Sustained Inflation Reduces Pulmonary Blood Flow during Resuscitation with an Intact Cord
Abstract
1. Introduction
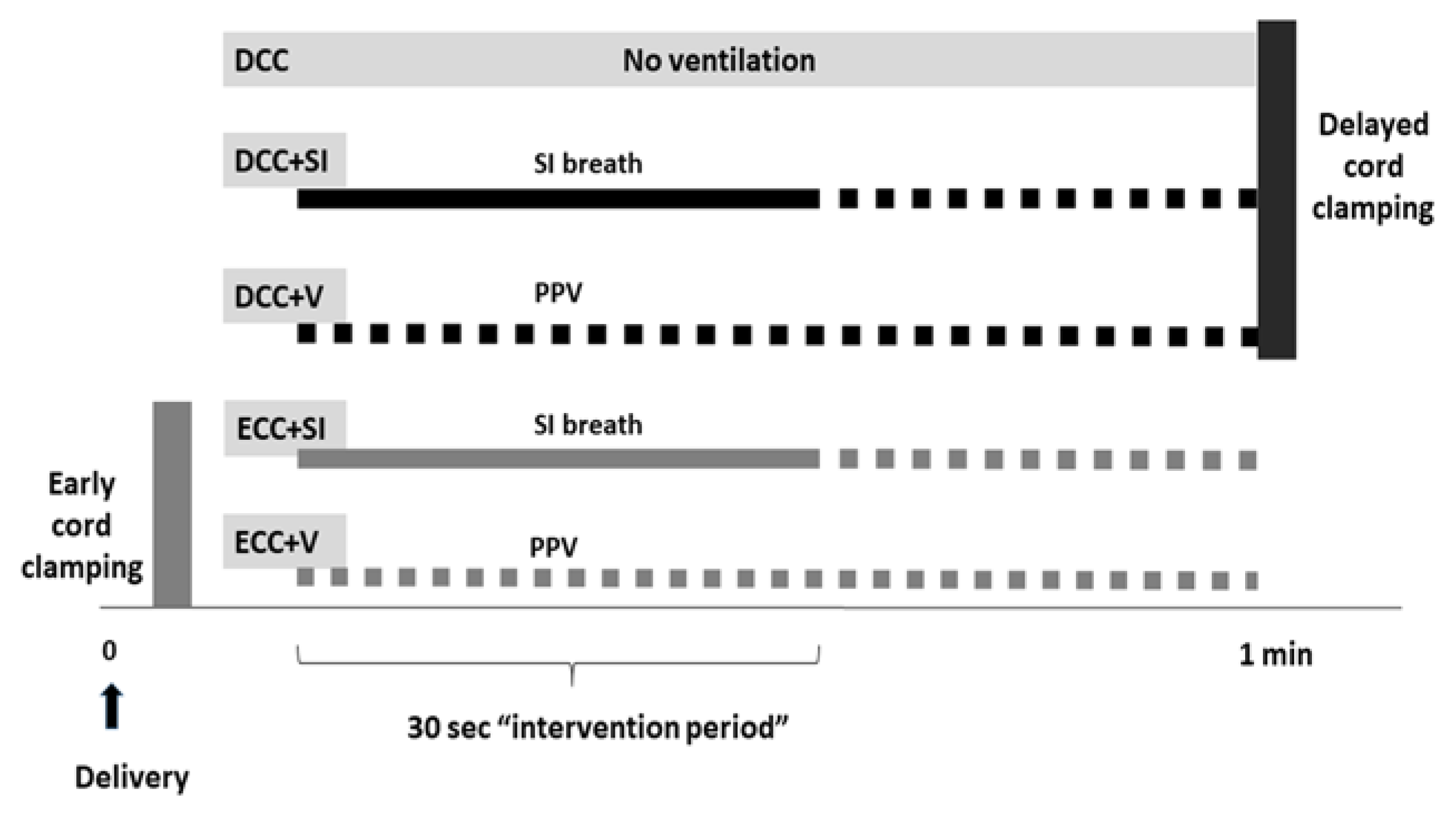
2. Materials and Methods
- (i)
- RBC volume = Amount of biotinylated RBC injected ÷ concentration of biotinylated RBC in the sample after equilibration
- (ii)
- Blood volume = RBC volume × 100 ÷ Hct
- (iii)
- Residual placental volume = Fetoplacental blood volume—neonatal blood volume
- (iv)
- Fraction of neonatal retained blood = Neonatal blood volume ÷ Fetoplacental blood volume.
