Why Unidimensional Pain Measurement Prevails in the Pediatric Acute Pain Context and What Multidimensional Self-Report Methods Can Offer
Abstract
:1. Introduction
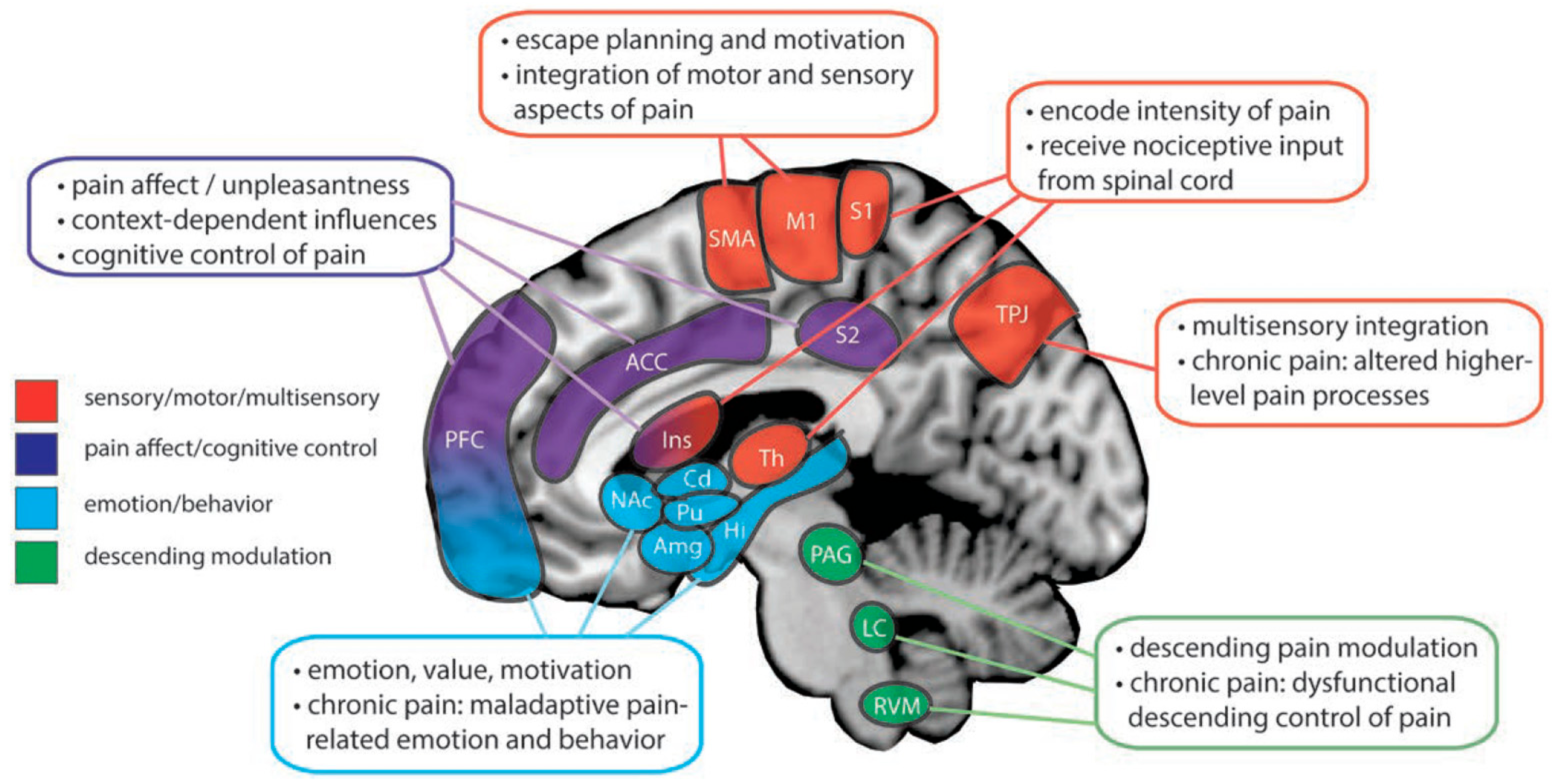
2. Pain: A Multidimensional Experience
3. Concordance and Discordance between Various Dimensions of the Acute Pain Experience
4. Pediatric Multidimensional Acute Pain Measurement in the Clinical Context
4.1. Acute Pain Dimensions Associated with Transition to Chronicity
4.2. Multidimensional Pain Assessment and Therapeutic Decision-Making
5. Pediatric Acute Pain Assessment for Clinical Trials
6. Possible Reasons for Why Acute Pain Assessment in Children Is Often Unidimensional
7. Future Directions
7.1. Development of Age-Appropriate Self-Report Affective and Evaluative Pain Assessment Tools
7.2. Future Research into the Clinical Application and Value of Multidimensional Pediatric Acute Pain Assessment
7.3. Education of Health Professionals Regarding Multidimensional Pain Assessment
7.4. Expansion of Multidimensional Pain Assessment to Include the Child’s Social Context
7.5. Limitations
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Association for the Study of Pain. Part iii: Pain terms, a current list with definitions and notes on usage. In Classification of Chronic Pain: Descriptors of Chronic Pain Syndromes and Definitions of Pain Terms, 2nd ed.; Merskey, H., Bogduk, N., Eds.; IASP Press: Seattle, WA, USA, 1994; pp. 209–214. [Google Scholar]
- Walco, G.A.P.; Conte, P.M.P.; Labay, L.E.P.; Engel, R.P.; Zeltzer, L.K.M.D. Procedural distress in children with cancer: Self-report, behavioral observations, and physiological parameters. Clin. J. Pain Novemb. Dec. 2005, 21, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Howard, R.F.; Liossi, C. Persistent postsurgical pain in children and young people: Prediction, prevention, and management. Pain Rep. 2017, 2, e616. [Google Scholar] [CrossRef] [PubMed]
- Schiavenato, M.; von Baeyer, C.L.; Craig, K.D. Self-report is a primary source of information about pain, but it is not infallible: A comment on “response to voepel-lewis’s letter to the editor, ‘bridging the gap between pain assessment and treatment: Time for a new theoretical approach?’”. West. J. Nurs. Res. 2013, 35, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Twycross, A.P.; Voepel-Lewis, T.P.; Vincent, C.P.; Franck, L.S.P.; von Baeyer, C.L.P. A debate on the proposition that self-report is the gold standard in assessment of pediatric pain intensity. Clin. J. Pain 2015, 31, 707–712. [Google Scholar] [CrossRef]
- Stinson, J.N.; Kavanagh, T.; Yamada, J.; Gill, N.; Stevens, B. Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain 2006, 125, 143–157. [Google Scholar] [CrossRef]
- Von Baeyer, C.L.; Jaaniste, T.; Vo, H.L.T.; Brunsdon, G.; Lao, H.C.; Champion, G.D. Systematic review of self-report measures of pain intensity in 3- and 4-year-old children: Bridging a period of rapid cognitive development. J. Pain 2017, 18, 1017–1026. [Google Scholar] [CrossRef]
