 (14) | ||
| Masters/Doctoral | 40 (57) | 4 (33) | 20 (69) | 16 (55) | 7 (39) | 25 (63) | 9 (64) | ||
| Household Income | 0.188 | 0.448 | |||||||
| <50,000 | 6 (8) | 3 (25) | 2 (7) | 1 (3) | 1 (6) | 4 (11) | 1 (7) | ||
| 50,000–75,000 | 8 (11) | 2 (17) | 4 (14) | 2 (7) | 3 (17) | 4 (11) | 1 (7) | ||
| >75,000 | 53 (76) | 6 (50) | 22 (76) | 25 (86) | 12 (67) | 30 (78) | 11 (80) | ||
| Don’t know/refused to answer | 3 (4) | 1 (8) | 1 (3) | 1 (4) | 2 (11) | 0 | 1 (7) | ||
| Race | 0.871 | 0.538 | |||||||
| White | 58 (82) | 9 (75) | 25 (86) | 24 (83) | 13 (72) | 32 (84) | 13 (93) | ||
| Black | 6 (9) | 2 (17) | 2 (7) | 2 (7) | 2 (11) | 3 (8) | 1 (7) | ||
| Asian | 6 (9) | 1 (8) | 2 (7) | 3 (10) | 3 (17) | 3 (8) | 0 | ||
| Trimester 1 | Trimester 2 | Trimester 3 | ||||
|---|---|---|---|---|---|---|
| Std. ß | 95% CI | Std. ß | 95% CI | Std. ß | 95% CI | |
| SED | ||||||
| Gross Motor | −2.10 | 5.69, 1.49 | −2.50 | −6.08, 1.07 | −1.55 | −4.85, 1.96 |
| Fine Motor | 0.26 | −3.55, 4.06 | −1.92 | −5.66, 1.82 | −1.96 | −5.42, 1.79 |
| Perception-Action | −1.35 | −4.08, 1.38 | −1.36 | −4.09, 1.36 | −0.79 | −3.36, 1.89 |
| MVPA | ||||||
| Gross Motor | 1.90 | −1.58, 5.38 | 0.29 | −3.27, 3.84 | −1.03 | −4.74, 2.68 |
| Fine Motor | 4.33 | 0.81, 7.84 | 3.72 | 0.10, 7.33 | 2.70 | −1.20, 6.60 |
| Perception-Action | 3.78 | 1.29, 6.27 | 2.87 | 0.27, 5.47 | 1.42 | −1.47, 4.32 |
| Low | Medium | High | ||||
|---|---|---|---|---|---|---|
| Coef. | 95% CI | Coef. | 95% CI | Coef. | 95% CI | |
| SED Trajectories | ||||||
| Gross Motor | ||||||
| Model 1 | Reference | - | −2.07 | −14.21, 10.07 | −1.63 | −13.75, 10.49 |
| Model 2 | Reference | - | −1.96 | −14.33, 10.51 | −1.43 | −14.42, 10.51 |
| Fine Motor | ||||||
| Model 1 | Reference | - | −4.65 | −15.64, 6.33 | −3.39 | −14.35, 7.57 |
| Model 2 | Reference | - | −5.19 | −16.44, 6.06 | −4.37 | −16.02, 7.27 |
| Perception-Action | ||||||
| Model 1 | Reference | - | −1.01 | −10.12, 8.09 | −2.77 | −11.85, 6.32 |
| Model 2 | Reference | - | −1.59 | −10.89, 7.71 | −3.83 | −13.45, 5.80 |
| MVPA Trajectories | ||||||
| Gross Motor | ||||||
| Model 1 | Reference | - | −0.95 | −10.65, 8.74 | 3.57 | −8.40, 15.54 |
| Model 2 | Reference | - | −0.70 | −10.61, 9.20 | 4.09 | −8.42, 16.61 |
| Fine Motor | ||||||
| Model 1 | Reference | - | 7.22 | −1.26, 15.70 | 11.60 | 1.13, 22.07 |
| Model 2 | Reference | - | 7.36 | −1.31, 16.03 | 11.91 | 0.96, 22.86 |
| Perception-Action | ||||||
| Model 1 | Reference | - | 5.38 | −1.68, 12.45 | 8.80 | 0.08, 17.52 |
