Safety and Dose-Dependent Effects of Echinacea for the Treatment of Acute Cold Episodes in Children: A Multicenter, Randomized, Open-Label Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Formulation
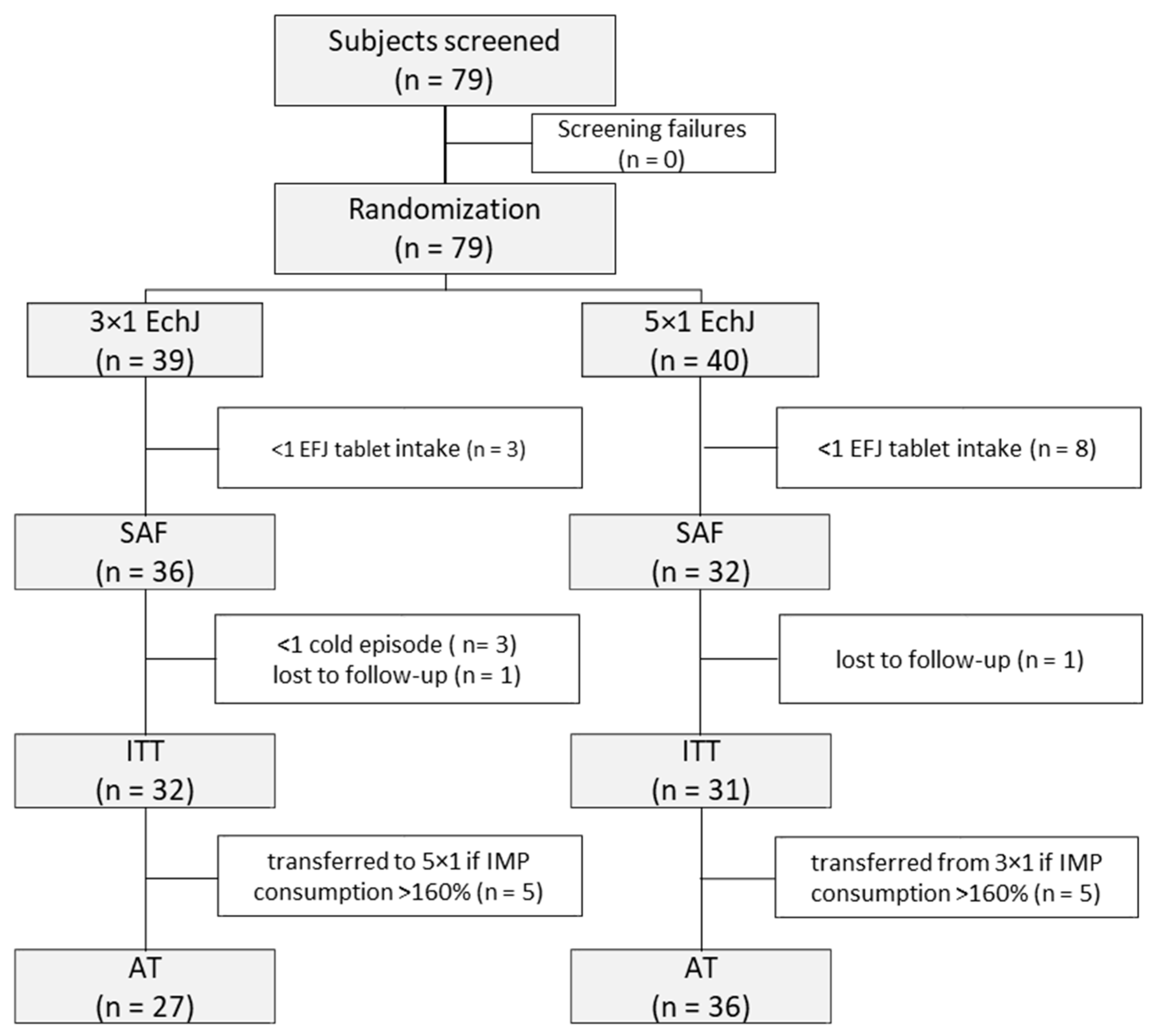
2.3. Study Design and Patient Recruitment
2.4. Assessments
2.5. Randomization and Sample Size Calculations
2.6. Data Evaluation
3. Results
3.1. Clinical Safety Outcomes
3.2. Clinical Effects
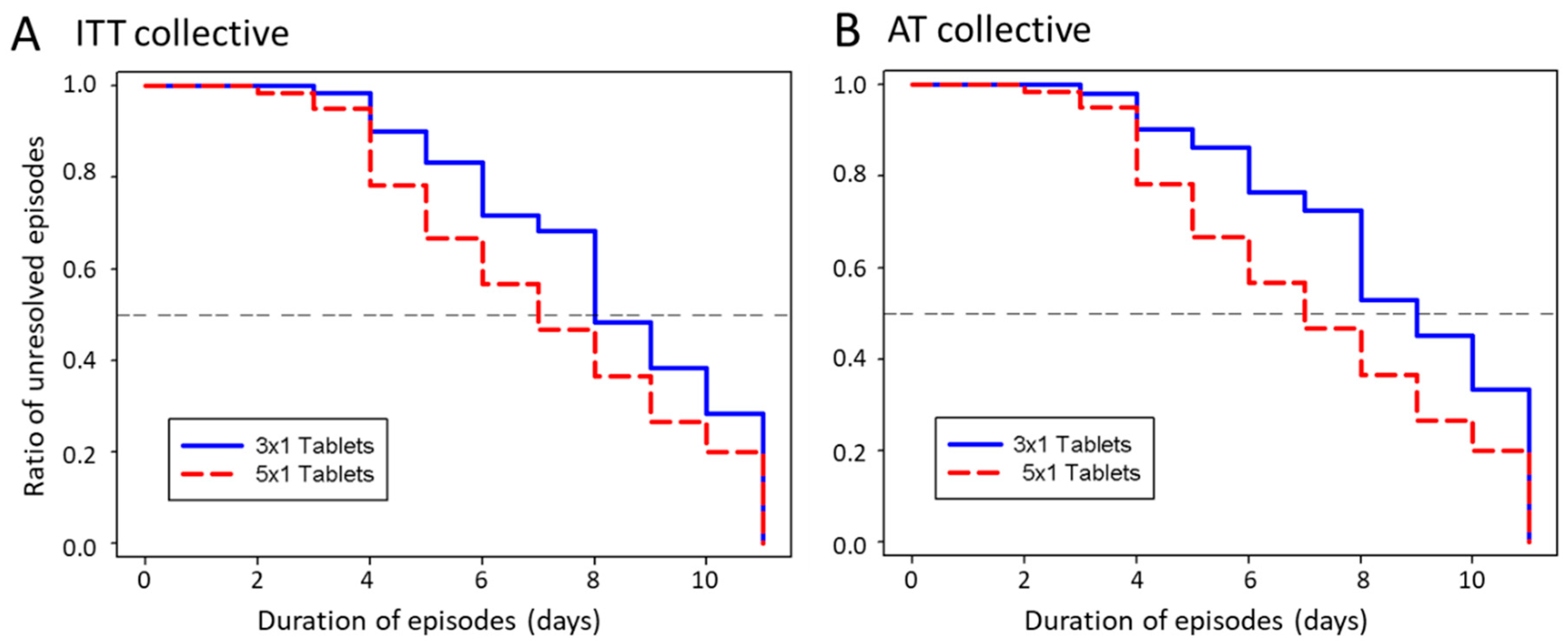
3.2.1. Duration of Episodes
3.2.2. Cumulative Number of Sick Days
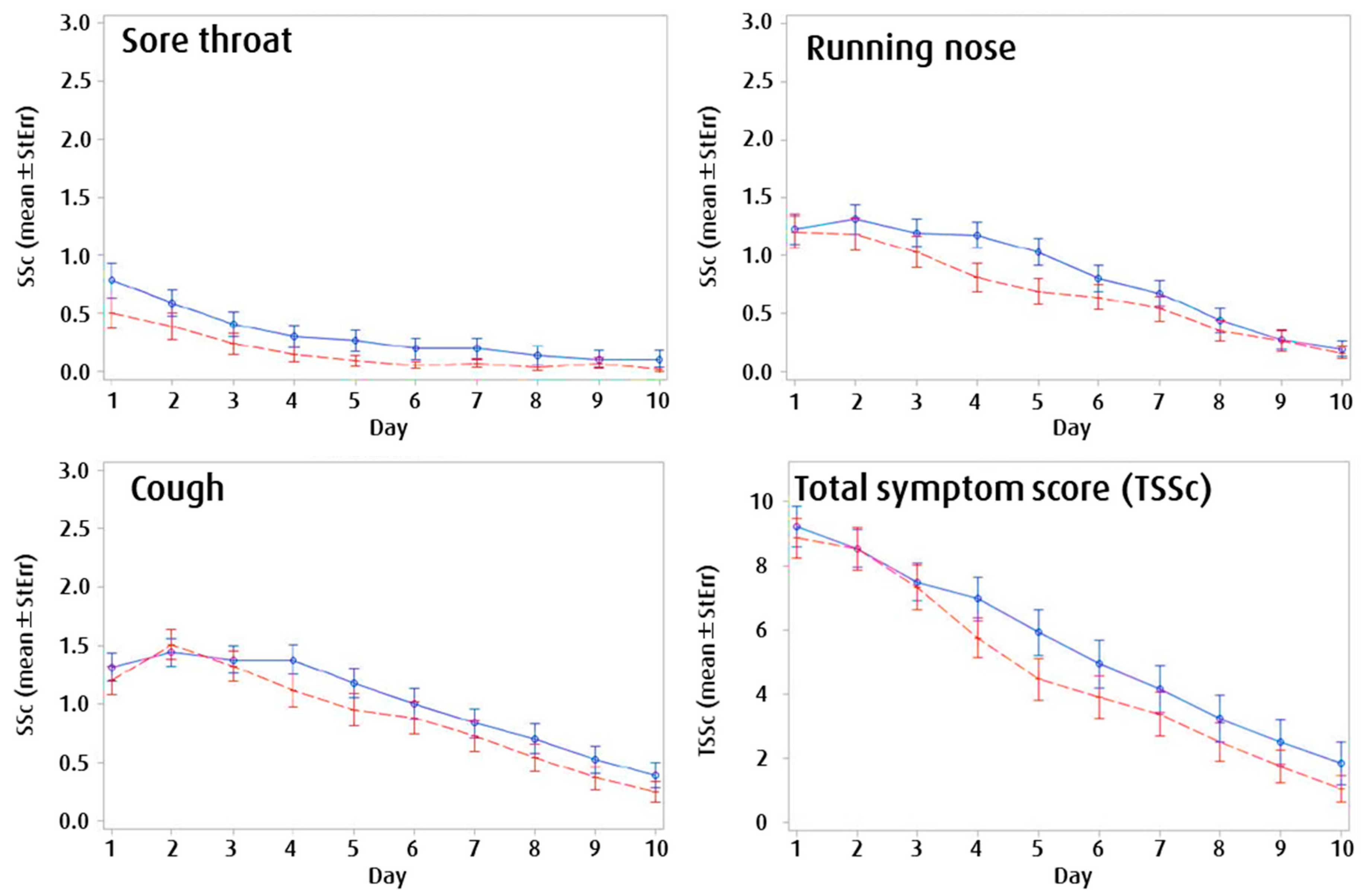
3.2.3. Symptom Development
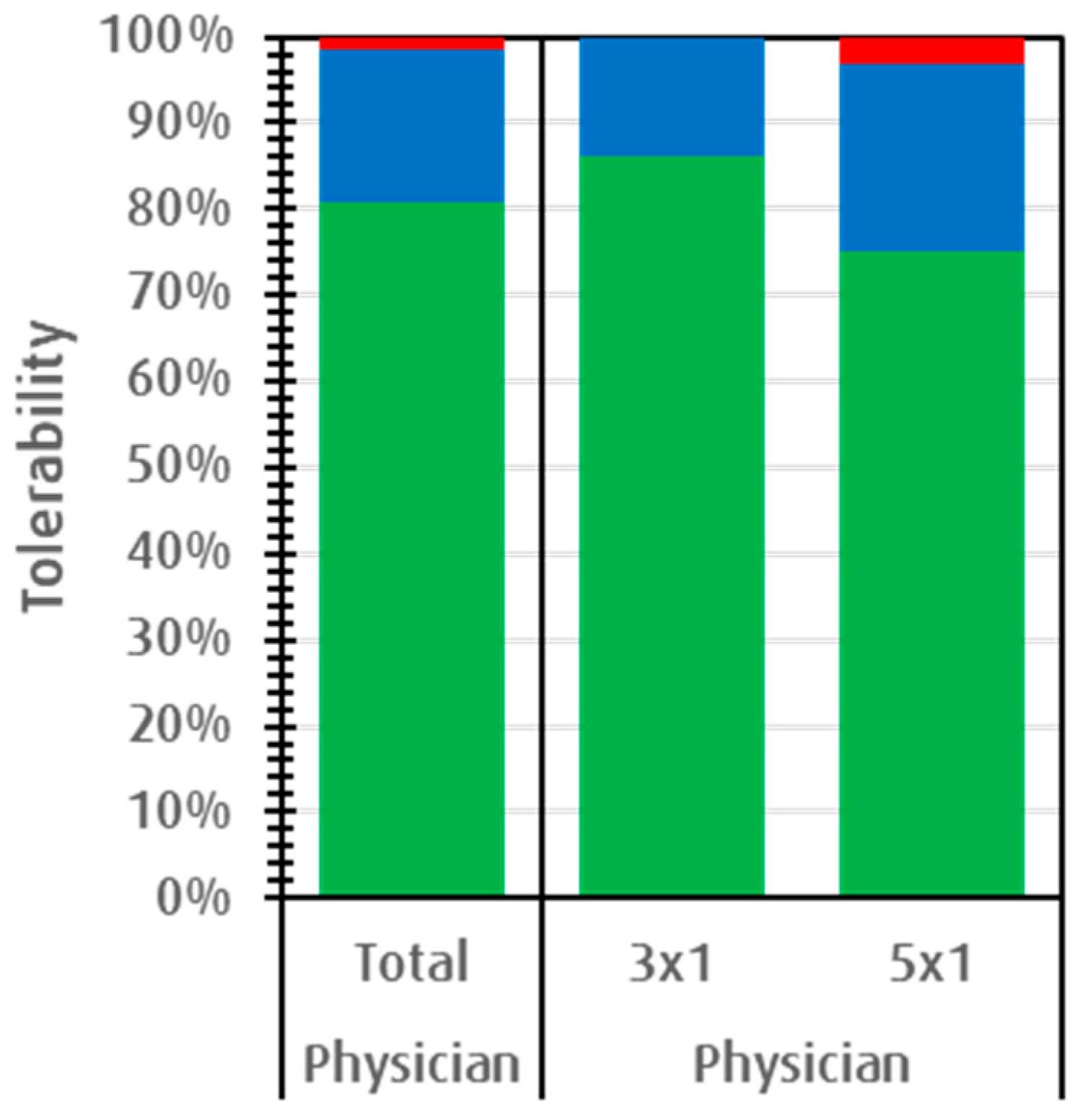
3.2.4. Subjective Effectiveness and Acceptance
3.2.5. Adverse Events and Concomitant Medication
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monto, A.S. Epidemiology of viral respiratory infections. Am. J. Med. 2002, 112, 4–12. [Google Scholar] [CrossRef]
- Goldmann, A.D. Epidemiology and Prevention of Pediatric Viral Respiratory Infections in Health-Care Institutions. Emerg. Infect. Dis. 2001, 7, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, T.; Järvinen, A. The common cold. Lancet 2003, 361, 51–59. [Google Scholar] [CrossRef]
- Fendrick, A.M.; Monto, A.S.; Nightengale, B.; Sarnes, M. The Economic Burden of Non–Influenza-Related Viral Respiratory Tract Infection in the United States. Arch. Intern. Med. 2003, 163, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Kaye, M.; Skidmore, S.; Osman, H.; Weinbren, M.; Warren, R. Surveillance of respiratory virus infections in adult hospital admissions using rapid methods. Epidemiol. Infect. 2006, 134, 792–798. [Google Scholar] [CrossRef]
