Pediatric Subcutaneous Abscess: Still a Clinical Exam-Based Diagnosis and Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Variables.
2.3. Statistical Analysis
3. Results
3.1. Demographics and Patient Characteristics
3.2. Diagnostic Evaluation
3.3. Treatment and Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mistry, R.D. Skin and Soft Tissue Infections. Pediatr. Clin. N. Am. 2013, 60, 1063–1082. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.L.; Chambers, H.F.; Maselli, J.H.; Gonzales, R. National Trends in Ambulatory Visits and Antibiotic Prescribing for Skin and Soft-Tissue Infections. Arch. Intern. Med. 2008, 168, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.J.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; McDougal, L.K.; Carey, R.B.; Talan, D.A. Methicillin-ResistantS. Aureus Infections among Patients in the Emergency Department. N. Engl. J. Med. 2006, 355, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Iverson, K.; Haritos, D.; Thomas, R.; Kannikeswaran, N. The effect of bedside ultrasound on diagnosis and management of soft tissue infections in a pediatric ED. Am. J. Emerg. Med. 2012, 30, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.F.; Sivitz, A.; Alade, K.; Doniger, S.J.; Tessaro, M.O.; Rabiner, J.E.; Arroyo, A.; Castillo, E.M.; Thompson, C.A.; Yang, M.; et al. Comparison of Ultrasound Guidance vs. Clinical Assessment Alone for Management of Pediatric Skin and Soft Tissue Infections. J. Emerg. Med. 2018, 55, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.; Markwell, S.; Peter, J.; Barenkamp, S. Randomized, Controlled Trial of Antibiotics in the Management of Community-Acquired Skin Abscesses in the Pediatric Patient. Ann. Emerg. Med. 2010, 55, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.R.; Durica, S.R.; Thompson, D.M.; Bogie, A.; Naifeh, M.; Vuolo, M.; Staff, J. Blood