Evaluation of Fractional Exhaled Nitric Oxide in Pediatric Asthma and Allergic Rhinitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Test
2.2. Skin Prick Test
2.3. Pulmonary Function Tests
2.4. Methacholine Provocation Test
2.5. Fractional Exhaled Nitric Oxide
2.6. Statistical Analysis
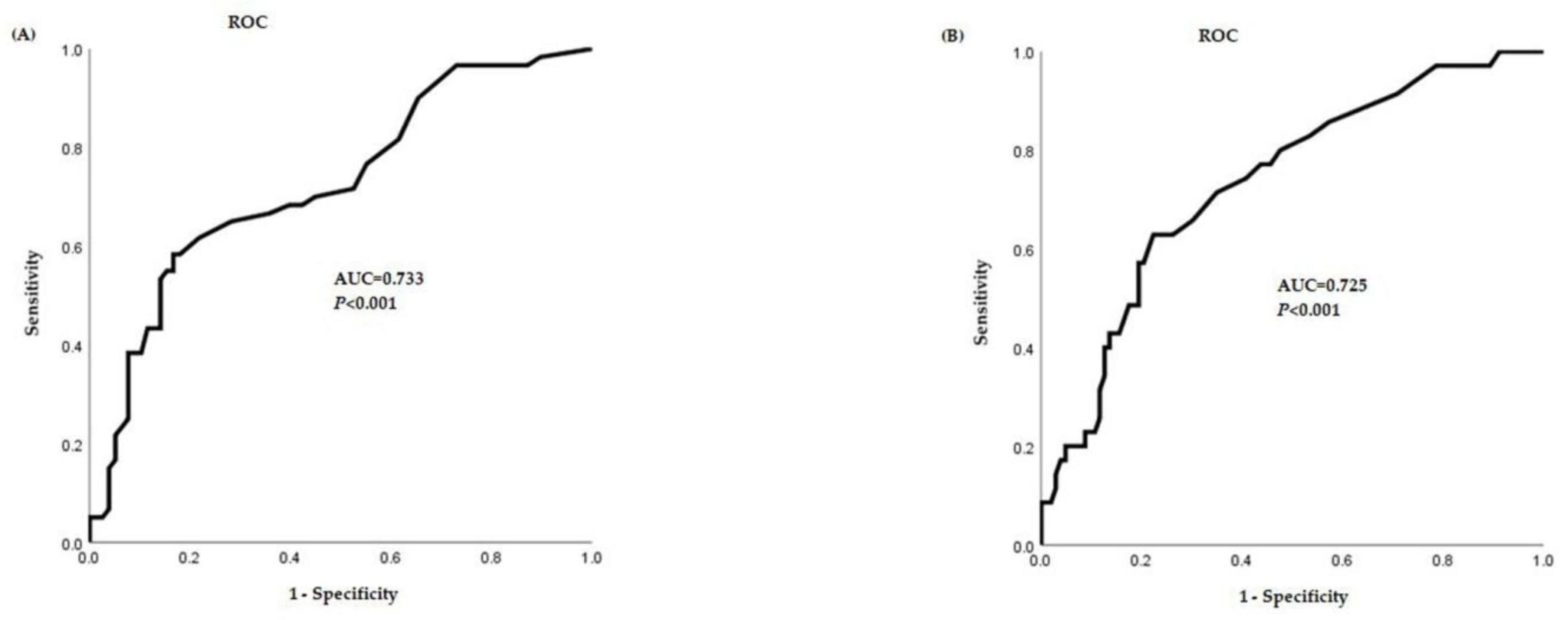
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hahn, Y.S. Measurements of fractional exhaled nitric oxide in pediatric asthma. Korean J. Pediatr. 2013, 56, 424. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.W.; Song, W.J.; Kim, M.H.; Lim, K.H.; Yang, M.S.; Jung, J.W.; Lee, J.; Suh, D.I.; Shin, Y.S.; Kim, S.-H.; et al. The KAAACI Standardization Committee Report on the procedure and application of fractional exhaled nitric oxide measurement. Allergy Asthma Respir. Dis. 2017, 5, 185–192. [Google Scholar] [CrossRef]
- Berlyne, G.S.; Parameswaran, K.; Kamada, D.; Efthimiadis, A.; Hargreave, F.E. A comparison of exhaled nitric oxide and induced sputum as markers of airway inflammation. J. Allergy Clin. Immunol. 2000, 106, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Warke, T.; Fitch, P.; Brown, V.; Taylor, R.; Lyons, J.; Ennis, M.; Shields, M.D. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax 2002, 57, 383–387. [Google Scholar] [CrossRef]
- Society, A.T. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R.; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.G.; Yoon, S.; Sim, J.H.; Woo, S.I. Fractional exhaled nitric oxide and forced expiratory volume in 1 second/forced vital capacity have predictive value of asthma exacerbation in Korean school children. Asia Pac. Allergy 2020, 10, e7. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Paton, J.Y.; Niven, R.; Pinnock, H. Guidelines for the diagnosis and management of asthma: A look at the key differences between BTS/SIGN and NICE. Thorax 2018, 73, 293–297. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Jensen, R.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general US population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Crapo, R. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am. J. Respir. Crit. Care Med. 2000, 161, 309–329. [Google Scholar] [PubMed]
- Guo, F.H.; Comhair, S.A.; Zheng, S.; Dweik, R.A.; Eissa, N.T.; Thomassen, M.J.; Calhoun, W.; Erzurum, S.C. Molecular mechanisms of increased nitric oxide (NO) in asthma: Evidence for transcriptional and post-translational regulation of NO synthesis. J. Immunol. 2000, 164, 5970–5980. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.N.; Adcock, I.M.; Wilson, N.M.; Oates, T.; Scallan, M.; Bush, A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am. J. Respir. Crit. Care Med. 2001, 164, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Kwon, J.W.; Kim, E.J.; Lee, S.M.; Kim, S.H.; Lee, S.Y.; Kim, S.H.; Park, H.W.; Chang, Y.S.; Kim, W.K.; et al. Clinical application of exhaled nitric oxide measurements in a Korean population. Allergy Asthma Immunol. Res. 2015, 7, 3–13. [Google Scholar] [CrossRef]