- Aroll, B. Common cold. BMJ Clin. Evid. 2011, 2011, 1510. [Google Scholar]
- Little, P.; Stuart, B.; Hobbs, F.D.; Butler, C.C.; Hay, A.D.; Campbell, J.; Delaney, B.; Broomfield, S.; Barratt, P.; Hood, K.; et al. Predictors of suppurative complications for acute sore throat in primary care: Prospective clinical cohort study. BMJ 2013, 347, f6867. [Google Scholar] [CrossRef]
- Thompson, M.; Vodicka, T.A.; Blair, P.S.; Buckley, D.I.; Heneghan, C.; Hay, A.D. Duration of symptoms of respiratory tract infections in children: Systematic review. BMJ 2013, 347, f7027. [Google Scholar] [CrossRef]
- Goldsobel, A.B.; Chipps, B.E. Cough in the Pediatric Population. J. Pediatr. 2009, 156, 352–358.e1. [Google Scholar] [CrossRef]
- Fashner, J.; Ericson, K.; Werner, S. Treatment of the common cold in children and adults. Am. Fam. Physician 2012, 86, 153–159. [Google Scholar]
- Shehab, N.; Schaefer, M.K.; Budnitz, D.S.; Kegler, S.R. Adverse Events From Cough and Cold Medications After a Market Withdrawal of Products Labeled for Infants. Pediatrics 2010, 126, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Gummin, D.D.; Mowry, J.; Spyker, D.A.; Brooks, D.; Osterthaler, K.M.; Banner, W. Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin. Toxicol. 2018, 56, 1213–1415. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Chang, Y.J.; Cho, H.M.; Hwang, Y.W.; Moon, Y.S. Non-steroidal anti-inflammatory drugs for the common cold (Review). Cochrane Libr. 2013. [Google Scholar] [CrossRef]
