Using Combinations of Both Clinical and Radiographic Parameters to Develop a Diagnostic Prediction Model Demonstrated an Excellent Performance in Early Detection of Patients with Blount’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Patients
2.3. Study Variables and Candidate Predictors
2.4. Clinical Endpoints
2.5. Statistical Methods
2.5.1. Study Size Estimation
2.5.2. Fundamental Statistical Analysis
2.5.3. Model Development
- Missing data management
- Continuous predictors management
- Predictive model development
- Model performance and internal validation
- Model presentation
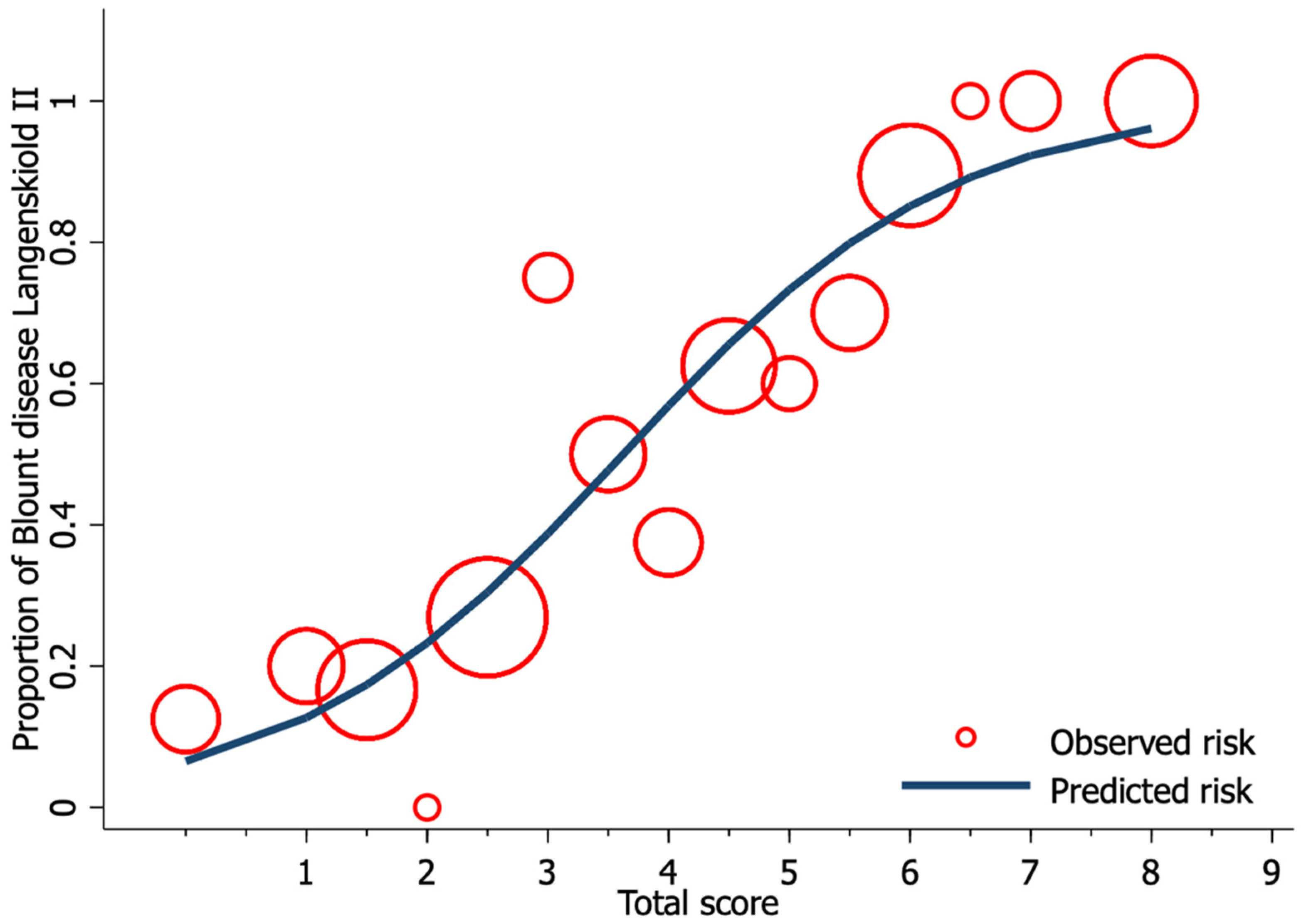
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dettling, S.; Weiner, D.S. Management of bow legs in children: A primary care protocol. J. Fam. Pr. 2017, 66, E1–E6. [Google Scholar]
- Brooks, W.C.; Gross, R.H. Genu Varum in Children: Diagnosis and Treatment. J. Am. Acad. Orthop. Surg. 1995, 3, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Langenskioeld, A.; Riska, E.B. Tibia Vara (Osteochondrosis Deformans tibiae): A Survey of Seventy-One Cases. J. Bone Jt. Surg.-Am. Vol. 1964, 46, 1405–1420. [Google Scholar] [CrossRef]
- Park, B.K.; Park, K.-B.; Kwak, Y.H.; Jin, S.; Kim, H.W.; Park, H. A comparative evaluation of tibial metaphyseal-diaphyseal angle changes between physiologic bowing and Blount disease. Medicine 2019, 98, e15349. [Google Scholar] [CrossRef] [PubMed]
- Alturki, Y.A. Unusual case of a 13-year-old male with Blount′s disease who was unable to walk: A prevention lesson. Saudi J. Med. Med. Sci. 2016, 4, 137–138. [Google Scholar] [CrossRef]
- Levine, A.M.; Drennan, J.C. Physiological bowing and tibia vara. The metaphyseal-diaphyseal angle in the measurement of bowleg deformities. J. Bone Jt. Surg.-Am. Vol. 1982, 64, 1158–1163. [Google Scholar] [CrossRef]
- Wongcharoenwatana, J.; Kaewpornsawan, K.; Chotigavanichaya, C.; Eamsobhana, P.; Laoharojanaphand, T.; Musikachart, P.; Ariyawatkul, T. Medial Metaphyseal Beak Angle as a Predictor for Langenskiöld Stage II of Blount’s Disease. Orthop. Surg. 2020, 12, 1703–1709. [Google Scholar] [CrossRef]
- Feldman, M.D.; Schoenecker, P.L. Use of the metaphyseal-diaphyseal angle in the evaluation of bowed legs. J. Bone Jt. Surg.-Am. Vol. 1993, 75, 1602–1609. [Google Scholar] [CrossRef]
- Hägglund, G.; Ingvarsson, T.; Ramgren, B.; Zayer, M. Metaphyseal-diaphyseal angle in Blount’s disease. A 30-year follow-up of 13 unoperated children. Acta Orthop. Scand. 1997, 68, 167–169. [Google Scholar] [CrossRef] [Green Version]