- Kovesi, T.; Kulka, R.; Dales, R. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9-to 12-year-old children. Chest 2008, 133, 169–175. [Google Scholar] [CrossRef]
- Buchvald, F.; Baraldi, E.; Carraro, S.; Gaston, B.; De Jongste, J.; Pijnenburg, M.W.; Silkoff, P.E.; Bisgaard, H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J. Allergy Clin. Immunol. 2005, 115, 1130–1136. [Google Scholar] [CrossRef]
- Kim, J.O.; Woo, S.I.; Hahn, Y.S. Relevance of exhaled nitric oxide levels to asthma control test scores and spirometry values in children with atopic asthma. Pediatr. Allergy Respir. Dis. 2011, 21, 24–31. [Google Scholar] [CrossRef][Green Version]
- Steerenberg, P.; Janssen, N.; De Meer, G.; Fischer, P.; Nierkens, S.; Van Loveren, H.; Opperhuizen, A.; Brunekreef, B.; van Amsterdam, J.G.C. Relationship between exhaled NO, respiratory symptoms, lung function, bronchial hyperresponsiveness, and blood eosinophilia in school children. Thorax 2003, 58, 242–245. [Google Scholar] [CrossRef]
- del Giudice, M.M.; Brunese, F.; Piacentini, G.; Pedullà, M.; Capristo, C.; Decimo, F.; Capristo, A.F. Fractional exhaled nitric oxide (FENO), lung function and airway hyperresponsiveness in naïve atopic asthmatic children. J. Asthma 2004, 41, 759–765. [Google Scholar] [CrossRef]
- Covar, R.A.; Szefler, S.J.; Martin, R.J.; Sundstrom, D.; Silkoff, P.E.; Murphy, J.; Young, D.A.; Spahn, J.D. Relations between exhaled nitric oxide and measures of disease activity among children with mild-to-moderate asthma. J. Pediatr. 2003, 142, 469–475. [Google Scholar] [CrossRef]
- Horvath, I.; de Jongste, J. Exhaled Biomarkers: European Respiratory Monograph; European Respiratory Society: Lausanne, Switzerland, 2010; pp. 14–17. [Google Scholar]
- Janson, C.; Kalm-Stephens, P.; Foucard, T.; Norbäck, D.; Alving, K.; Nordvall, S.L. Exhaled nitric oxide levels in school children in relation to IgE sensitisation and window pane condensation. Respir. Med. 2005, 99, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Tosca, M.A.; Capasso, M. Exhaled nitric oxide in children with allergic rhinitis and/or asthma: A relationship with bronchial hyperreactivity. J. Asthma 2010, 47, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Van Amsterdam, J.; Janssen, N.; De Meer, G.; Fischer, P.; Nierkens, S.; Van Loveren, H.; Opperhuizen, A.; Steerenberg, P.A.; Brunekreef, B. The relationship between exhaled nitric oxide and allergic sensitization in a random sample of school children. Clin. Exp. Allergy 2003, 33, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Karrasch, S.; Linde, K.; Rücker, G.; Sommer, H.; Karsch-Völk, M.; Kleijnen, J.; Jörres, R.A.; Schneider, A. Accuracy of FENO for diagnosing asthma: A systematic review. Thorax 2017, 72, 109–116. [Google Scholar] [CrossRef]