- Hayward, G.; Thompson, M.J.; Perera, R.; Del Mar, C.B.; Glasziou, P.P.; Heneghan, C.J. Corticosteroids for the common cold. Cochrane Database Syst. Rev. 2015, 10, CD008116. [Google Scholar] [CrossRef] [PubMed]
- Touboul-Lundgren, P.; Bruno, P.; Bailly, L.; Dunais, B.; Pradier, C. Paediatric antibiotic prescriptions in primary care in the Alpes-Maritimes area of southeastern France between 2008 and 2013. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 509–516. [Google Scholar] [CrossRef]
- Kenealy, T.W.; Arroll, B. Antibiotic use for common cold. Common Cold 2009, 237–247. [Google Scholar] [CrossRef]
- Huntley, A.L.; Coon, J.T.; Ernst, E. The Safety of Herbal Medicinal Products Derived from Echinacea Species. Drug Saf. 2005, 28, 387–400. [Google Scholar] [CrossRef]
- Pleschka, S.; Stein, M.; Schoop, R.; Hudson, J.B. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian Influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol. J. 2009, 6, 197. [Google Scholar] [CrossRef]
- Sharma, M.; Shawn, A.; Anderson, S.A.; Schoop, R.; Hudson, J.B. Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antivir. Res. 2009, 83, 165–170. [Google Scholar] [CrossRef]
- Sharma, M.; Schoop, R.; Hudson, J.B. Echinacea as an antiinflammatory agent: The influence of physiologically relevant parameters. Phytother. Res. 2009, 23, 863–867. [Google Scholar] [CrossRef]
- Ritchie, M.R.; Gertsch, J.; Klein, P.; Schoop, R. Effects of Echinaforce® treatment on ex vivo-stimulated blood cells. Phytomedicine 2011, 18, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Diaz, R.A.; Rodarte, N.W.; Robles, S.C.; Aguilar-Salinas, C.A. The Ethical Use of Placebo in Pediatric Research. J. Clin. Res. Bioeth. 2011, 2. [Google Scholar] [CrossRef]