- Cook, C.E. Potential Pitfalls of Clinical Prediction Rules. J. Man. Manip. Ther. 2008, 16, 69–71. [Google Scholar] [CrossRef] [Green Version]
- World Medical Association World Medical Association Declaration of Helsinki. JAMA 2013, 310, 2191–2194. [CrossRef] [Green Version]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef]
- Kgoedi, M.; Rischbieter, P.; Goller, R. Body mass index and Blount’s disease: A single academic hospital experience. SA Orthop. J. 2019, 18, 15–20. [Google Scholar] [CrossRef]
- Kim, S.Y. The Definition of Obesity. Korean J. Fam. Med. 2016, 37, 309. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.H.; Choi, I.H.; Cho, T.-J.; Chung, C.Y.; Yoo, W.J. Development of Tibiofemoral Angle in Korean Children. J. Korean Med. Sci. 2008, 23, 714–717. [Google Scholar] [CrossRef] [Green Version]
- Vasiliadis, A.V.; Maris, A.; Gadikoppula, S. Tibia Vara or Blount’s Disease: Why an Early Diagnosis and Treatment Are Important? Clin. Pr. 2020, 10, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.; Sabharwal, S. Treatment of Infantile Blount Disease: An Update. J. Pediatr. Orthop. 2017, 37, S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Doyle, B.S.; Volk, A.G.; Smith, C.F. Infantile Blount Disease: Long-Term Follow-up of Surgically Treated Patients at Skeletal Maturity. J. Pediatr. Orthop. 1996, 16, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Abak, A.A.; Khoshhal, K.I. Acute “three-in-one” surgery for the treatment of severe Blount’s disease: Surgical technique and report of two cases. J. Taibah Univ. Med. Sci. 2020, 15, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Althubaiti, N.; Ghamri, R. Family Physicians’ Approaches to Mental Health Care and Collaboration with Psychiatrists. Cureus 2019, 11, e4755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, S.D.; Lavernia, C.J.; Burke, S.W.; Skinner, H.B.; Haddad, R.J. A Biomechanical Analysis of the Etiology of Tibia Vara. J. Pediatr. Orthop. 1983, 3, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Pirpiris, M.; Jackson, K.R.; Farng, E.; Bowen, R.E.; Otsuka, N.Y. Body Mass Index and Blount Disease. J. Pediatr. Orthop. 2006, 26, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Bradway, J.K.; Klassen, R.A.; Peterson, H.A. Blount disease: A review of the English literature. J. Pediatr. Orthop. 1987, 7, 472–480. [Google Scholar] [CrossRef] [PubMed]

| Patient Demographic | Mean | ±SD |
|---|---|---|
| Age (month) | 26.0 | 6.1 |
