Vitamin D Status in Children in Greece and Its Relationship with Sunscreen Application
Abstract
1. Introduction
1.1. Vitamin D
1.2. Ultraviolet (UV) Radiation
1.3. Sunscreens
2. Materials and Methods
Statistical Analysis
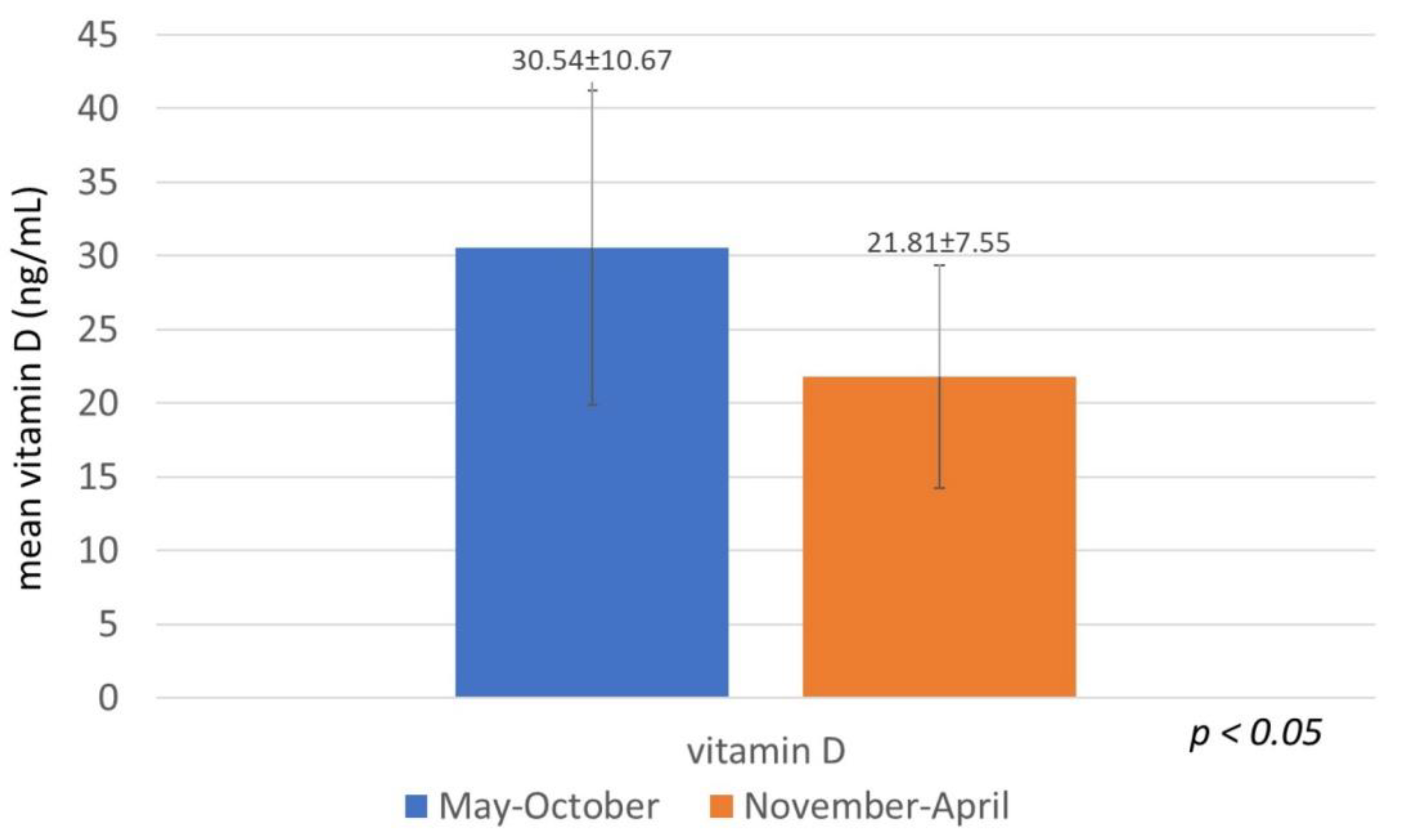
3. Results
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melmed, S.; Auchus, R.J.; Goldfine, A.B.; Koenig, R.J.; Rosen, C.J. Williams Textbook of Endocrinology, 14th ed.; Elsevier-Health Science Division: Philadelphia, PA, USA, 2019; p. 1792. [Google Scholar]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Lagunova, Z.; Porojnicu, A.; Aksnes, L.; Holick, M.; Iani, V.; Bruland, Ø.S.; Moan, J. Effect of vitamin D supplementation and ultraviolet B exposure on serum 25-hydroxyvitamin D concentrations in healthy volunteers: A randomized, crossover clinical trial. Br. J. Dermatol. 2013, 169, 434–440. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Holick, M.F. McCollum Award Lecture, 1994: Vitamin D—New horizons for the 21st century. Am. J. Clin. Nutr. 1994, 60, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Sucheston-Campbell, L. Vitamin D and the RNA transcriptome: More than mRNA regulation. Front. Physiol. 2014, 5, 181. [Google Scholar] [CrossRef]
- Holick, M.F. Environmental factors that influence the cutaneous production of vitamin D. Am. J. Clin. Nutr. 1995, 61, 638S–645S. [Google Scholar] [CrossRef]
- Matsuoka, L.Y.; Holich, M.F.; Wortsman, J. Regular Use of Sunscreen on Vitamin D Levels. Arch. Dermatol. 1995, 131, 1337–1338. [Google Scholar] [CrossRef]
- Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Vitamin D in the Healthy European Paediatric Population. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 692–701. [Google Scholar] [CrossRef]
- D’Orazio, J.A.; Jarrett, S.G.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M. Sunlight and Vitamin D. Derm. Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Holick, M. Sunlight, UV Radiation, Vitamin D, and Skin Cancer: How Much Sunlight Do We Need? Adv. Exp. Med. Biol. 2020, 1268, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Holick, M. Ultraviolet B Radiation: The Vitamin D Connection. Adv. Exp. Med. Biol. 2017, 996, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.S.; Tomecki, K.J. Sunscreens in the United States: Current Status and Future Outlook. Adv. Exp. Med. Biol. 2020, 1268, 355–379. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, L.Y.; Ide, L.; Wortsman, J.; MacLaughlin, J.A.; Holick, M.F. Sunscreens Suppress Cutaneous Vitamin D3Synthesis*. J. Clin. Endocrinol. Metab. 1987, 64, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Balk, S.J.; The Council on Environmental Health; Dermatology, S.O. Ultraviolet Radiation: A Hazard to Children and Adolescents. Pediatrics 2011, 127, e791–e817. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Defining Childhood Obesity. BMI for Children and Teens. Available online: https://www.cdc.gov/obesity/childhood/defining.html (accessed on 27 December 2020).