- Taylor, J.A.; Weber, W.J.; Martin, E.T.; Mccarty, R.L.; Englund, J.A. Development of a Symptom Score for Clinical Studies to Identify Children with a Documented Viral Upper Respiratory Tract Infection. Pediatr. Res. 2010, 68, 252–257. [Google Scholar] [CrossRef]
- Kurugöl, Z.; Akilli, M.; Bayram, N.; Koturoglu, G. The prophylactic and therapeutic effectiveness of zinc sulphate on common cold in children. Acta Paediatr. 2006, 95, 1175–1181. [Google Scholar] [CrossRef]
- Grüber, C.; Keil, T.; Kulig, M.; Roll, S.; Wahn, U.; Wahn, V.; MAS-90 Study Group. History of respiratory infections in the first 12 yr among children from a birth cohort. Pediatr. Allergy Immunol. 2008, 19, 505–512. [Google Scholar] [CrossRef]
- Schapowal, A.; Klein, P.; Johnston, S.L. Echinacea Reduces the Risk of Recurrent Respiratory Tract Infections and Complications: A Meta-Analysis of Randomized Controlled Trials. Adv. Ther. 2015, 32, 197–200. [Google Scholar] [CrossRef]
- Gertsch, J.; Schoop, R.; Kuenzle, U.; Suter, A. Echinacea alkylamides modulate TNF-α gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Lett. 2004, 577, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Hersberger, K.E.; Botomino, A.; Sarkar, R.; Tschudi, P.; Bucher, H.C.; Briel, M. Prescribed medications and pharmacy interventions for acute respiratory tract infections in Swiss primary care. J. Clin. Pharm. Ther. 2009, 34, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Glinz, D.; Reyes, S.L.; Saccilotto, R.; Widmer, A.F.; Zeller, A.; Bucher, H.C.; Hemkens, L.G. Quality of antibiotic prescribing of Swiss primary care physicians with high prescription rates: A nationwide survey. J. Antimicrob. Chemother. 