3. Results
3.1. Airway Pressure
3.2. Primary Outcomes
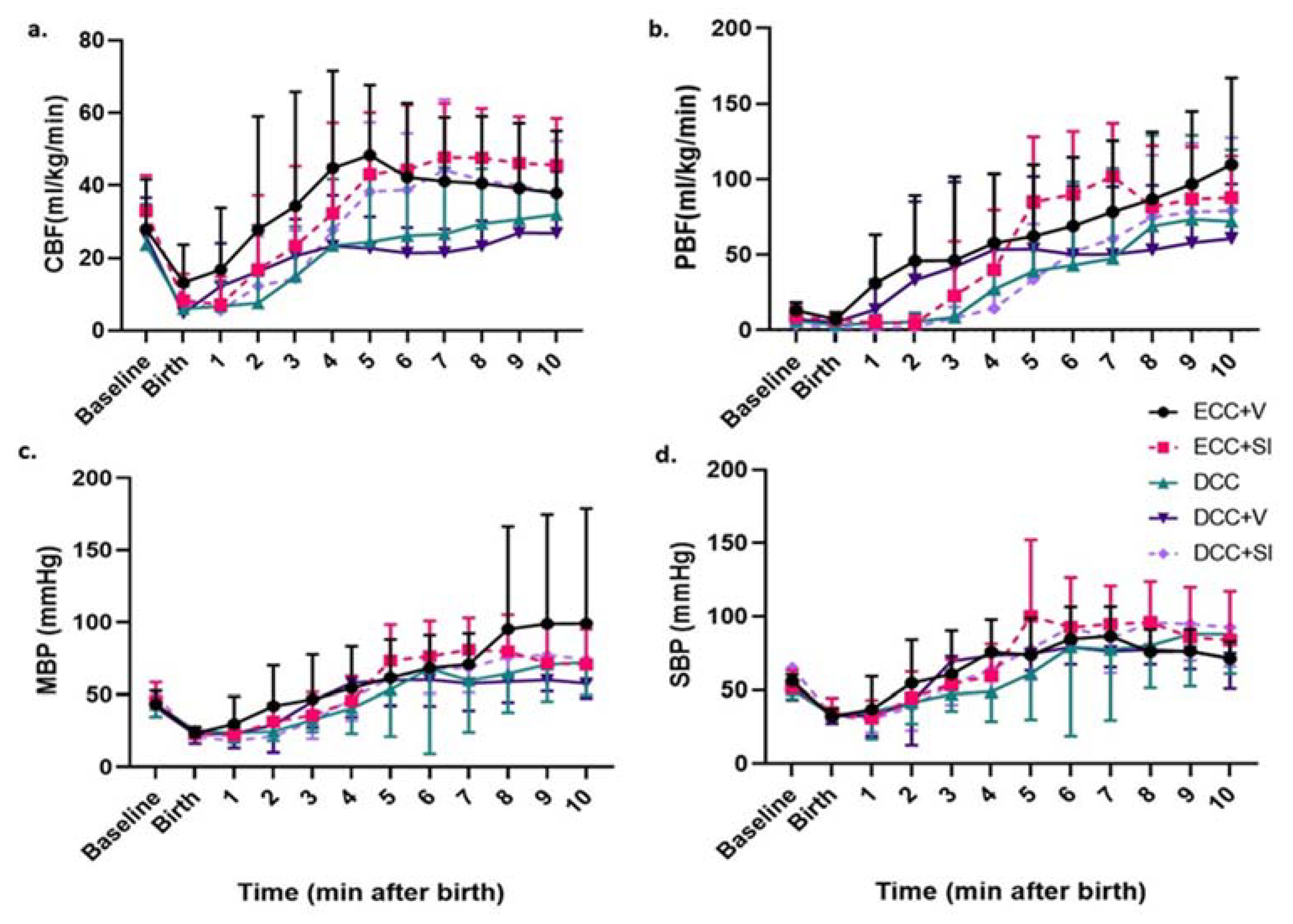
3.3. Hemodynamics during Intervention Period
3.4. Post Resuscitation Hemodynamics
3.5. Gas Exchange and Lactate
3.6. Magnitude of Placental Transfusion
3.7. Mode of Ventilation
3.8. Timing of Cord Clamping
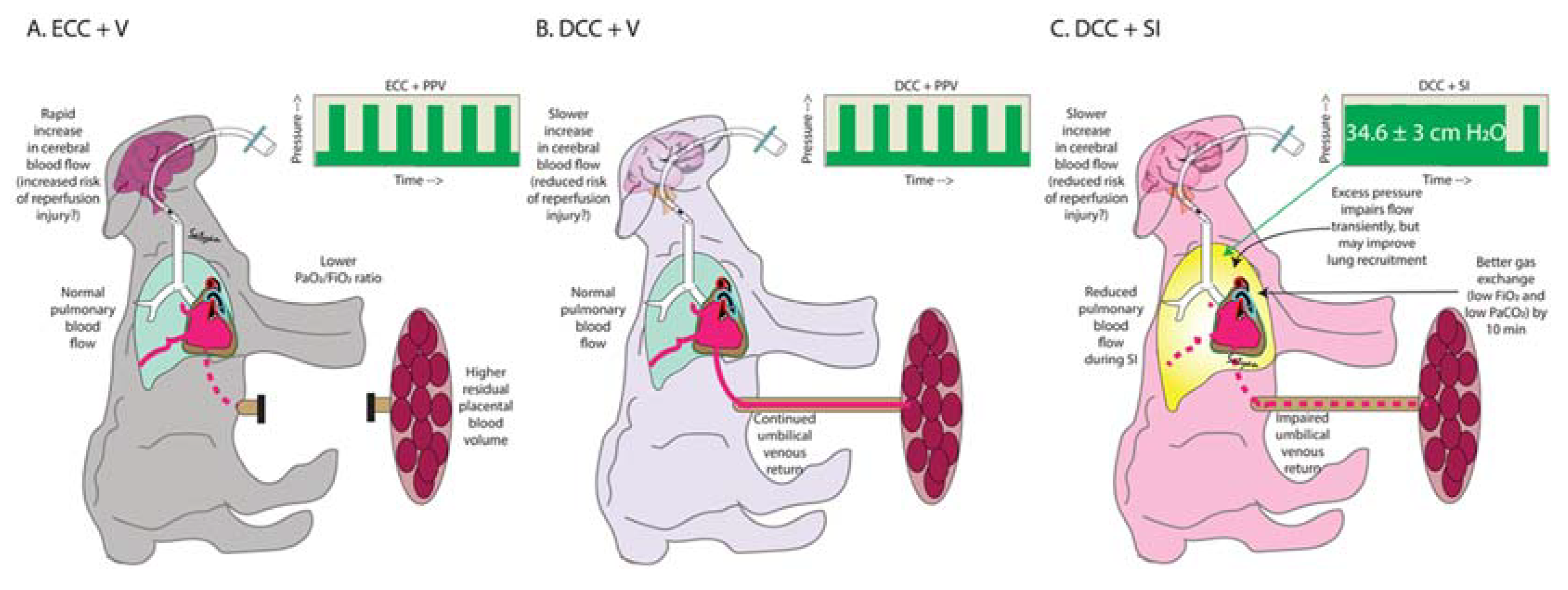
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nair, J.; Kumar, V.H.S. Current and emerging therapies in the management of hypoxic ischemic encephalopathy in neonates. Children 2018, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D. Reducing global neonatal mortality is possible. Neonatology 2011, 99, 250–257. [Google Scholar] [CrossRef]
- Vento, M.; Saugstad, O.D. Resuscitation of the term and preterm infant. Semin. Fet. Neonatal Med. 2010, 15, 216–222. [Google Scholar] [CrossRef]
- Ersdal, H.L.; Mduma, E.; Svensen, E.; Perlman, J.M. Early initiation of basic resuscitation interventions including face mask ventilation may reduce birth asphyxia related mortality in low-income countries: A prospective descriptive observational study. Resuscitation 2012, 83, 869–873. [Google Scholar] [CrossRef]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rudiger, M.; Skare, C.; Szczapa, T.; Te Pas, A.; Trevisanuto, D.; et al. European resuscitation council guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef]
- Klingenberg, C.; Sobotka, K.S.; Ong, T.; Allison, B.J.; Schmolzer, G.M.; Moss, T.J.; Polglase, G.R.; Dawson, J.A.; Davis, P.G.; Hooper, S.B. Effect of sustained inflation duration; resuscitation of near-term asphyxiated lambs. Arch. Dis. Child. Fetal. Neonatal. Ed. 2013, 98, F222–F227. [Google Scholar] [CrossRef]
- Schmolzer, G.M.; O’Reilly, M.; Labossiere, J.; Lee, T.F.; Cowan, S.; Qin, S.; Bigam, D.L.; Cheung, P.Y. Cardiopulmonary resuscitation with chest compressions during sustained inflations: A new technique of neonatal resuscitation that improves recovery and survival in a neonatal porcine model. Circulation 2013, 128, 2495–2503. [Google Scholar] [CrossRef]