| Model 2 | Reference | - | 5.43 | −1.79, 12.65 | 8.90 | −0.22, 18.03 |
| Trimester 1 | Trimester 2 | Trimester 3 | ||||
|---|---|---|---|---|---|---|
| Std. Coef. | 95% CI | Std. Coef. | 95% CI | Std. Coef. | 95% CI | |
| SED | ||||||
| Gross Motor | ||||||
| Model 1 | −1.46 | −5.67, 2.79 | −2.00 | −6.33, 2.33 | −0.52 | −4.68, 3.63 |
| Model 2 | −1.59 | −6.0, 2.85 | −1.99 | −6.38, 2.41 | −0.80 | −5.09, 3.48 |
| Fine Motor | ||||||
| Model 1 | 0.47 | −3.40, 4.34 | 0.03 | −3.91, 3.97 | −0.12 | −3.95, 3.73 |
| Model 2 | 0.31 | −3.76, 4.38 | −0.07 | −4.07, 3.92 | −0.29 | −4.25, 3.67 |
| Perception-Action | ||||||
| Model 1 | −1.36 | −4.53, 1.81 | −0.68 | −3.95, 2.59 | −0.01 | −3.18. 3.17 |
| Model 2 | −1.70 | −5.02, 1.62 | −0.79 | −4.10, 2.52 | −0.28 | −3.54, 2.98 |
| MVPA | ||||||
| Gross Motor | ||||||
| Model 1 | 1.20 | −2.92, 5.32 | −1.21 | −5.57, 3.15 | −2.27 | −5.46, 2.68 |
| Model 2 | 1.32 | −2.93, 5.54 | −1.30 | −6.24, 3.64 | −1.39 | −6.62, 2.09 |
| Fine Motor | ||||||
| Model 1 | 3.72 | 0.09, 7.34 | 3.24 | −0.64, 7.12 | 2.04 | −1.71, 5.79 |
| Model 2 | 3.72 | 0.00, 7.44 | 3.62 | −0.76, 8.01 | 1.98 | −2.09, 6.04 |
| Perception-Action | ||||||
| Model 1 | 3.77 | 0.83, 6.71 | 2.91 | −0.27, 6.10 | 1.07 | −2.11, 4.24 |
| Model 2 | 3.78 | 0.76, 6.79 | 3.19 | −0.41, 6.79 | 0.79 | −2.63, 4.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, M.A.; Whitaker, K.M.; Taverno Ross, S.E.; Davis, K.; Libertus, K.; Barone Gibbs, B. Maternal Sedentary Behavior and Physical Activity across Pregnancy and Early Childhood Motor Development. Children 2021, 8, 549. https://doi.org/10.3390/children8070549
Jones MA, Whitaker KM, Taverno Ross SE, Davis K, Libertus K, Barone Gibbs B. Maternal Sedentary Behavior and Physical Activity across Pregnancy and Early Childhood Motor Development. Children. 2021; 8(7):549. https://doi.org/10.3390/children8070549
Chicago/Turabian StyleJones, Melissa A., Kara M. Whitaker, Sharon E. Taverno Ross, Kelliann Davis, Klaus Libertus, and Bethany Barone Gibbs. 2021. "Maternal Sedentary Behavior and Physical Activity across Pregnancy and Early Childhood Motor Development" Children 8, no. 7: 549. https://doi.org/10.3390/children8070549
APA StyleJones, M. A., Whitaker, K. M., Taverno Ross, S. E., Davis, K., Libertus, K., & Barone Gibbs, B. (2021). Maternal Sedentary Behavior and Physical Activity across Pregnancy and Early Childhood Motor Development. Children, 8(7), 549. https://doi.org/10.3390/children8070549