Cultures in the Evaluation of Uncomplicated Skin and Soft Tissue Infections. Pediatrics 2013, 132, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, W.; Liu, Y.; Siemieniuk, R.A.C.; Li, L.; Martínez, J.P.D.; Guyatt, G.H.; Sun, X. Antibiotics for uncomplicated skin abscesses: Systematic review and network meta-analysis. BMJ Open 2018, 8, e020991. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Thode, H.C. Systemic antibiotics after incision and drainage of simple abscesses: A meta-analysis. Emerg. Med. J. 2013, 31, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.J.; O’Leary, S.T.; Caldwell, B.; Knepper, B.C.; Pawlowski, S.W.; Burman, W.J.; Jenkins, T.C. Clinical Characteristics and Antibiotic Utilization in Pediatric Patients Hospitalized with Acute Bacterial Skin and Skin Structure Infection. Pediatr. Infect. Dis. J. 2014, 33, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Fenster, D.B.; Renny, M.H.; Ng, C.; Roskind, C.G. Scratching the surface: A review of skin and soft tissue infections in children. Curr. Opin. Pediatr. 2015, 27, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Avila, J.; Chottiner, M.; Peksa, G.D. Point-of-Care Ultrasonography for the Diagnosis of Skin and Soft Tissue Abscesses: A Systematic Review and Meta-analysis. Ann. Emerg. Med. 2020, 76, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Barbic, D.; Chenkin, J.; Cho, D.D.; Jelic, T.; Scheuermeyer, F.X. In patients presenting to the emergency department with skin and soft tissue infections what is the diagnostic accuracy of point-of-care ultrasonography for the diagnosis of abscess compared to the current standard of care? A systematic review and meta-analysis. BMJ Open 2017, 7, e013688. [Google Scholar] [CrossRef] [PubMed]
