Short-Term and Long-Term Effectiveness of Intensive Interdisciplinary Pain Treatment for Children and Adolescents with Chronic Headache: A Longitudinal Observation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Sample
2.3. Procedure
2.4. Intensive Interdisciplinary Pain Treatment (IIPT)
2.5. Measures
2.5.1. Average Pain Intensity
2.5.2. Pain-Related School Absence
2.5.3. Pain-Related Disability
2.5.4. General Anxiety
2.5.5. Depression
2.6. Ethics
2.7. Data Analysis
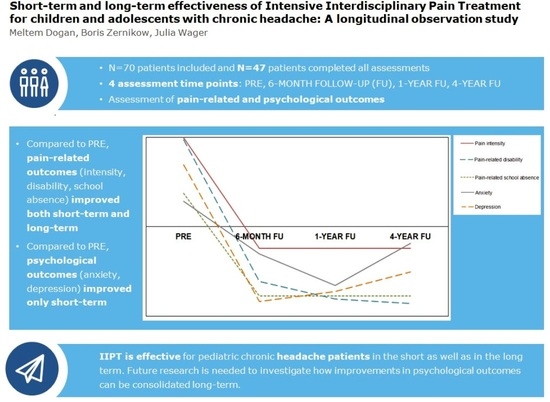
3. Results
3.1. Demographic and Clinical Characteristics
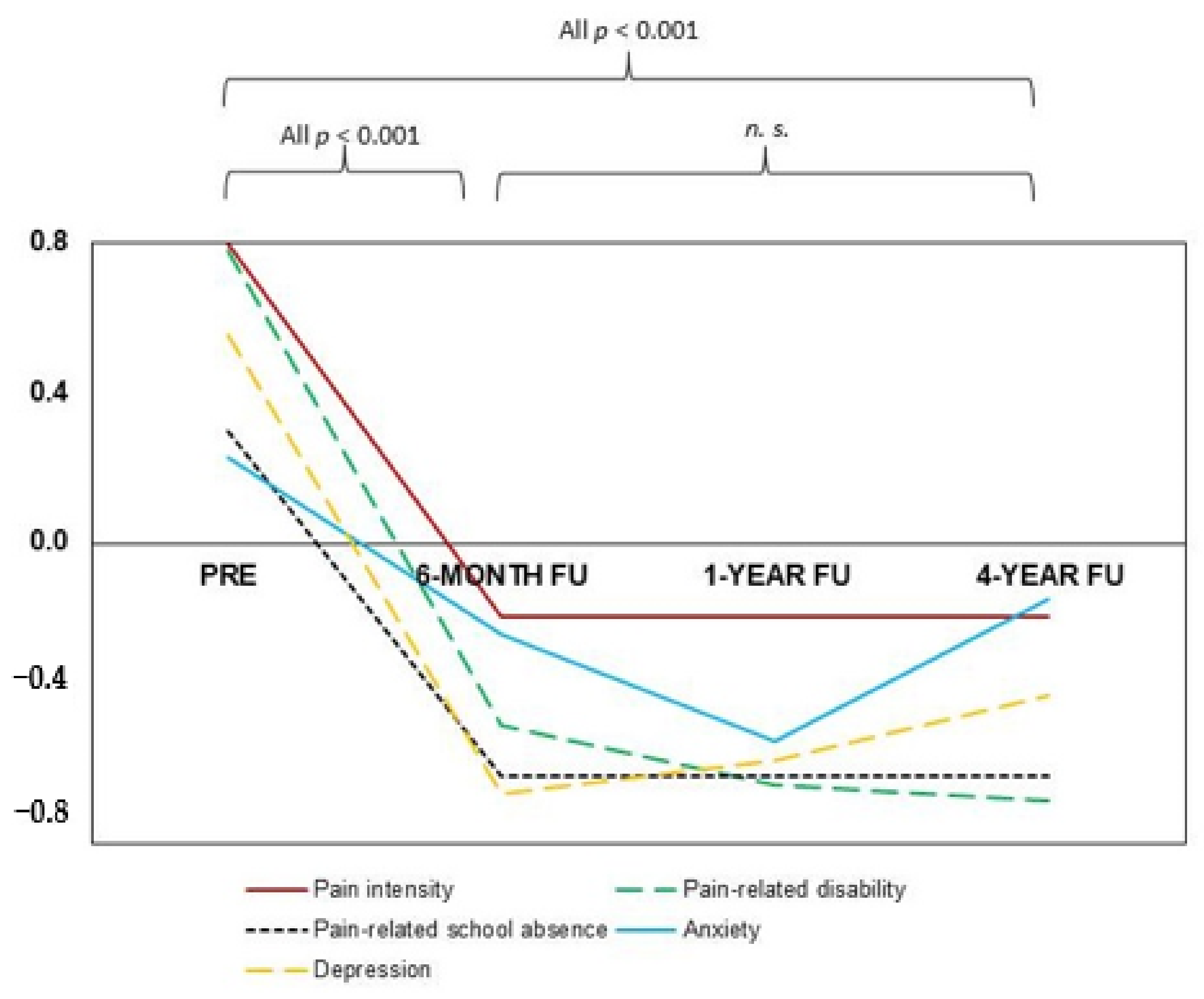
3.2. Short-Term and Long-Term Effectiveness of IIPT
3.2.1. Pain-Related Outcome Parameters
3.2.2. Psychological Outcome Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huguet, A.; Miró, J. The severity of chronic pediatric pain: An epidemiological study. J. Pain 2008, 9, 226–236. [Google Scholar] [CrossRef]
- Könning, A.; Rosenthal, N.; Brown, D.; Stahlschmidt, L.; Wager, J. Severity of chronic pain in german adolescent school students: A cross-sectional study. Clin. J. Pain 2020, 37, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zernikow, B.; Wager, J.; Hechler, T.; Hasan, C.; Rohr, U.; Dobe, M.; Meyer, A.; Hübner-Möhler, B.; Wamsler, C.; Blankenburg, M. Characteristics of highly impaired children with severe chronic pain: A 5-year retrospective study on 2249 pediatric pain patients. BMC Pediatr. 2012, 16, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.K.; Goldschneider, K.R.; Kashikar-Zuck, S.; Kotagal, U.; Tessman, C.; Jones, B. Healthcare utilization and indirect burden among families of pediatric patients with chronic pain. J. Musculoskelet. Pain 2008, 16, 155–164. [Google Scholar] [CrossRef]
- Pakalnis, A.; Butz, C.; Splaingard, D.; Kring, D.; Fong, J. Emotional problems and prevalence of medication overuse in pediatric chronic daily headache. J. Child Neurol. 2007, 22, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Hechler, T.; Kanstrup, M.; Holley, A.L.; Simons, L.E.; Wicksell, R.; Hirschfeld, G.; Zernikow, B. Systematic review on intensive interdisciplinary pain treatment of children with chronic pain. Pediatrics 2015, 136, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Zernikow, B.; Ruhe, A.-K.; Stahlschmidt, L.; Schmidt, P.; Staratzke, T.; Frosch, M.; Wager, J. Clinical and economic long-term treatment outcome of children and adolescents with disabling chronic pain. Pain Med. 2018, 19, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Hechler, T.; Ruhe, A.; Schmidt, P.; Hirsch, J.; Wager, J.; Dobe, M.; Krummenauer, F.; Zernikow, B. Inpatient-based intensive interdisciplinary pain treatment for highly impaired children with severe chronic pain: Randomized controlled trial of efficacy and economic effects. Pain 2014, 155, 118–128. [Google Scholar] [CrossRef] [PubMed]
- IASP. Task Force on Multimodal Pain Treatment Defines Terms for Chronic Pain Care. Available online: https://www.iasp-pain.org/PublicationsNews/NewsDetail.aspx?ItemNumber=6981 (accessed on 1 February 2021).
