Cost of Illness in Young Children: A Prospective Birth Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Illness
2.3. Parent Work Absenteeism
2.4. Estimation of Costs
2.5. Pregnancy Interventions
2.6. Environmental Risk Factors
2.7. Statistics
3. Results
3.1. Baseline Characteristics
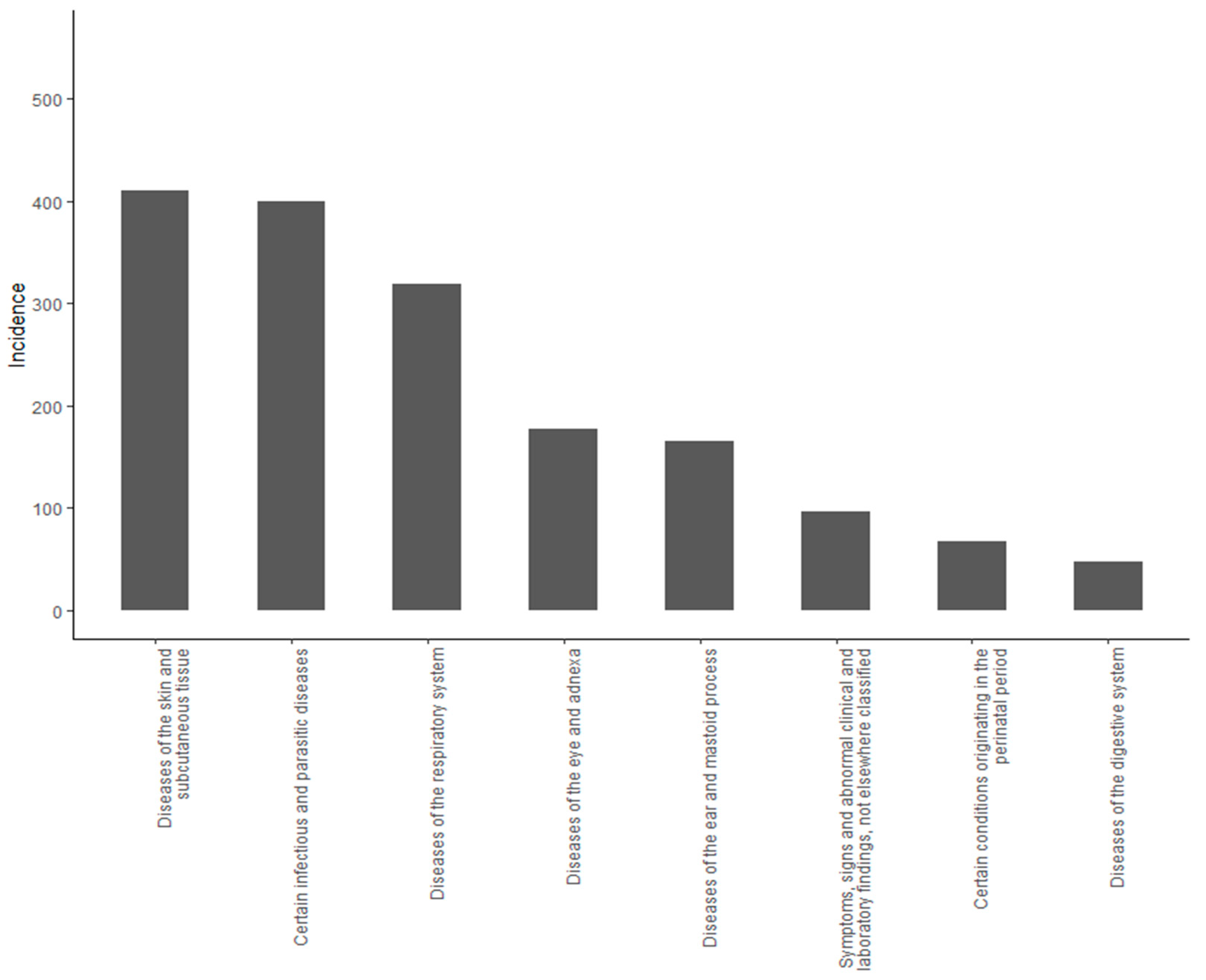
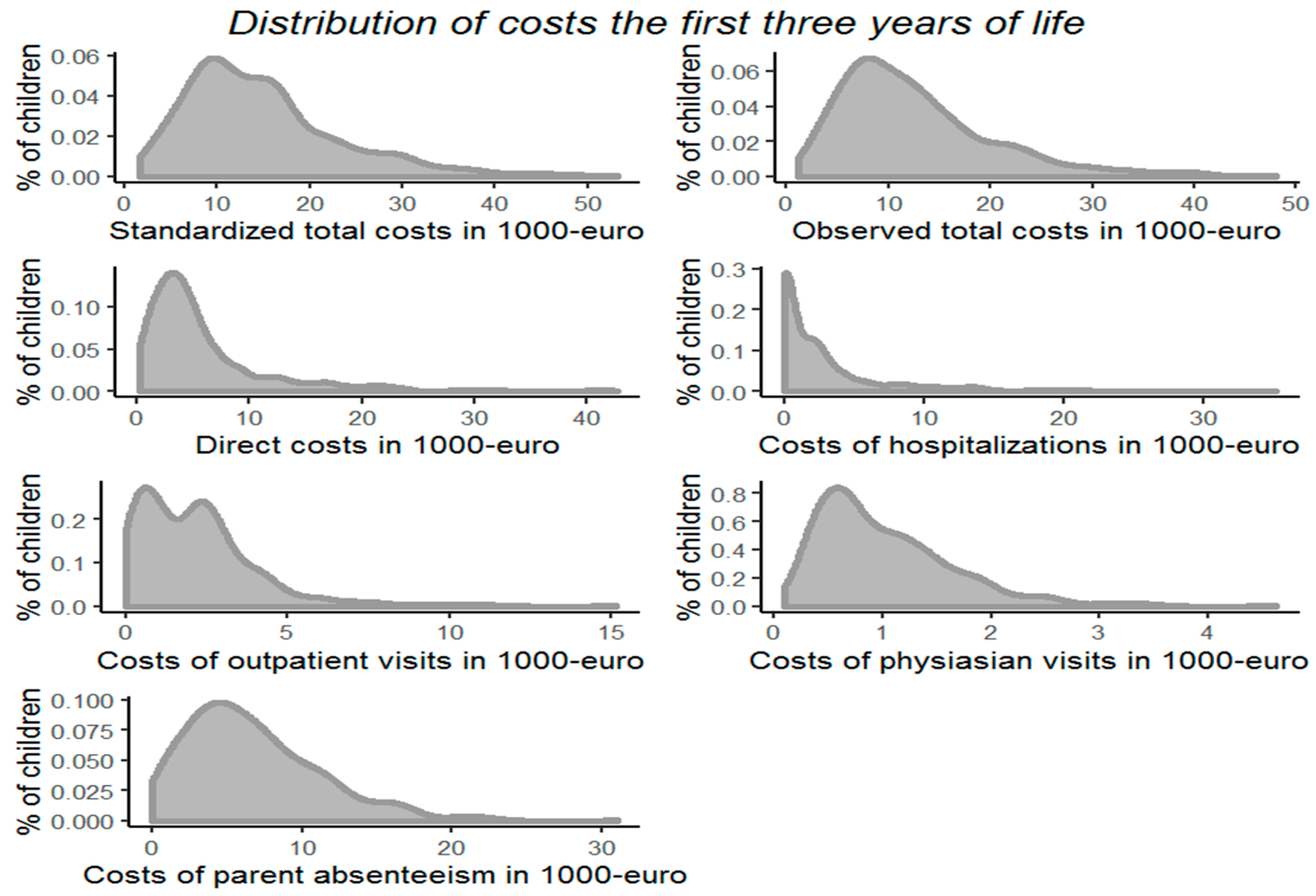
3.2. Costs of Illness