- Wathen, D.; Halloran, D.R. Blood culture associations in children with a diagnosis of cellulitis in the era of methicillin-resistant Staphylococcus aureus. Hosp. Pediatrics 2013, 3, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.J.; Zaoutis, T.E.; Troxel, A.B.; Loh, A.; Keren, R. Empiric Antimicrobial Therapy for Pediatric Skin and Soft-Tissue Infections in the Era of Methicillin-Resistant Staphylococcus aureus. Pediatrics 2009, 123, e959–e966. [Google Scholar] [CrossRef] [PubMed]

| Variable | All (n = 200) | Variable | All (n = 200) | |
|---|---|---|---|---|
| Age (years) | 4.75 ± 5.4 | Surrounding Cellulitis | 110 (55.0%) | |
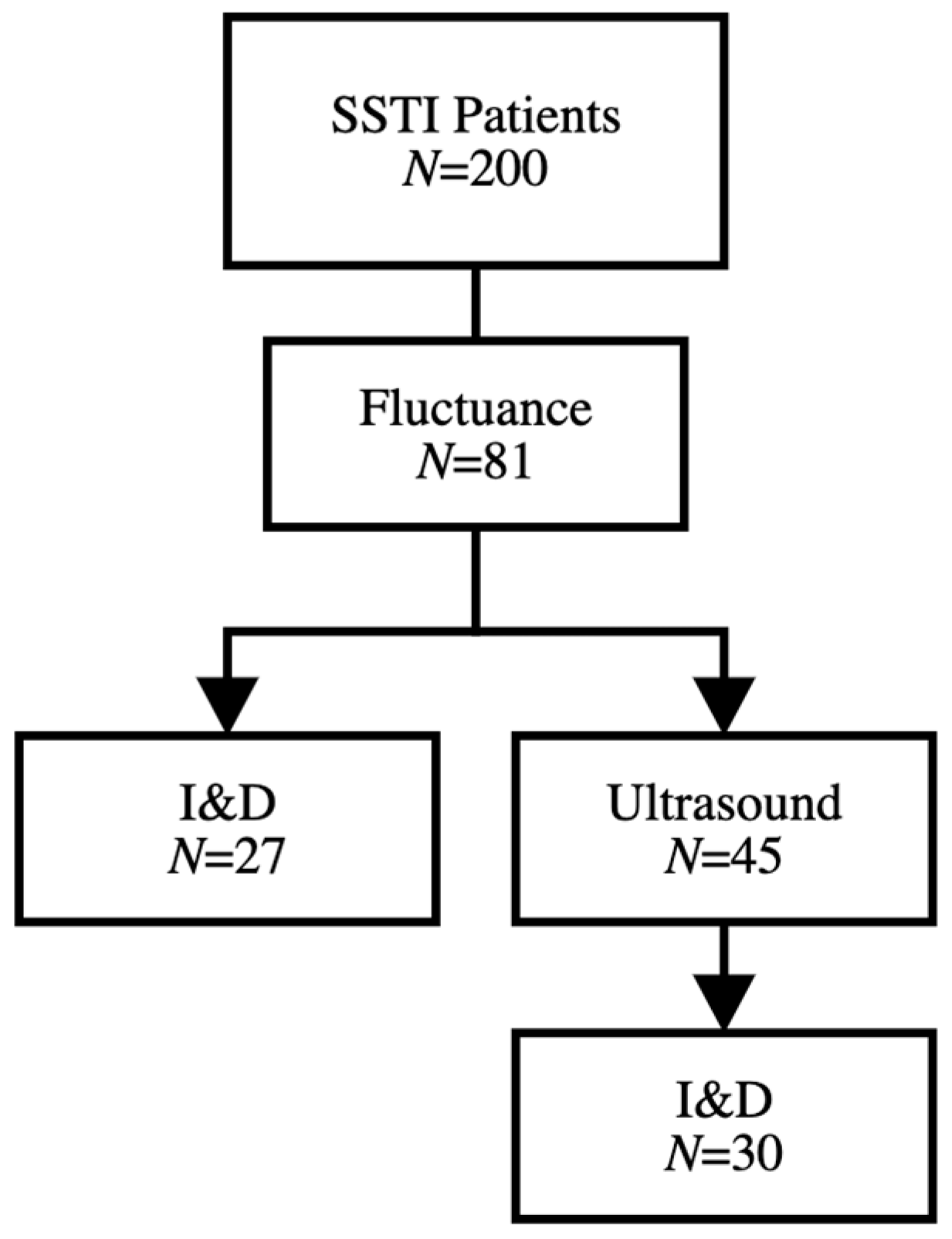
| Gender | Male | 88 (44.0%) | Fluctuance | 81 (40.5%) |
| Ethnicity | Hispanic | 84 (42.0%) | Multiple Abscesses | 29 (14.5%) |
| BMI | 20.1 ± 10.0 | Heart rate (beats/minute) | 123 ± 26.3 | |
| Co-morbidities | 11 (5.5%) | Respiratory Rate (breaths/minute) | 25.4 ± 5.3 | |
| Previous oral antibiotic use | 58 (29.0%) | WBC count (109 cells/L) | 17.5 ± 6.9 | |
| Duration of oral antibiotics | 3.97 ± 4.9 | CRP level (mg/L) | 6.4 ± 5.2 | |
| History of MRSA | 12 (6.0%) | Area of Abscess (cm2) | 24.4 ± 34.9 | |
| History of prior abscess | 60 (30.0%) | Wound Culture obtained | 83 (41.5%) | |
| Subjective Fever | 101 (50.5%) | Blood Culture obtained | 43 (21.5%) |
| Variable | All (n = 200) | I&D (n = 121) | No I&D (n = 79) | p Value | |
|---|---|---|---|---|---|
| Exam Findings | Fluctuance | 81 (40.5%) | 58 (47.9%) | 23 (29.1%) | 0.005 |
| Cellulitis | 110 (55.0%) | 70 (57.9%) | 40 (50.6%) | 0.07 | |
| Fluctuance + Cellulitis | 42 (21.0%) | 31 (25.6%) | 11 (13.9%) | 0.052 | |
| Temperature (°C) | 37.2 ± 0.8 | 37.2 ± 0.8 | 37.2 ± 0.8 | 0.96 | |
| Heart rate (beats/min) | 123 ± 26.3 | 123.0 ± 24.5 | 123.1 ± 28.6 | 0.98 | |