2017, 72, 3205–3212. [Google Scholar] [CrossRef]
- Jawad, M.; Schoop, R.; Suter, A.; Klein, P.; Eccles, R. Safety and Efficacy Profile of Echinacea purpurea to Prevent Common Cold Episodes: A Randomized, Double-Blind, Placebo-Controlled Trial. Evid. Based Complementary Altern. Med. 2012, 2012, 841315. [Google Scholar] [CrossRef]
| Patient Collective | SAF | ITT | ||
|---|---|---|---|---|
| EFJ 1 Dosage Group | 3 × 1 | 5 × 1 | 3 × 1 | 5 × 1 |
| N | 36 | 32 | 32 | 31 |
| Age in years, mean (±SD) | 7.5 (2.62) | 5.9 (1.85) | 7.4 (2.65) | 5.9 (1.88) |
| Height in cm, mean (±SD) | 125.6 (20.2) | 115.6 (13.0) | 125.2 (20.3) | 115.5 (13.2) |
| Body weight in kg, mean (±SD) | 25.9 (10.4) | 20.5 (5.2) | 25.6 (10.8) | 20.5 (5.2) |
| Past cold episodes 2, n, mean (±SD) | 3.1 (0.17) | 3.2 (0.21) | 3.0 (0.19) | 3.1 (0.20) |
| Kindergarten/School (%) | 88.9 | 78.1 | 87.5 | 77.4 |
| Daily Dosage Regimen | ||||
|---|---|---|---|---|
| Collective | Parameter | 3 × 1 EFJ 1 | 5 × 1 EFJ 1 | Wilcoxon Test (p Value) |
| ITT 2 | Number of cold episodes | 61 | 59 | |
| CSD 4 per study group, days ± SD | 494 | 410 | 0.022 | |
| CSD 4 per patient, days ± SD | 13.9 ± 6.41 | 12.5 ± 6.88 | 0.362 | |
| Episode duration, days ± SD | 8.10 ± 3.52 | 6.90 ± 3.48 | 0.046 | |
| Responder/non-responder rate, % 5 | 80.3/19.7 | 89.3/10.7 | 0.21 | |