- Stahlschmidt, L.; Zernikow, B.; Wager, J. Specialized rehabilitation programs for children and adolescents with severe disabling chronic pain: Indications, treatment and outcomes. Children 2016, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Benore, E.; Brenner, A.; Banez, G.A.; Wang, L.; Worley, S. It takes two: Parent functioning within the pediatric chronic pain experience and interdisciplinary rehabilitation treatment. Rehabil. Psychol. 2018, 63, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Shulman, J.; Conroy, C.; Cybulski, A.; Smith, K.R.; Jervis, K.; Johnson, H.; Zurakowski, D.; Sethna, N.F. Does intensive interdisciplinary pain treatment improve pediatric headache-related disability? Disabil. Rehabil. 2020, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hechler, T.; Dobe, M.; Kosfelder, J.; Damschen, U.; Hübner, B.; Blankenburg, M.; Sauer, C.; Zernikow, B. Effectiveness of a 3-week Multimodal Inpatient Pain Treatment for Adolescents Suffering From Chronic Pain. Clin. J. Pain 2009, 25, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Dobe, M.; Zernikow, B. Practical Treatment Options for Chronic Pain in Children and Adolescents: An Interdisciplinary Therapy Manual; Springer: Berlin/Heidelberg, Germany, 2019; Volume 2, pp. 1–299. [Google Scholar]
- Tsze, D.S.; von Baeyer, C.L.; Pahalyants, V.; Dayan, P.S. Validity and reliability of the verbal numerical rating scale for children aged 4 to 17 years with acute pain. Ann. Emerg. Med. 2018, 71, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Hübner, B.; Hechler, T.; Dobe, M.; Damschen, U.; Kosfelder, J.; Denecke, H.; Schroeder, S.; Zernikow, B. Pain-related disability in adolescents suffering from chronic pain: Preliminary examination of the pediatric pain disability index (p-pdi). Schmerz 2009, 23, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Stahlschmidt, L.; Friedrich, Y.; Zernikow, B.; Wager, J. Assessment of pain-related disability in pediatric chronic pain: A comparison of the functional disability inventory and the pediatric pain disability index. Clin. J. Pain 2018, 34, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Wieczerkowski, W.; Nickel, H.; Janowksi, A.; Fittkau, B.; Rauer, W. Anxiety Questionnaire for Pupils; Westermann: Göttingen, Germany, 1981; Volume 6. [Google Scholar]
- Stiensmeier-Pelster, J.; Schürmann, M.; Duda, K. Depression Inventory for Children and Adolescents; Hogrefe: Göttingen, Germany, 2000. [Google Scholar]
- Hurtubise, K.; Blais, S.; Noel, M.; Brousselle, A.; Dallaire, F.; Rasic, N.; Camden, C. Is it worth it? A comparison of an intensive interdisciplinary pain treatment and a multimodal treatment for youths with pain-related disability. Clin. J. Pain 2020, 36, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Thabrew, H.; Ruppeldt, P.; Sollers, J.J. Systematic review of biofeedback interventions for addressing anxiety and depression in children and adolescents with long-term physical conditions. Appl. Psychophysiol. Biofeedback 2018, 43, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Moessner, M.; Schiltenwolf, M.; Neubauer, E. Internet-based aftercare for patients with back pain—A pilot study. Telemed. e-Health 2012, 18, 413–419. [Google Scholar] [CrossRef]