| Respiratory Rate (breaths/min) | 25.4 ± 5.3 | 25.0 ± 4.9 | 26.0 ± 5.6 | 0.22 | |
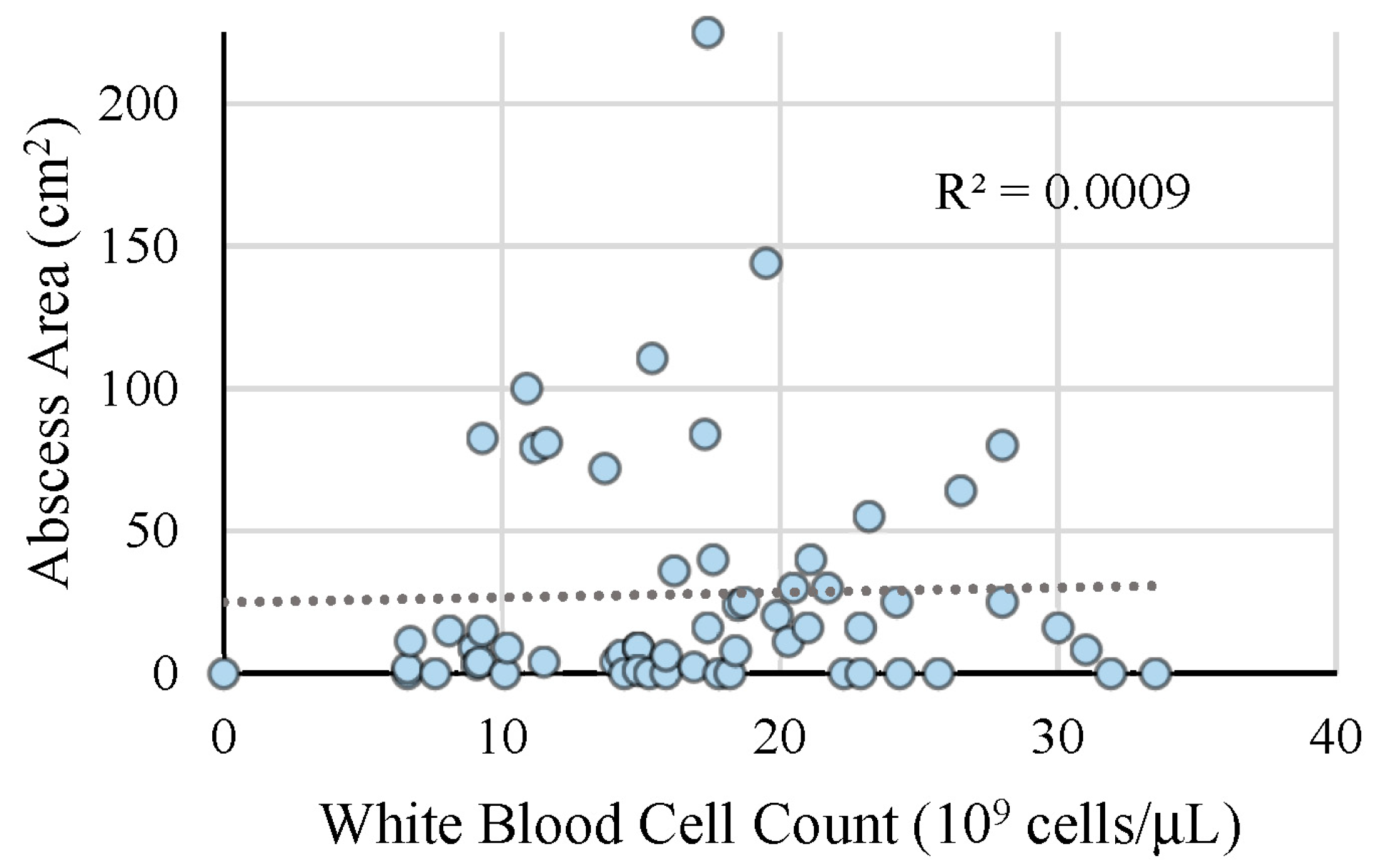
| WBC count (109 cells/L) | 17.5 ± 6.9 | 18.6 ± 6.4 | 15.7 ± 7.5 | 0.14 | |
| CRP level (mg/L) | 6.4 ± 5.2 | 6.9 ± 5.8 | 5.6 ± 3.9 | 0.53 | |
| Area of Abscess (cm2) | 24.4 ± 34.9 | 28.8 ± 39.4 | 17.2 ± 24.5 | 0.05 | |
| Ultrasound Performed | 108 (54.0%) | 65 (53.7%) | 43 (54.4%) | 1.0 |
| Variable | All (n = 200) | Admitted (n = 55) | Not Admitted (n = 145) | p Value | |
|---|---|---|---|---|---|
| Age (years) | 4.75 ± 5.4 | 3.4 ± 4.5 | 5.3 ± 5.6 | 0.02 | |
| Gender | Male | 88 (44.0%) | 21 (38.2%) | 67 (46.2%) | |
| Ethnicity | Hispanic | 84 (42.0%) | 16 (29.1%) | 68 (53.1%) | |
| BMI | 20.1 ± 10.0 | 20.5 ± 11.8 | 19.5 ± 6.7 | 0.68 | |
| Co-morbidities | 11 (5.5%) | 5 (9.1%) | 6 (4.1%) | ||
| Previous oral antibiotic use | 58 (29.0%) | 20 (36.4 %) | 38 (26.2%) | ||
| History of MRSA | 12 (6.0%) | 5 (9.1%) | 7 (4.8%) | ||
| History of prior abscess | 60 (30.0%) | 14 (25.5%) | 46 (31.7%) | ||
| Subjective Fever | 101 (50.5%) | 40 (72.7%) | 61 (42.1%) | ||
| Surrounding Cellulitis | 110 (55.0%) | 46 (83.6%) | 64 (44.1%) | ||
| Fluctuance | 81 (40.5%) | 27 (49.1%) | 54 (37.2.0%) | ||
| Multiple Abscesses | 29 (14.5%) | 11 (20.0%) | 18 (9.0%) | ||
| Temperature (°C) | 37.2 ± 0.8 | 37.1 ± 0.7 | 37.3 ± 1.0 | 0.14 | |
| Heart rate (beats/minute) | 123 ± 26.3 | 132.5 ± 23.2 | 119.5 ± 26.4 | 0.002 | |
| Respiratory Rate (breaths/minute) | 25.4 ± 5.3 | 27.5 ± 6.0 | 24.6 ± 4.7 | 0.001 | |
| WBC count (109 cells/L) | 17.5 ± 6.9 | 19.1 ± 6.9 | 14.2 ± 5.8 | 0.01 | |
| CRP level (mg/L) | 6.4 ± 5.2 | 8.0 ± 5.2 | 3.8 ± 4.0 | 0.04 | |
| Area of Abscess (cm2) | 24.4 ± 34.9 | 47.8 ± 50.5 | 16.2 ± 22.7 | 0.001 | |
| Ultrasound performed | 108 (54.0%) | 36 (65.4%) | 72 (49.7%) | ||