3.3. Pregnancy Nutritional Interventions
3.4. Environmental Risk Factors
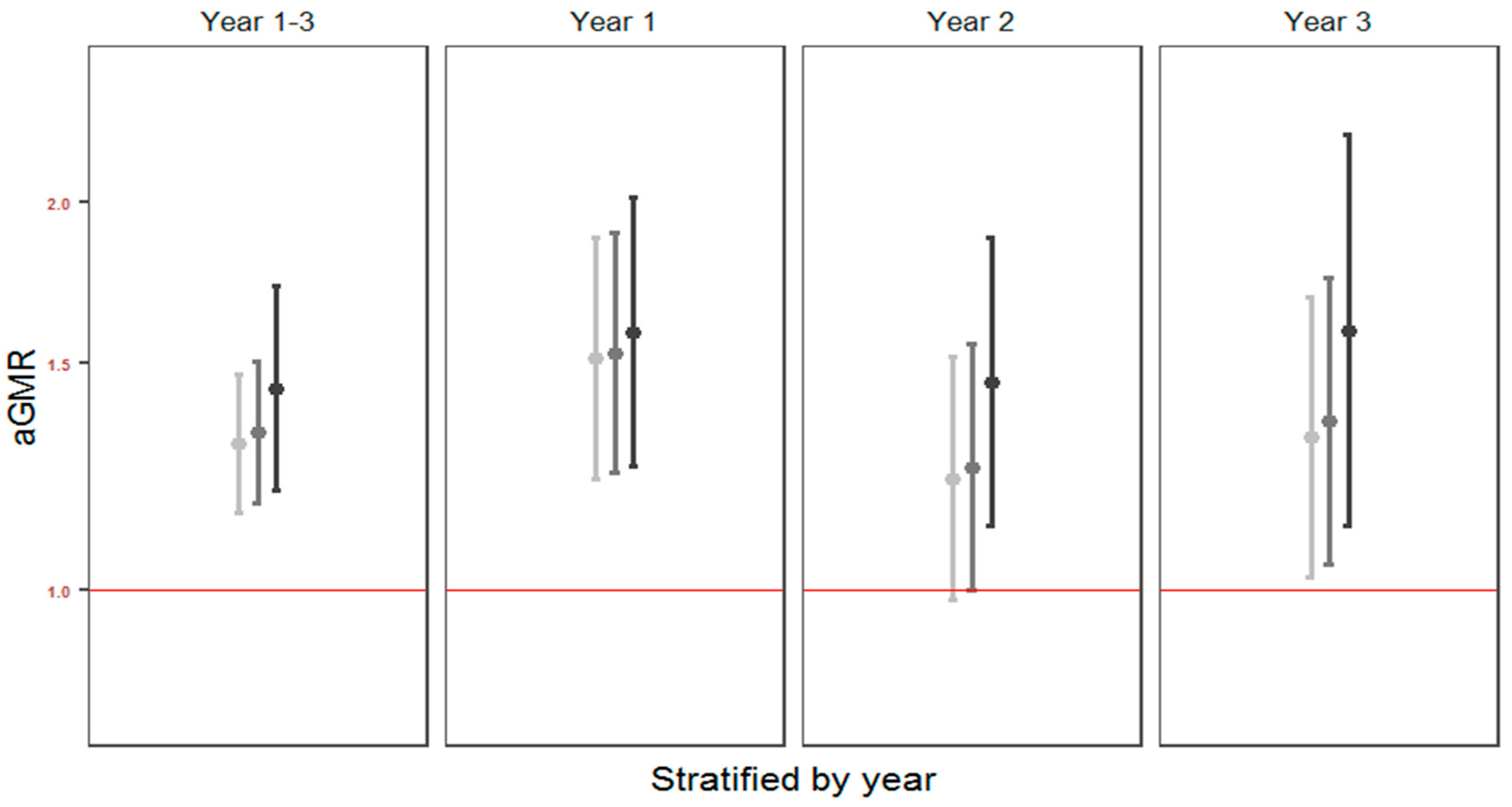
3.5. Cesarean Delivery
4. Discussion
4.1. Primary Findings
4.2. Strengths and Limitations
4.3. Interpretation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Vissing, N.H.; Chawes, B.L.; Rasmussen, M.A.; Bisgaard, H. Epidemiology and Risk Factors of Infection in Early Childhood. Pediatrics 2018, 141. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, P.; Zeramdini, R.; Carrin, G. Basic patterns in national health expenditure. Bull. World Health Organ. 2002, 80, 134–142. [Google Scholar]
- Danish Regions. Available online: https://www.regioner.dk/media/2209/2015-pres-paa-sundhedsvaesenet.pdf (accessed on 25 December 2020).