- Peterson, C.A.; Belenchia, A.M. Vitamin D Deficiency & Childhood Obesity: A Tale of Two Epidemics. Mo. Med. 2014, 111, 49–53. [Google Scholar]
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front. Endocrinol. 2019, 10, 103. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Service, H.N.M. Climate Data by City, Sunshine Duration. Available online: http://www.hnms.gr/emy/el/agriculture/agriculture_city?poli=Pyrgos (accessed on 27 December 2020).
- Bener, A.; Al-Ali, M.; Hoffmann, G.F. Vitamin D deficiency in healthy children in a sunny country: Associated factors. Int. J. Food Sci. Nutr. 2009, 60, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef] [PubMed]
- Al-Taiar, A.; Rahman, A.; Al-Sabah, R.; Shaban, L.; Al-Harbi, A. Vitamin D status among adolescents in Kuwait: A cross-sectional study. BMJ Open 2018, 8, e021401. [Google Scholar] [CrossRef] [PubMed]
- Alagöl, F.; Shihadeh, Y.; Boztepe, H.; Tanakol, R.; Yarman, S.; Azizlerli, H.; Sandalci, Ö. Sunlight exposure and vitamin D deficiency in Turkish women. J. Endocrinol. Investig. 2000, 23, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Wulf, H.C.; Philipsen, P.A. Improving Photoprotection and Implications for 25(OH)D Formation. Anticancer. Res. 2019, 40, 511–518. [Google Scholar] [CrossRef]
- Hansen, L.; Tjønneland, A.; Køster, B.; Brot, C.; Andersen, R.; Lundqvist, M.; Christensen, J.; Olsen, A. Sun Exposure Guidelines and Serum Vitamin D Status in Denmark: The StatusD Study. Nutrients 2016, 8, 266. [Google Scholar] [CrossRef]
- Marks, R.; Foley, P.A.; Jolley, D.; Knight, K.R.; Harrison, J.; Thompson, S. The Effect of Regular Sunscreen Use on Vitamin D Levels in an Australian Population. Arch. Dermatol. 1995, 131, 415–421. [Google Scholar] [CrossRef]
- Jayaratne, N.; Russell, A.; Van Der Pols, J.C. Sun protection and vitamin D status in an Australian subtropical community. Prev. Med. 2012, 55, 146–150. [Google Scholar] [CrossRef]
- Bonilla, C.; Ness, A.R.; Wills, A.K.; Lawlor, D.A.; Lewis, S.J.; Smith, G.D. Skin pigmentation, sun exposure and vitamin D levels in children of the Avon Longitudinal Study of Parents and Children. BMC Public Health 2014, 14, 597. [Google Scholar] [CrossRef]
- Kligman, E.W.; Watkins, A.; Johnson, K.; Kronland, R. The impact of lifestyle factors on serum 25-hydroxy vitamin D levels in older adults: A preliminary study. Fam. Pr. Res. J. 1989, 9, 11–19. [Google Scholar]