- Vali, P.; Chandrasekharan, P.; Rawat, M.; Gugino, S.; Koenigsknecht, C.; Helman, J.; Mathew, B.; Berkelhamer, S.; Nair, J.; Lakshminrusimha, S. Continuous chest compressions during sustained inflations in a perinatal asphyxial cardiac arrest lamb model. Pediatr. Crit. Care Med. 2017, 18, e370–e377. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Alison, B.J.; Wallace, E.M.; Crossley, K.J.; Gill, A.W.; Kluckow, M.; te Pas, A.B.; Morley, C.J.; Polglase, G.R.; Hooper, S.B. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J. Physiol. 2013, 591, 2113–2126. [Google Scholar] [CrossRef]
- Vali, P.; Gugino, S.; Koenigsknecht, C.; Helman, J.; Chandrasekharan, P.; Rawat, M.; Lakshminrusimha, S.; Nair, J. The perinatal asphyxiated lamb model: A model for newborn resuscitation. J. Vis. Exp. 2018, 138, 57553. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.; Lee, H.C.; Escobedo, M.B.; Hoover, A.V.; Kamath-Rayne, B.D.; Kapadia, V.S.; Magid, D.J.; Niermeyer, S.; Schmolzer, G.M.; Szyld, E.; et al. Part 5: Neonatal resuscitation: 2020 american heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020, 142, S524–S550. [Google Scholar] [CrossRef] [PubMed]
- Hudson, I.R.; Cavill, I.A.; Cooke, A.; Holland, B.M.; Hoy, T.G.; Trevett, D.; Turner, T.L.; Wardrop, C.A. Biotin labeling of red cells in the measurement of red cell volume in preterm infants. Pediatr. Res. 1990, 28, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Polglase, G.R.; Blank, D.A.; Barton, S.K.; Miller, S.L.; Stojanovska, V.; Kluckow, M.; Gill, A.W.; LaRosa, D.; Te Pas, A.B.; Hooper, S.B. Physiologically based cord clamping stabilises cardiac output and reduces cerebrovascular injury in asphyxiated near-term lambs. Arch. Dis. Child. Fetal. Neonatal. Ed. 2018, 103, F530–F538. [Google Scholar] [CrossRef] [PubMed]
- Andersson, O.; Rana, N.; Ewald, U.; Malqvist, M.; Stripple, G.; Basnet, O.; Subedi, K.; Kc, A. Intact cord resuscitation versus early cord clamping in the treatment of depressed newborn infants during the first 10 min of birth (nepcord III)—A randomized clinical trial. Matern. Health Neonatol. Perinatol. 2019, 5, 15. [Google Scholar] [CrossRef]