- Barnes, P.J.; Jonsson, B.; Klim, J.B. The costs of asthma. Eur. Respir. J. 1996, 9, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; Sullivan, S.D.; Smith, D.H.; Weiss, K.B. The economic burden of asthma in US children: Estimates from the National Medical Expenditure Survey. J. Allergy Clin. Immunol. 1999, 104, 957–963. [Google Scholar] [CrossRef]
- Ungar, W.J.; Coyte, P.C.; Pharmacy Medication Monitoring Program Advisory Board. Prospective study of the patient-level cost of asthma care in children. Pediatr. Pulmonol. 2001, 32, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.B.; Allen, K.M.; Carter, R.C.; Nolan, T.M. The cost of community-managed viral respiratory illnesses in a cohort of healthy preschool-aged children. Respir. Res. 2008, 9, 11. [Google Scholar] [CrossRef]
- Sevelsted, A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Cesarean section and chronic immune disorders. Pediatrics 2015, 135, e92–e98. [Google Scholar] [CrossRef]
- Bisgaard, H.; Bønnelykke, K. Fish Oil in Pregnancy and Asthma in Offspring. N. Engl. J. Med. 2017, 376, 1191–1192. [Google Scholar]
- Chawes, B.L.; Bønnelykke, K.; Stokholm, J.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA 2016, 315, 353–361. [Google Scholar] [CrossRef]
- Eder, W.; Ege, M.J.; von Mutius, E. The asthma epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Vissing, N.H.; Carson, C.G.; Bischoff, A.L.; Følsgaard, N.V.; Kreiner-Møller, E.; Chawes, B.L.; Stokholm, J.; Pedersen, L.; Bjarnadóttir, E.; et al. Deep phenotyping of the unselected COPSAC2010 birth cohort study. Clin. Exp. Allergy 2013, 43, 1384–1394. [Google Scholar] [CrossRef]
- Kildemoes, H.W.; Sørensen, H.T.; Hallas, J. The Danish National Prescription Registry. Scand. J. Public Health 2011, 39, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.S.; Olivarius ND, F.; Krasnik, A. The Danish National Health Service Register. Scand. J. Public Health 2011, 39, 34–37. [Google Scholar] [CrossRef]
- Lynge, E.; Sandegaard, J.L.; Rebolj, M. The Danish National Patient Register. Scand. J. Public Health 2011, 39, 30–33. [Google Scholar] [CrossRef] [PubMed]
- (No title). Available online: https://www.statistikbanken.dk/statbank5a/selectvarval/saveselections.asp (accessed on 23 October 2019).