- Kim, S.; Carson, K.; Chien, A. Prevalence and correlates of sun protections with sunburn and vitamin D deficiency in sun-sensitive individuals. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.A.; Luz, F.B.; De Oliveira, C.M.M.; Xavier, A.R.; Kanaan, S.; Miot, H.A. Evaluation of vitamin D plasma levels after mild exposure to the sun with photoprotection. An. Bras. Dermatol. 2019, 94, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, L.; Olsen, C.M.; Gordon, L.; Green, A.C.; Aitken, J.; Neale, R.; Whiteman, D.; Janda, M. Letter to the Editor in response to “When to apply sunscreen: A consensus statement for Australia and New Zealand”. Aust. N. Z. J. Public Health 2019, 43, 504. [Google Scholar] [CrossRef]
- Passeron, T.; Bouillon, R.; Callender, V.; Cestari, T.F.; Diepgen, T.; Green, A.C.; Van Der Pols, J.C.; Bernard, B.; Ly, F.; Bernerd, F.; et al. Sunscreen photoprotection and vitamin D status. Br. J. Dermatol. 2019, 181, 916–931. [Google Scholar] [CrossRef]
- Young, A.R.; Narbutt, J.; Harrison, G.; Lawrence, K.P.; Bell, M.; O’Connor, C.; Olsen, P.; Grys, K.; Baczynska, K.; Rogowski-Tylman, M.; et al. Optimal sunscreen use, during a sun holiday with a very high ultraviolet index, allows vitamin D synthesis without sunburn. Br. J. Dermatol. 2019, 181, 1052–1062. [Google Scholar] [CrossRef]
- Linos, E.; Keiser, E.; Kanzler, M.; Sainani, K.L.; Lee, W.; Vittinghoff, E.; Chren, M.-M.; Tang, J.Y. Sun protective behaviors and vitamin D levels in the US population: NHANES 2003–2006. Cancer Causes Control. 2011, 23, 133–140. [Google Scholar] [CrossRef]
- Kannan, S.; Lim, H.W. Photoprotection and vitamin D: A review. Photodermatol. Photoimmunol. Photomed. 2014, 30, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Grossman, Z.; Hadjipanayis, A.; Stiris, T.; Del Torso, S.; Mercier, J.-C.; Valiulis, A.; Shamir, R. Vitamin D in European children—statement from the European Academy of Paediatrics (EAP). Eur. J. Nucl. Med. Mol. Imaging 2017, 176, 829–831. [Google Scholar] [CrossRef] [PubMed]

| Parameter | N (%) | VitD Mean (SD) | p |
|---|---|---|---|
| Gender | |||
| Males | 184 (48.9) | 27.5 (10.6) | 0.156 |
| Females | 192 (51.1) | 26 (10.1) | |
| Month | |||
| January | 31 (8.2) | 17.9 (6.8) | p < 0.001 ++ |
| February | 24 (6.4) | 21.8 (7.4) | |
| March | 29 (7.7) | 22.7 (5.8) | |
| April | 24 (6.4) | 20.6 (7.5) | |
| May | 35 (9.3) | 26.5 (13.9) | |
| June | 39 (10.4) | 27.6 (10.4) | |
| July | 40 (10.6) | 33 (9.4) | |
| August | 25 (6.6) | 30.1 (8.2) | |
| September | 44 (11.7) | 34.6 (8.7) | |
| October | 28 (7.4) | 30.1 (10.6) | |
| November | 38 (10.1) | 25.7 (6.9) | |
| December | 19 (5.1) | 20.5 (9.4) | |
| Season | |||
| Winter | 74 (19.7) | 19.8 (7.8) | p < 0.001 ++ |
| Spring | 88 (23.4) | 23.6 (10.4) | |