- Vyas, H.; Milner, A.D.; Hopkin, I.E.; Boon, A.W. Physiologic responses to prolonged and slow-rise inflation in the resuscitation of the asphyxiated newborn infant. J. Pediatr. 1981, 99, 635–639. [Google Scholar] [CrossRef]
- Lista, G.; Boni, L.; Scopesi, F.; Mosca, F.; Trevisanuto, D.; Messner, H.; Vento, G.; Magaldi, R.; Del Vecchio, A.; Agosti, M.; et al. Sustained lung inflation at birth for preterm infants: A randomized clinical trial. Pediatrics 2015, 135, e457–e464. [Google Scholar] [CrossRef]
- Kirpalani, H.; Ratcliffe, S.J.; Keszler, M.; Davis, P.G.; Foglia, E.E.; Te Pas, A.; Fernando, M.; Chaudhary, A.; Localio, R.; van Kaam, A.H.; et al. Effect of sustained inflations vs intermittent positive pressure ventilation on bronchopulmonary dysplasia or death among extremely preterm infants: The sail randomized clinical trial. JAMA 2019, 321, 1165–1175. [Google Scholar] [CrossRef]
- Brouwer, E.; Te Pas, A.B.; Polglase, G.R.; McGillick, E.V.; Bohringer, S.; Crossley, K.J.; Rodgers, K.; Blank, D.; Yamaoka, S.; Gill, A.W.; et al. Effect of spontaneous breathing on umbilical venous blood flow and placental transfusion during delayed cord clamping in preterm lambs. Arch. Dis. Child. Fetal. Neonatal. Ed. 2020, 105, 26–32. [Google Scholar] [CrossRef]
- Feihl, F.; Eckert, P.; Brimioulle, S.; Jacobs, O.; Schaller, M.D.; Melot, C.; Naeije, R. Permissive hypercapnia impairs pulmonary gas exchange in the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2000, 162, 209–215. [Google Scholar] [CrossRef]
- Polglase, G.R.; Schmolzer, G.M.; Roberts, C.T.; Blank, D.A.; Badurdeen, S.; Crossley, K.J.; Miller, S.L.; Stojanovska, V.; Galinsky, R.; Kluckow, M.; et al. Cardiopulmonary resuscitation of asystolic newborn lambs prior to umbilical cord clamping; the timing of cord clamping matters! Front. Physiol. 2020, 11, 902. [Google Scholar] [CrossRef]