- Wald, E.R.; Guerra, N.; Byers, C. Frequency and severity of infections in day care: Three-year follow-up. J. Pediatr. 1991, 118, 509–514. [Google Scholar] [CrossRef]
- Caudri, D.; Wijga, A.; Scholtens, S.; Kerkhof, M.; Gerritsen, J.; Ruskamp, J.; Brunekreef, B.; Smit, H.A.; de Jongste, J.C. Early daycare is associated with an increase in airway symptoms in early childhood but is no protection against asthma or atopy at 8 years. Am. J. Respir. Crit. Care Med. 2009, 180, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Nafstad, P.; Hagen, J.A.; Oie, L.; Magnus, P.; Jaakkola, J.J. Day care centers and respiratory health. Pediatrics 1999, 103, 753–758. [Google Scholar] [CrossRef]
- Nafstad, P.; Jaakkola, J.J.; Hagen, J.A.; Botten, G.; Kongerud, J. Breastfeeding, maternal smoking and lower respiratory tract infections. Eur. Respir. J. 1996, 9, 2623–2629. [Google Scholar] [CrossRef]
- Zutavern, A.; Rzehak, P.; Brockow, I.; Schaaf, B.; Bollrath, C.; von Berg, A.; Link, E.; Kraemer, U.; Borte, M.; Herbarth, O.; et al. Day care in relation to respiratory-tract and gastrointestinal infections in a German birth cohort study. Acta Paediatr. 2007, 96, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Cushing, A.H.; Samet, J.M.; Lambert, W.E.; Skipper, B.J.; Hunt, W.C.; Young, S.A.; McLaren, L.C. Breastfeeding reduces risk of respiratory illness in infants. Am. J. Epidemiol. 1998, 147, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.J.; Leeder, S.R.; Corkhill, R.T. The relationship between breast and bottle feeding and respiratory illness in the first year of life. J. Epidemiol. Community Health 1979, 33, 180–182. [Google Scholar] [CrossRef]
- Jones, L.L.; Hashim, A.; McKeever, T.; Cook, D.G.; Britton, J.; Leonardi-Bee, J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: Systematic review and meta-analysis. Respir. Res. 2011, 12, 5. [Google Scholar] [CrossRef]
- Bergroth, E.; Remes, S.; Pekkanen, J.; Kauppila, T.; Büchele, G.; Keski-Nisula, L. Respiratory tract illnesses during the first year of life: Effect of dog and cat contacts. Pediatrics 2012, 130, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N. Engl. J. Med. 2016, 375, 2530–2539. [Google Scholar] [CrossRef]
- World Health Organization. The International Statistical Classification of Diseases and Related Health Problems; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Svanes, C.; Jarvis, D.; Chinn, S.; Burney, P. Childhood environment and adult atopy: Results from the European Community Respiratory Health Survey. J. Allergy Clin. Immunol. 1999, 103, 415–420. [Google Scholar] [CrossRef]
- Backman, H.; Räisänen, P.; Hedman, L.; Stridsman, C.; Andersson, M.; Lindberg, A.; Lundbäck, B.; Rönmark, E. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016-results from three population surveys. Clin. Exp. Allergy 2017, 47, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Stensballe, L.G.; Sørup, S.; Aaby, P.; Benn, C.S.; Greisen, G.; Jeppesen, D.L.; Birk, N.M.; Kjærgaard, J.; Nissen, T.N.; Pihl, G.T.; et al. BCG vaccination at birth and early childhood hospitalisation: A randomised clinical multicentre trial. Arch. Dis. Child. 2017, 102, 224–231. [Google Scholar] [CrossRef]
- Merenstein, D.J.; Gatti, M.E.; Mays, D.M. The association of mode of delivery and common childhood illnesses. Clin. Pediatr. 2011, 50, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Baumfeld, Y.; Walfisch, A.; Wainstock, T.; Segal, I.; Sergienko, R.; Landau, D.; Sheiner, E. Elective cesarean delivery at term and the long-term risk for respiratory morbidity of the offspring. Eur. J. Pediatr. 2018, 177, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Betrán, A.P.; Ye, J.; Moller, A.B.; Zhang, J.; Gülmezoglu, A.M.; Torloni, M.R. The Increasing Trend in Caesarean Section Rates: Global, Regional and National Estimates: 1990-2014. PLoS ONE 2016, 11, e0148343. [Google Scholar] [CrossRef] [PubMed]
- Boatin, A.A.; Schlotheuber, A.; Betran, A.P.; Moller, A.B.; Barros, A.J.D.; Boerma, T.; Torloni, M.R.; Victora, C.G.; Hosseinpoor, A.R. Within country inequalities in caesarean section rates: Observational study of 72 low and middle income countries. BMJ 2018, 360, k55. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Statement on Caesarean Section Rates. Available online: https://apps.who.int/iris/bitstream/handle/10665/161442/WHO_RHR_15.02_eng.pdf;jsessionid=19EBBAB2A1E190C16B278564F1B81362?sequence=1 (accessed on 25 December 2020).