- Orr, S.L.; Kabbouche, M.A.; O’Brien, H.L.; Kacperski, J.; Powers, S.W.; Hershey, A.D. Paediatric migraine: Evidence-based management and future directions. Nat. Rev. Neurol. 2018, 14, 515–527. [Google Scholar] [CrossRef]

| All | Participants 4-Year Follow-up | Dropouts 4-Year Follow-Up | p-Value | |
|---|---|---|---|---|
| n = 70 | n = 47 | n = 23 | ||
| Sex [n (%)] | 0.096 | |||
| Female | 54 (77.1%) | 39 (83%) | 15 (65.2%) | |
| Male | 16 (22.9%) | 8 (17%) | 8 (34.8%) | |
| Age [M (SD)] | 14.5 (2.1) | 14.5 (2.2) | 14.5 (2.0) | 0.914 |
| Pain onset a [M (SD)] | 32.3 (34.2) | 27.7 (31.0) | 41.6 (39.1) | 0.112 |
| Average pain intensity b [M (SD)] | 6.5 (2.1) | 6.7 (2.1) | 6.0 (2.0) | 0.149 |
| Missed school days c [M (SD)] | 8.2 (7.0) | 8.6 (7.0) | 7.6 (7.1) | 0.572 |
| Pain-related disability d [M (SD)] | 36.8 (9.1) | 37.5 (8.9) | 35.4 (9.5) | 0.364 |
| General anxiety f [M (SD)] | 53.2 (10.1) | 54.0 (9.0) | 51.5 (12.2) | 0.344 |
| Depression e [M (SD)] | 55.4 (9.9) | 55.7 (10.2) | 54.7 (9.4) | 0.702 |
| PRE | 6-Month Follow-Up | 1-Year Follow-Up | 4-Year Follow-Up | |
|---|---|---|---|---|
| n = 70 | n = 65 | n = 58 | n = 47 | |
| Pain characteristics | ||||
| Average pain intensity (last 7 days) a | 6.6 (2.2) | 4.1 (3.2) | 3.9 (3.0) | 3.4 (3.1) |
| Missed school days (last 4 weeks) | 8.2 (7.0) | 0.8 (2.1) | 1.1 (2.1) | 2.1 (3.8) |
| Pain-related disability b | 37.9 (8.6) | 22.3 (10.4) | 22.8 (12.2) | 22.8 (12.0) |
| Psychological characteristics | ||||
| General anxiety c | 55.1 (10.3) | 48.0 (11.2) | 49.3 (13.2) | 50.5 (6.6) |
| Depression d | 53.7 (8.4) | 45.3 (11.0) | 46.2 (13.0) | 48.2 (11.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogan, M.; Zernikow, B.; Wager, J. Short-Term and Long-Term Effectiveness of Intensive Interdisciplinary Pain Treatment for Children and Adolescents with Chronic Headache: A Longitudinal Observation Study. Children 2021, 8, 220. https://doi.org/10.3390/children8030220
Dogan M, Zernikow B, Wager J. Short-Term and Long-Term Effectiveness of Intensive Interdisciplinary Pain Treatment for Children and Adolescents with Chronic Headache: A Longitudinal Observation Study. Children. 2021; 8(3):220. https://doi.org/10.3390/children8030220
Chicago/Turabian StyleDogan, Meltem, Boris Zernikow, and Julia Wager. 2021. "Short-Term and Long-Term Effectiveness of Intensive Interdisciplinary Pain Treatment for Children and Adolescents with Chronic Headache: A Longitudinal Observation Study" Children 8, no. 3: 220. https://doi.org/10.3390/children8030220
APA StyleDogan, M., Zernikow, B., & Wager, J. (2021). Short-Term and Long-Term Effectiveness of Intensive Interdisciplinary Pain Treatment for Children and Adolescents with Chronic Headache: A Longitudinal Observation Study. Children, 8(3), 220. https://doi.org/10.3390/children8030220