- Katheria, A.C.; Rich, W.D.; Bava, S.; Lakshminrusimha, S. Placental transfusion for asphyxiated infants. Front Pediatr. 2019, 7, 473. [Google Scholar] [CrossRef] [PubMed]
- Linderkamp, O. Placental transfusion: Determinants and effects. Clin. Perinatol. 1982, 9, 559–592. [Google Scholar] [CrossRef]
- Katheria, A.C.; Lakshminrusimha, S.; Rabe, H.; McAdams, R.; Mercer, J.S. Placental transfusion: A review. J. Perinatol. 2017, 37, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Creasy, R.K.; Drost, M.; Green, M.V.; Morris, J.A. Effect of ventilation on transfer of blood from placenta to neonate. Am. J. Physiol. 1972, 222, 186–188. [Google Scholar] [CrossRef][Green Version]


| ECC + V (n = 5) | ECC + SI (n = 6) | DCC (n = 5) | DCC + V (n = 5) | DCC + SI (n = 7) | |
|---|---|---|---|---|---|
| Weight (kg) | 4.1 ± 0.8 | 4.3 ± 1 | 3.7 ± 0.8 | 3.6 ± 0.4 | 3.7 ± 0.4 |
| Male | 2 | 2 | 3 | 3 | 5 |
| Multiple gestation | 3 | 3 | 3 | 4 | 4 |
| Incidence of arrest (epinephrine doses) | 2 (1) | 3 (2) | 2 (2) | 2 (2) | 3 (1) |
| Baseline hemodynamics | |||||
| Left carotid blood flow (mL/kg/min) | 28 ± 14 | 33 ± 10 | 24 ± 11 | 26 ± 11 | 33 ± 9 |
| Mean systemic blood pressure (mmHg) | 49 | 45 | 43 | 41 | 49 |
| Mean umbilical venous blood flow (mL/kg/min) | 29.8 ± 20 | 28.4 ± 17 | 34.2 ± 14.3 | 34.7 ± 11.1 | 49.2 ± 12.7 |
| Mean umbilical arterial blood flow (mL/kg/min) | 34.3 ± 29.6 | 22 ± 9 | 32.6 ± 9.8 | 31.4 ± 11.4 | 40.1 ± 18.5 |
| Asphyxia blood gas | |||||
| pH | 6.86 ± 0.1 | 6.88 ± 0.1 | 6.89 ± 0.0 | 6.88 ± 0.1 | 6.88 ± 0.1 |
| pCO2 (mm Hg) | 111 ± 19 | 121 ± 11 | 123 ± 4 | 112 ± 34 | 119 ± 25 |
| Lactate (mmol/L) | 8.5 ± 2.5 | 9.0 ± 2.2 | 8.9 ± 2.3 | 9.8 ± 2.1 | 12.4 ± 2 |
| ECC + V (n = 5) | ECC + SI (n = 6) | DCC (n = 5) | DCC + V (n = 5) | DCC + SI (n = 7) | |
|---|---|---|---|---|---|
| Primary Outcomes | |||||
| Time to reach baseline CBF (s) | 115 ± 124 | 190 ± 83 | 295 ± 180 | 246 ± 246 | 207 ± 119 |
| Time to HR > 100 bpm (s) | 149 ± 101 | 151 ± 116 | 247 ± 161 | 243 ± 212 | 173 ± 126 |