| Gender (n, %) | ||
| Male | 48 | 60.8 |
| Female | 31 | 39.2 |
| BMI 1 (kg/m2) | 24.9 | 4.5 |
| Laterality (n, %) | ||
| Blount’s disease of right leg | 9 | 11.4 |
| Blount’s disease of left leg | 19 | 24.1 |
| Bilateral Blount’s disease | 28 | 35.4 |
| Bilateral physiologic bowlegs | 23 | 29.1 |
| FTA 2 (°) | 11.6 | 5.7 |
| MDA 3 (°) | 12.4 | 3.6 |
| MMB 4 (°) | 122.9 | 6.1 |
| Characteristics (n = 158 Sides) | Missing Data | Blount Disease (n = 84 Sides) | Physiologic Bow-Leg (n = 74 Sides) | p-Value | |||
|---|---|---|---|---|---|---|---|
| n | (%) | Mean | ±SD | Mean | ±SD | ||
| Clinical characteristics | |||||||
| Age (months) | 0 | 0 | 27.0 | 5.2 | 24.9 | 6.9 | 0.030 |
| Age ≥ 24 months (n, %) | 57 | 67.9 | 37 | 50.0 | 0.024 | ||
| Gender (n, %) | |||||||
| Male | 0 | 0 | 48 | 57.1 | 48 | 64.9 | |
| Female | 0 | 0 | 36 | 42.9 | 26 | 35.1 | 0.333 |
| BMI 1 | 62 | 39.24 | 24.9 | 4.3 | 25.0 | 4.9 | 0.900 |
| BMI ≥ 23 kg/m2 (n. %) | 39 | 63.93 | 21 | 60.0 | 0.827 | ||
| Laterality (n, %) | |||||||
| Right | 0 | 0 | 37 | 44.1 | 42 | 56.8 | |
| Left | 0 | 0 | 47 | 55.9 | 32 | 43.2 | 0.151 |
| Radiographic Characteristics | |||||||
| FTA 2 (°) | 0 | 0 | 13.5 | 6.2 | 9.2 | 7.3 | <0.001 |
| FTA ≥ 5° (n, %) | 75 | 89.3 | 49 | 66.2 | <0.001 | ||
| MDA 3 (°) | 0 | 0 | 14.5 | 4.0 | 10.0 | 4.4 | <0.001 |
| MDA < 11° (n, %) | 13 | 15.5 | 43 | 15.5 | |||
| MDA 11–16° (n, %) | 40 | 47.6 | 27 | 36.5 | |||
| MDA > 16° (n, %) | 31 | 36.9 | 4 | 5.4 | <0.001 | ||
| MMB 4 (°) | 0 | 0 | 127.4 | 6.1 | 118.3 | 6.2 | <0.001 |
| MMB ≥ 122° (n, %) | 64 | 76.2 | 18 | 24.3 | <0.001 | ||
| Characteristics | Univariable Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| (n = 158 sides) | uOR | 95% CI | p-value | mOR | 95% CI | p-value | ||
| Age ≥ 24 months | 2.11 | 1.11 | 4.03 | 0.023 | 2.75 | 1.09 | 6.95 | 0.033 |
| Male | 0.72 | 0.38 | 1.37 | 0.322 | 0.70 | 0.27 | 1.79 | 0.459 |
| BMI 1 ≥ 23 kg/m2 | 1.71 | 0.73 | 3.99 | 0.213 | 2.36 | 0.70 | 8.05 | 0.165 |
| Right side | 0.60 | 0.32 | 1.13 | 0.112 | 0.77 | 0.33 | 1.77 | 0.533 |
| FTA 2 ≥ 5° | 4.25 | 1.83 | 9.87 | <0.001 | 1.37 | 0.45 | 4.19 | 0.580 |
| MDA 3 | ||||||||
| MDA < 11° | Ref. | |||||||
| MDA 11–16° | 4.90 | 2.23 | 10.79 | <0.001 | 2.66 | 0.91 | 7.80 | 0.074 |
| MDA > 16° | 25.63 | 7.63 | 86.14 | <0.001 | 11.65 | 2.44 | 55.63 | 0.002 |
| MMB 4 ≥ 122° | 9,96 | 4.79 | 20.68 | <0.001 | 4.47 | 1.59 | 11.52 | 0.005 |
| Characteristics | Multivariable Analysis | Score | ||||
|---|---|---|---|---|---|---|