- Woo, S.I.; Lee, J.H.; Kim, H.; Kang, J.W.; Sun, Y.H.; Hahn, Y.S. Utility of fractional exhaled nitric oxide (FENO) measurements in diagnosing asthma. Respir. Med. 2012, 106, 1103–1109. [Google Scholar] [CrossRef]

| Parameters | BA (n = 29) | AR (n = 43) | BA + AR (n = 38) | Control (n = 28) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 8.9 ± 3.3 | 10.7 ± 3.7 | 10.9 ± 4.0 | 10.9 ± 3.6 | 0.124 |
| Sex (male) | 22 | 27 | 30 | 11 | 0.005 |
| Height (cm) | 135.0 ± 21.3 | 145.2 ± 20.7 | 145.7 ± 20.3 | 146.2 ± 20.6 | 0.114 |
| Weight (kg) | 38.9 ± 23.4 | 43.2 ± 18.4 | 46.0 ± 21.1 | 43.6 ± 16.6 | 0.550 |
| BMI (z-score) | 0.94 (−2.0–3.0) | 0.64 (−1.5−2.4) | 0.93 (−1.5−2.2) | 0.35 (−1.4−2.4) | 0.414 |
| Eosinophil (/㎕) | 142.5 (8.0−1930.0) | 243.5 (17.0−620.0) | 399.0 (0−2148.0) | 133.5 (0−566.0) | 0.085 |
| Total IgE (IU/mL) | 143.0 (70.6−335.5) | 215.0 (97.0−440.3) | 410.0 (195−1132) | 58 (24.6−85.4) | <0.001 |
| ECP (µg/L) | 32.5 (12.5−62.8) | 10.3 (3.8−16.2) | 14.7 (8.3−23.1) | 11.4 (3.0−50.3) | 0.290 |
| Number of positive skin prick test | 2.4 ± 2.5 (n: 14) | 6.1 ± 6.3 (n: 26) | 5.6 ± 5.1 (n: 27) | 0.7 ± 1.5 (n: 20) | <0.001 |
| Number of positive CAP | 1.5 ± 1.2 (n: 16) | 1.9 ± 1.2 (n: 19) | 1.9 ± 1.1 (n: 13) | 1.1 ± 1.5 (n: 8) | 0.385 |
| FVC (%) | 95.8 ± 16.0 | 95.0 ± 11.9 | 100.8 ± 17.5 | 98.9 ± 10.1 | 0.262 |
| FEV1 (%) | 94.0 ± 17.2 | 99.4 ± 13.8 | 94.0 ± 20.3 | 102.3 ± 12.8 | 0.116 |
| FEV1/FVC | 84.7 ± 9.1 | 91.1 ± 5.4 | 81.8 ± 9.5 | 91.1 ± 7.3 | <0.001 |
| PC20 (mg/mL) | 6.4 (1.6−12.3) | 25.0 | 5.7 (1.2−11.3) | 23.7 | 0.004 |
| FeNO(ppb) | 19 (10−38) | 13 (8−19) | 27 (13−47) | 11 (8−17) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y.Y.; Ahn, J.Y. Evaluation of Fractional Exhaled Nitric Oxide in Pediatric Asthma and Allergic Rhinitis. Children 2021, 8, 3. https://doi.org/10.3390/children8010003
Jang YY, Ahn JY. Evaluation of Fractional Exhaled Nitric Oxide in Pediatric Asthma and Allergic Rhinitis. Children. 2021; 8(1):3. https://doi.org/10.3390/children8010003
Chicago/Turabian StyleJang, Yoon Young, and Ji Young Ahn. 2021. "Evaluation of Fractional Exhaled Nitric Oxide in Pediatric Asthma and Allergic Rhinitis" Children 8, no. 1: 3. https://doi.org/10.3390/children8010003
APA StyleJang, Y. Y., & Ahn, J. Y. (2021). Evaluation of Fractional Exhaled Nitric Oxide in Pediatric Asthma and Allergic Rhinitis. Children, 8(1), 3. https://doi.org/10.3390/children8010003