| Time to mean BP > 40 mmHg (s) | 204 ± 189 | 217 ± 78 | 355 ± 181 | 281 ± 224 | 192 ± 107 |
| During intervention | |||||
| Airway pressure (cmH2O) | 19.5 ± 5 | 37.7 ± 3 | n/a | 21.8 ± 6 | 34.6 ± 3 ** |
| CBF (mean ± SD) mL/kg/min | 12.1 ± 11.6 | 8.1 ± 10.8 | 4.9 ± 3.7 | 4.9 ± 2.7 | 6.7 ± 8.2 |
| PBF (mean ± SD) mL/kg/min | 2.1 ± 4.1 | 5.0 ± 6.7 | 3.6 ± 4.4 | 6.6 ± 6.4 | 1.3 ± 3.1 * |
| UV flow (mean ± SD) mL/kg/min | n/a | n/a | 4.5 ± 3.4 | 2.9 ± 2.2 | 1.6 ± 3.6 * |
| UA flow (mean ± SD) mL/kg/min | n/a | n/a | 3.1 ± 2.5 | 0.7 ± 1.6 | 1.3 ± 3.4 |
| Mean BP (mean ± SD) mmHg | 25 ± 13 | 21 ± 4 | 25 ± 6 | 21 ± 7 | 20 ± 9 |
| ECC + V (n = 5) | ECC + SI (n = 6) | DCC (n = 5) | DCC + V (n = 5) | DCC + SI (n = 6) | |
|---|---|---|---|---|---|
| Blood gas parameters at 2 min | |||||
| pH | 6.9 ± 0.1 | 6.9 ± 0.1 | 6.9 ± 0.1 | 7.0 ± 0.2 | 6.9 ± 0.1 |
| pCO2 (mmHg) | 107 ± 16 | 110 ± 14 | 130 ± 24 | 87 ± 24 | 92 ± 26 |
| pO2 (mmHg) | 29 ± 24 | 27 ± 6 | 22 ± 14 | 29 ± 11 | 20 ± 8 |
| FiO2 | 0.5 ± 0.4 | 0.8 ± 0.3 | 0.5 ± 0.3 | 0.5 ± 0.4 | 0.8 ± 0.4 |
| P/F ratio | 106 ± 133 | 41 ± 24 | 51 ± 38 | 79 ± 65 | 39 ± 38 |
| Lactate (mmol/L) | 8.3 ± 1.8 | 8.7 ± 1.5 | 10.2 ± 2.4 | 8.9 ± 1.5 | 11.7 ± 2.5 |
| Blood gas parameters at 5 min | |||||
| pH | 6.9 ± 0.2 | 6.9 ± 0.1 | 6.9 ± 0.1 | 6.9 ± 0.2 | 7 ± 0.2 |
| pCO2 (mmHg) | 102 ± 27 | 95 ± 11 | 99 ± 39 | 90 ± 42 | 66 ± 36 |
| pO2 (mmHg) | 61 ± 51 | 85 ± 50 | 37 ± 31 | 46 ± 30 | 45 ± 17 |
| FiO2 | 0.6 ± 0.4 | 0.7 ± 0.3 | 0.6 ± 0.3 | 0.4 ± 0.2 | 0.5 ± 0.4 |
| P/F ratio | 126 ± 132 | 138 ± 60 | 93 ± 106 | 149 ± 110 | 127 ± 113 |
| Lactate (mmol/L) | 8.9 ± 2.3 | 8.8 ± 1.9 | 9.4 ± 3.4 | 9.8 ± 3.8 | 11.2 ± 3 |
| Blood gas parameters at 10 min | |||||
| pH | 7.0 ± 0.2 | 6.9 ± 0.1 | 6.9 ± 0.1 | 6.9 ± 0.2 | 7.0 ± 0.1 |
| pCO2 (mmHg) | 81 ± 15 | 82 ± 27 | 83 ± 39 | 73 ± 34 | 44 ± 15 * |
| pO2 (mmHg) | 82 ± 36 | 106 ± 68 | 52 ± 30 | 76 ± 49 | 134 ± 80 |
| FiO2 | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.8 ± 0.4 | 0.7 ± 0.4 | 0.3 ± 0.1 |
| P/F ratio | 180 ± 72 | 227 ± 165 | 98 ± 83 | 165 ± 156 | 400 ± 151 *# |