- Khan, K.A.; Petrou, S.; Dritsaki, M.; Johnson, S.J.; Manktelow, B.; Draper, E.S.; Smith, L.K.; Seaton, S.E.; Marlow, N.; Dorling, J.; et al. Economic Costs Associated with Moderate and Late Preterm Birth: A Prospective Population-Based Study. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1495–1505. [Google Scholar] [CrossRef]
- Frey, H.A.; Klebanoff, M.A. The Epidemiology, Etiology, and Costs of Preterm Birth. Semin. Fetal. Neonatal Med. 2016, 21, 68–73. [Google Scholar] [CrossRef] [PubMed]
| Study Population | |
|---|---|
| Fish-oil supplement, n (%) | 282 (50.5) |
| High-dose vitamin D supplement, n (%) | 230 (41.1) |
| Maternal smoking, n (%) | 16 (2.9) |
| Maternal age (years), mean ± sd | 32.30 ± 4.12 |
| Maternal atopic disease, n (%) | 312 (55.9) |
| Maternal prepregnancy BMI, mean ± sd | 24.56 ± 4.43 |
| Preeclampsia, n (%) | 29 (5.2) |
| Cesarean delivery, n (%) | 110 (19.7) |
| Male, n (%) | 282 (50.4) |
| Premature, n (%) | 21 (3.8) |
| GA (weeks), mean ± sd | 39.87 ± 1.71 |
| Birth weight (100g), mean ± sd | 35.26 ± 5.52 |
| Season of birth, n (%) | |
| Summer | 114 (20.4) |
| Autumn | 120 (21.5) |
| Winter | 171 (30.6) |
| Spring | 154 (27.5) |
| Older siblings, n (%) | 313 (56.0) |
| Cat at birth, n (%) | 111 (19.9) |
| Dog at birth, n (%) | 97 (17.4) |
| Breastfed (months), mean ± sd | 3.42 ± 1.93 |
| Social circumstances, mean ± sd | 0.02 ± 0.95 |
| Day care (years), mean ± sd | 0.90 ± 0.24 |
| Type of daycare (nursery), n (%) | 352 (66.0) |
| Standardized Total Costs | Observed Total Costs | Direct Costs | Days Home from Daycare | |||||
|---|---|---|---|---|---|---|---|---|
| Environmental Factor | aGMR (95% CI) | Interpretation | aGMR (95% CI) | Interpretation | aGMR (95% CI) | Interpretation | aOR (95% CI) | Interpretation |
| (p-Value) | (Euro) | (p-Value) | (Euro) | (p-Value) | (Euro) | (p-Value) | (Days) | |
| High-dose vitamin D suppl. | 1.05 (0.95; 1.17) 0.32 | 708 (−660; 2223) | 1.07 (0.96; 1.19) 0.20 | 733 (−378; 1966) | 1.04 (0.89; 1.21) 0.62 | 158 (−439; 853) | 0.99 (0.88; 1.12) 0.90 | −0.2 (−3.0; 3.0) |
| Maternal smoking | 1.19 (0.88; 1.61) 0.25 | 2602 (−1555;8210) | 1.12 (0.83; 1.53) 0.46 | 1304 (−1819; 5557) | 1.60 (1.02; 2.5) 0.039 | 2450 (101; 6117) | 1.10 (0.79; 1.57) 0.60 | 2.05 (−5.5; 14.8) |