| (n = 158 sides) | β | 95% CI | p-value | Transformed β | Assigned score | |
| Age ≥ 24 months) | 1.05 | 0.15 | 1.94 | 0.022 | 1.34 | 1.5 |
| BMI 1 ≥ 23 kg/m2 | 0.78 | −0.30 | 1.87 | 0.154 | 1.00 | 1 |
| MDA 2 | ||||||
| MDA < 11° | Reference | 0 | ||||
| MDA 11–16° | 1.16 | 0.17 | 2.16 | 0.022 | 1.49 | 1.5 |
| MDA > 16° | 2.60 | 1.10 | 4.11 | 0.001 | 3.34 | 3.5 |
| MMB 3 ≥ 122° | 1.50 | 0.58 | 2.43 | 0.001 | 1.93 | 2 |
| Risk Categories | Score | Blount | Physiologic Bow-Leg | LR+ | 95% CI | LR− | 95% CI | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||||||||
| Low risk | <2.5 | 6 | 7.1 | 31 | 41.9 | 0.17 | 0.06 | 0.45 | 5.86 | 2.27 | 18.01 | <0.001 |
| Moderate risk | 2.5–5.5 | 38 | 45.2 | 41 | 55.4 | 0.82 | 0.46 | 1.45 | 1.22 | 0.69 | 2.18 | 0.462 |
| High risk | >5.5 | 40 | 47.6 | 2 | 2.7 | 17.62 | 4.41 | 70.41 | 0.06 | 0.01 | 0.23 | <0.001 |
| Mean ± SE | 5.2 | 0.2 | 2.5 | 0.2 | <0.001 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adulkasem, N.; Wongcharoenwatana, J.; Ariyawatkul, T.; Chotigavanichaya, C.; Kaewpornsawan, K.; Eamsobhana, P. Using Combinations of Both Clinical and Radiographic Parameters to Develop a Diagnostic Prediction Model Demonstrated an Excellent Performance in Early Detection of Patients with Blount’s Disease. Children 2021, 8, 890. https://doi.org/10.3390/children8100890
Adulkasem N, Wongcharoenwatana J, Ariyawatkul T, Chotigavanichaya C, Kaewpornsawan K, Eamsobhana P. Using Combinations of Both Clinical and Radiographic Parameters to Develop a Diagnostic Prediction Model Demonstrated an Excellent Performance in Early Detection of Patients with Blount’s Disease. Children. 2021; 8(10):890. https://doi.org/10.3390/children8100890
Chicago/Turabian StyleAdulkasem, Nath, Jidapa Wongcharoenwatana, Thanase Ariyawatkul, Chatupon Chotigavanichaya, Kamolporn Kaewpornsawan, and Perajit Eamsobhana. 2021. "Using Combinations of Both Clinical and Radiographic Parameters to Develop a Diagnostic Prediction Model Demonstrated an Excellent Performance in Early Detection of Patients with Blount’s Disease" Children 8, no. 10: 890. https://doi.org/10.3390/children8100890
APA StyleAdulkasem, N., Wongcharoenwatana, J., Ariyawatkul, T., Chotigavanichaya, C., Kaewpornsawan, K., & Eamsobhana, P. (2021). Using Combinations of Both Clinical and Radiographic Parameters to Develop a Diagnostic Prediction Model Demonstrated an Excellent Performance in Early Detection of Patients with Blount’s Disease. Children, 8(10), 890. https://doi.org/10.3390/children8100890