| Lactate (mmol/L) | 8.5 ± 2.6 | 8 ± 2.1 | 10 ± 3.7 | 9.1 ± 2.5 | 11.8 ± 3.3 |
| A. All Lambs | ECC (n = 9) | DCC (n = 10) |
| Fetoplacental blood volume (mL/kg) | 82 ± 20 | 76 ± 18 |
| Newborn blood volume (mL/kg) | 61 ± 14 | 60 ± 17 |
| Residual placental blood volume (mL/kg) | 21 ± 15 | 16 ± 13 |
| Fraction of fetoplacental volume in newborn | 0.76 ± 0.1 | 0.8 ± 0.2 |
| B. Non-arrested | ECC (n = 5) | DCC (n = 7) |
| Fetoplacental blood volume (mL/kg) | 92 ± 18 | 74 ± 19 |
| Newborn blood volume (mL/kg) | 58 ± 13 | 58 ± 12 |
| Residual placental blood volume (mL/kg) | 35 ± 8 | 16 ± 14 * |
| Fraction of fetoplacental volume in newborn | 0.65 ± 0.1 | 0.84 ± 0.2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, J.; Davidson, L.; Gugino, S.; Koenigsknecht, C.; Helman, J.; Nielsen, L.; Sankaran, D.; Agrawal, V.; Chandrasekharan, P.; Rawat, M.; et al. Sustained Inflation Reduces Pulmonary Blood Flow during Resuscitation with an Intact Cord. Children 2021, 8, 353. https://doi.org/10.3390/children8050353
Nair J, Davidson L, Gugino S, Koenigsknecht C, Helman J, Nielsen L, Sankaran D, Agrawal V, Chandrasekharan P, Rawat M, et al. Sustained Inflation Reduces Pulmonary Blood Flow during Resuscitation with an Intact Cord. Children. 2021; 8(5):353. https://doi.org/10.3390/children8050353
Chicago/Turabian StyleNair, Jayasree, Lauren Davidson, Sylvia Gugino, Carmon Koenigsknecht, Justin Helman, Lori Nielsen, Deepika Sankaran, Vikash Agrawal, Praveen Chandrasekharan, Munmun Rawat, and et al. 2021. "Sustained Inflation Reduces Pulmonary Blood Flow during Resuscitation with an Intact Cord" Children 8, no. 5: 353. https://doi.org/10.3390/children8050353
APA StyleNair, J., Davidson, L., Gugino, S., Koenigsknecht, C., Helman, J., Nielsen, L., Sankaran, D., Agrawal, V., Chandrasekharan, P., Rawat, M., Berkelhamer, S. K., & Lakshminrusimha, S. (2021). Sustained Inflation Reduces Pulmonary Blood Flow during Resuscitation with an Intact Cord. Children, 8(5), 353. https://doi.org/10.3390/children8050353