| Maternal age | 1.00 (0.99; 1.02) 0.43 | 66 (−97; 231) | 1.02 (1.01; 1.03) 0.004 | 195 (64; 329) | 1.02 (1.00; 1.03) 0.08 | 66 (−8; 142) | 1.00 (0.98; 1.01) 0.78 | −0.1 (−0.4; 0.3) |
| Maternal atopic disease | 1.10 (1.00; 1.22) 0.06 | 1390 (−31; 2962) | 1.07 (0.97; 1.19) 0.19 | 758 (−347; 1984) | 1.22 (1.05; 1.42) 0.009 | 899 (206; 1703) | 1.05 (0.93; 1.18) 0.42 | 1.03 (−1.7; 4.6) |
| Maternal pre pregnancy BMI | 1.00 (0.99; 1.01) 0.60 | 41 (−111; 196) | 1.00 (0.99; 1.01) 0.85 | 11 (−110; 135) | 1.01 (1.00; 1.03) 0.13 | 53 (−16; 124) | 1.00 (0.99; 1.01) 0.87 | 0.0 (−0.4; 0.3) |
| Preeclampsia | 1.29 (1.03; 1.61) 0.03 | 3841 (360; 8199) | 1.25 (0.99; 1.58) 0.06 | 2618 (−85; 6025) | 1.12 (0.80; 1.57) 0.51 | 484 (−821; 2310) | 1.29 (1.01; 1.68) 0.05 | 7.05 (0.1; 17.6) |
| Cesarean delivery | 1.30 (1.15; 1.47) <0.0001 | 4061 (2021; 6372) | 1.33 (1.17; 1.51) <0.0001 | 3424 (1764; 5310) | 1.45 (1.20; 1.74) 0.0001 | 1822 (824; 3022) | 1.17 (1.02; 1.35) 0.029 | 4.05 (0.5; 9.2) |
| Male | 1.16 (1.05; 1.28) 0.004 | 2105 (631; 3733) | 1.18 (1.06; 1.30) 0.0019 | 1852 (655; 3178) | 1.17 (1.01; 1.36) 0.0371 | 703 (40; 1471) | 1.03 (0.92; 1.16) 0.58 | 0.09 (−2.0; 4.1) |
| GA (weeks) | 0.93 (0.91; 0.96) <0.0001 | −901 (−1259; −534) | 0.92 (0.90; 0.95) <0.0001 | −789 (−1071; −499) | 0.84 (0.81; 0.88) <0.0001 | −645 (−785; −499) | 1.03 (0.99; 1.06) 0.14 | 0.07 (−0.2; 1.6) |
| Premature | 2.00 (1.55; 2.59) <0.0001 | 13492 (7392; 21378) | 2.21 (1.70; 2.87) <0.0001 | 12606 (7278; 19536) | 4.08 (2.81; 5.94) <0.0001 | 12600 (7391; 20175) | 0.86 (0.64; 1.17) 0.31 | −3.7 (−9.4; 4.5) |
| Birth weight (100g) | 0.98 (0.97; 0.99) 0.0003 | −225 (−343; −105) | 0.98 (0.97; 0.99) 0.0002 | −186 (−281; −90) | 0.96 (0.95; 0.98) <0.0001 | −144 (−196; −92) | 1.00 (0.99; 1.01) 0.69 | −0.1 (−0.3; 0.2) |
| Season of birth (ref: summer) | ||||||||
| Autumn | 1.07 (0.92; 1.25) 0.37 | 983 (−1087; 3398) | 1.10 (0.94; 1.29) 0.25 | 1032 (−658; 3014) | 1.09 (0.86; 1.37) 0.48 | 351 (−564; 1504) | 1.05 (0.88; 1.26) 0.56 | 1.40 (−3.0; 6.7) |
| Winter | 0.93 (0.81; 1.08) 0.35 | −895 (−2566; 1033) | 0.93 (0.81; 1.08) 0.36 | −692 (−2025; 852) | 0.95 (0.77; 1.18) 0.66 | −192 (−939; 734) | 0.94 (0.80; 1.11) 0.50 | −1.40 (−5.2; 2.9) |
| Spring | 0.98 (0.85; 1.14) 0.81 | −243 (−2036; 1831) | 0.99 (0.85; 1.14) 0.84 | −156 (−1591; 1511) | 0.98 (0.78; 1.21) 0.82 | 101 (−881; 869) | 1.00 (0.85; 1.18) 0.99 | 0.0 (−4.0; 4.8) |