| Summer | 104 (27.6) | 30.3 (9.7) | |
| Autumn | 110 (29.3) | 30.4 (9.4) | |
| Sunscreen ex beach | |||
| Yes | 76 (20.2) | 24.9 (10) | p < 0.001 + |
| No | 300 (79.8) | 33.8 (8.6) | |
| Sunscreen on the beach | |||
| Yes | 128 (34.05) | 23.7 (9.8) | p < 0.001 + |
| No | 248 (65.95) | 32.5 (8.9) |
| Vitamin D levels | ||||
|---|---|---|---|---|
| Deficiency N (%) | Insufficiency N (%) | Normal N (%) | p | |
| Gender | ||||
| Males | 46 (25) | 70 (38) | 68 (37) | 0.410 + |
| Females | 56 (29.2) | 77 (40.1) | 59 (30.7) | |
| Age, Mean (SD) | 9.1 (4.7) | 7.6 (4.9) | 6.4 (4.9) | p < 0.001 ++ |
| Month | ||||
| January | 20 (64.5) | 9 (29) | 2 (6.5) | p < 0.001 + |
| February | 11 (45.8) | 10 (41.7) | 3 (12.5) | |
| March | 8 (27.6) | 17 (58.6) | 4 (13.8) | |
| April | 13 (54.2) | 7 (29.2) | 4 (16.6) | |
| May | 12 (34.3) | 13 (37.1) | 10 (28.6) | |
| June | 6 (15.4) | 23 (59) | 10 (25.6) | |
| July | 3 (7.5) | 13 (32.5) | 24 (60) | |
| August | 1 (4) | 13 (52) | 11 (44) | |
| September | 1 (2.3) | 13 (29.5) | 30 (68.2) | |
| October | 5 (17.9) | 9 (32.1) | 14 (50) | |
| November | 10 (26.3) | 17 (44.8) | 11 (28.9) | |
| December | 12 (63.2) | 3 (15.7) | 4 (21.1) | |
| Season | ||||
| Winter | 43 (58.1) | 22 (29.7) | 9 (12.2) | p < 0.001 + |
| Spring | 33 (37.5) | 37 (42) | 18 (20.5) | |
| Summer | 10 (9.6) | 49 (47.1) | 45 (43.3) | |
| Autumn | 16 (14.5) | 39 (35.5) | 55 (50) | |
| Sunscreen ex beach | ||||
| Yes | 2 (2.6) | 25 (32.9) | 78 (26) | p < 0.001 + |
| No | 100 (33.3) | 122 (40.7) | 33.8 (8.6) | |
| Sunscreen on the beach | ||||
| Yes | 6 (4.7) | 49 (38.3) | 73 (57) | p < 0.001 + |
| No | 96 (38.7) | 98 (39.5) | 54 (21.8) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feketea, G.M.; Bocsan, I.C.; Tsiros, G.; Voila, P.; Stanciu, L.A.; Zdrenghea, M. Vitamin D Status in Children in Greece and Its Relationship with Sunscreen Application. Children 2021, 8, 111. https://doi.org/10.3390/children8020111
Feketea GM, Bocsan IC, Tsiros G, Voila P, Stanciu LA, Zdrenghea M. Vitamin D Status in Children in Greece and Its Relationship with Sunscreen Application. Children. 2021; 8(2):111. https://doi.org/10.3390/children8020111
Chicago/Turabian StyleFeketea, Gavriela Maria, Ioana Corina Bocsan, Georgios Tsiros, Panagiota Voila, Luminita Aurelia Stanciu, and Mihnea Zdrenghea. 2021. "Vitamin D Status in Children in Greece and Its Relationship with Sunscreen Application" Children 8, no. 2: 111. https://doi.org/10.3390/children8020111
APA StyleFeketea, G. M., Bocsan, I. C., Tsiros, G., Voila, P., Stanciu, L. A., & Zdrenghea, M. (2021). Vitamin D Status in Children in Greece and Its Relationship with Sunscreen Application. Children, 8(2), 111. https://doi.org/10.3390/children8020111