| I&D performed | 121 (60.5%) | 37 (67.3%) | 84 (57.9%) | ||
| Wound Culture | 83 (41.5%) | 41 (74.5%) | 42 (20.0%) | ||
| Blood Culture | 43 (21.5%) | 33 (60.0%) | 10 (6.9%) |
| Variable | Recurrence (n = 16) | No Recurrence (n = 184) | p Value |
|---|---|---|---|
| I&D | 12 (75.0%) | 42 (22.3%) | 0.17 |
| IV antibiotics | 5 (31.3%) | 51 (27.7%) | 0.005 |
| Oral antibiotics | 4 (25.0%) | 23 (12.5%) | 0.63 |
| Wound packed | 2 (12.5%) | 22 (12.0%) | 0.29 |
| Loop drain placed | 1 (6.3%) | 15 (8.2%) | 0.09 |
| Temperature (°C) | 37.4 ± 1.17 | 37.2 ± 0.83 | 0.30 |
| Heart rate (beats/minute) | 122.7 ± 34.1 | 126.8 ± 24.1 | 0.58 |
| Respiratory Rate (breaths/minute) | 24.6 ± 5.4 | 26.3 ± 6.0 | 0.29 |
| WBC count (109 cells/L) | 13.9 ± 6.0 | 18.8 ± 7.0 | 0.15 |
| CRP level (mg/L) | 2.14 ± 2.9 | 8.0 ± 5.5 | 0.17 |
| Area of Abscess (cm2) | 14.3 ± 15.5 | 40.0 ± 48.4 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, I.C.; Clark, R.A.; Chung, D.H.; Gaines, N. Pediatric Subcutaneous Abscess: Still a Clinical Exam-Based Diagnosis and Treatment. Children 2021, 8, 392. https://doi.org/10.3390/children8050392
Garcia IC, Clark RA, Chung DH, Gaines N. Pediatric Subcutaneous Abscess: Still a Clinical Exam-Based Diagnosis and Treatment. Children. 2021; 8(5):392. https://doi.org/10.3390/children8050392
Chicago/Turabian StyleGarcia, Isabel C., Rachael A. Clark, Dai H. Chung, and Nakia Gaines. 2021. "Pediatric Subcutaneous Abscess: Still a Clinical Exam-Based Diagnosis and Treatment" Children 8, no. 5: 392. https://doi.org/10.3390/children8050392
APA StyleGarcia, I. C., Clark, R. A., Chung, D. H., & Gaines, N. (2021). Pediatric Subcutaneous Abscess: Still a Clinical Exam-Based Diagnosis and Treatment. Children, 8(5), 392. https://doi.org/10.3390/children8050392