| Older siblings at birth | 1.08 (0.97; 1.19) 0.16 | 1015 (−372; 2549) | 1.11 (1.00; 1.23) 0.0496 | 1146 (4; 2412) | 1.06 (0.92; 1.24) 0.42 | 262 (−345; 967) | 1.22 (1.04; 1.44) 0.015 | 1.6 (−1.4; 5.0) |
| Cat at birth | 0.95 (0.84; 1.08) 0.45 | −639 (−2148; 1073) | 0.91 (0.80; 1.03) 0.14 | −969 (−2115; 336) | 0.95 (0.79; 1.15) 0.63 | −184 (−849; 618) | 1.02 (0.83; 1.26) 0.83 | 0.40 (−3.1; 4.5) |
| Dog at birth | 0.95 (0.83; 1.08) 0.43 | −701 (−2284; 1107) | 0.92 (0.80; 1.05) 0.21 | −879 (−2100; 521) | 1.21 (0.99; 1.47) 0.06 | 843 (−39; 1917) | 0.82 (0.66; 1.03) 0.08 | −4.2 (−7.2; −0.5) |
| Social circumstances | 0.99 (0.94; 1.05) 0.85 | −68 (−753; 654) | NA | NA | 0.97 (0.90; 1.05) 0.43 | −127 (−425; 196) | 1.00 (0.92; 1.09) 0.97 | −1.0 (−1.6; 1.5) |
| Solely breastfed (mths) | 1.00 (0.98; 1.03) 0.89 | 25 (−320; 379) | 1.00 (0.98; 1.03) 0.75 | 45 (−231; 329) | 0.97 (0.93; 1.01) 0.10 | −131 (−280; 25) | 1.00 (0.96; 1.04) 0.97 | 0.3 (−0.5; 1.0) |
| Introduction to daycare (mht) | 0.97 (0.95; 0.99) 0.0009 | −394 (−621; −164) | 0.97 (0.96; 0.99) 0.004 | −274 (−456; −89) | 1.00 (0.97; 1.02) 0.90 | −7 (−113; 102) | 0.96 (0.93; 1.00) 0.009 | −1.3 (−1.9; −0.7) |
| Type of daycare (nursery) | 1.23 (1.11; 1.37) 0.0001 | 3101 (1440; 4948) | 1.27 (1.13; 1.41) <0.0001 | 2773 (1409; 4293) | 0.94 (0.80; 1.11) 0.48 | −232 (−809; 446) | 1.33 (1.12; 1.58) 0.001 | 9.0 (5.1; 13.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nørgaard, S.K.; Vissing, N.H.; Chawes, B.L.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Cost of Illness in Young Children: A Prospective Birth Cohort Study. Children 2021, 8, 173. https://doi.org/10.3390/children8030173
Nørgaard SK, Vissing NH, Chawes BL, Stokholm J, Bønnelykke K, Bisgaard H. Cost of Illness in Young Children: A Prospective Birth Cohort Study. Children. 2021; 8(3):173. https://doi.org/10.3390/children8030173
Chicago/Turabian StyleNørgaard, Sarah Kristine, Nadja Hawwa Vissing, Bo Lund Chawes, Jakob Stokholm, Klaus Bønnelykke, and Hans Bisgaard. 2021. "Cost of Illness in Young Children: A Prospective Birth Cohort Study" Children 8, no. 3: 173. https://doi.org/10.3390/children8030173
APA StyleNørgaard, S. K., Vissing, N. H., Chawes, B. L., Stokholm, J., Bønnelykke, K., & Bisgaard, H. (2021). Cost of Illness in Young Children: A Prospective Birth Cohort Study. Children, 8(3), 173. https://doi.org/10.3390/children8030173