| AT 3 | Number of cold episodes | 51 | 69 | |
| CSD 4 per study group, days ± SD | 433 | 471 | 0.0022 | |
| CSD 4 per patient, days ± SD | 16.0 ± 1.58 | 13.1 ± 1.19 | 0.048 | |
| Episode duration, days ± SD | 8.50 ± 3.68 | 6.80 ± 3.28 | 0.02 | |
| Responder/non-responder rate 5, in % | 76.5/23.5 | 91.3/8.7 | 0.005 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weishaupt, R.; Bächler, A.; Feldhaus, S.; Lang, G.; Klein, P.; Schoop, R. Safety and Dose-Dependent Effects of Echinacea for the Treatment of Acute Cold Episodes in Children: A Multicenter, Randomized, Open-Label Clinical Trial. Children 2020, 7, 292. https://doi.org/10.3390/children7120292
Weishaupt R, Bächler A, Feldhaus S, Lang G, Klein P, Schoop R. Safety and Dose-Dependent Effects of Echinacea for the Treatment of Acute Cold Episodes in Children: A Multicenter, Randomized, Open-Label Clinical Trial. Children. 2020; 7(12):292. https://doi.org/10.3390/children7120292
Chicago/Turabian StyleWeishaupt, Ramon, Arnold Bächler, Simon Feldhaus, Günter Lang, Peter Klein, and Roland Schoop. 2020. "Safety and Dose-Dependent Effects of Echinacea for the Treatment of Acute Cold Episodes in Children: A Multicenter, Randomized, Open-Label Clinical Trial" Children 7, no. 12: 292. https://doi.org/10.3390/children7120292
APA StyleWeishaupt, R., Bächler, A., Feldhaus, S., Lang, G., Klein, P., & Schoop, R. (2020). Safety and Dose-Dependent Effects of Echinacea for the Treatment of Acute Cold Episodes in Children: A Multicenter, Randomized, Open-Label Clinical Trial. Children, 7(12), 292. https://doi.org/10.3390/children7120292