- Von Baeyer, C.L. Children’s self-reports of pain intensity: Scale selection, limitations and interpretation. Pain Res. Manag. 2006, 11, 157–162. [Google Scholar] [CrossRef]
- Gordon, D.B. Acute pain assessment tools: Let us move beyond simple pain ratings. Curr. Opin. Anesthesiol. 2015, 28, 565–569. [Google Scholar] [CrossRef]
- Radnovich, R.; Chapman, C.R.; Gudin, J.A.; Panchal, S.J.; Webster, L.R.; Pergolizzi, J.V., Jr. Acute pain: Effective management requires comprehensive assessment. Postgrad. Med. 2014, 126, 59–72. [Google Scholar] [CrossRef]
- Petti, E.B.A.; Scher, C.B.A.; Meador, L.M.P.H.; Van Cleave, J.H.P.R.N.; Reid, C.M.M.D.P. Can multidimensional pain assessment tools help improve pain outcomes in the perianesthesia setting? J. PeriAnesthesia Nurs. 2018, 33, 767–772. [Google Scholar] [CrossRef]
- Kent, M.L.; Tighe, P.J.; Belfer, I.; Brennan, T.J.; Bruehl, S.; Brummett, C.M.; Buckenmaier, C.C.; Buvanendran, A.; Cohen, R.I.; Desjardins, P.; et al. The acttion-aps-aapm pain taxonomy (aaapt) multidimensional approach to classifying acute pain conditions. J. Pain 2017, 18, 479–489. [Google Scholar] [CrossRef] [PubMed]
- IASP. Iasp’s Proposed New Definition of Pain Released for Comment. Available online: https://www.iasp-pain.org/PublicationsNews/NewsDetail.aspx?ItemNumber=9218 (accessed on 13 November 2019).
- Melzack, R.; Casey, K.L. Sensory, motivational, and central control determinants of pain: A new conceptual model. Ski. Senses 1968, 1, 423–443. [Google Scholar]
- Williams, A.C.; Craig, K.D. Updating the definition of pain. Pain 2016, 157, 2420–2423. [Google Scholar] [CrossRef]
- Kunz, M.; Lautenbacher, S.; LeBlanc, N.; Rainville, P. Are both the sensory and the affective dimensions of pain encoded in the face? Pain 2012, 153, 350–358. [Google Scholar] [CrossRef]
- Fields, H.L. Pain: An unpleasant topic. Pain 1999, 82, S61–S69. [Google Scholar] [CrossRef]
- Price, D.D. Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol. Interv. 2002, 2, 392. [Google Scholar] [CrossRef]
- Hofbauer, R.K.; Rainville, P.; Duncan, G.H.; Bushnell, M.C. Cortical representation of the sensory dimension of pain. J. Neurophysiol. 2001, 86, 402–411. [Google Scholar] [CrossRef]
- Peyron, R.; Laurent, B.; García-Larrea, L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol. Clin. Clin. Neurophysiol. 2000, 30, 263–288. [Google Scholar] [CrossRef]
- Morton, D.L.; Sandhu, J.S.; Jones, A.K.P. Brain imaging of pain: State of the art. J. Pain Res. 2016, 9, 613–624. [Google Scholar] [CrossRef]
- Martucci, K.T.; Mackey, S.C. Neuroimaging of pain: Human evidence and clinical relevance of central nervous system processes and modulation. Anesthesiology 2018, 128, 1241–1254. [Google Scholar] [CrossRef]
- Coghill, R.C.; Sang, C.N.; Maisog, J.M.; Iadarola, M.J. Pain intensity processing within the human brain: A bilateral, distributed mechanism. J. Neurophysiol. 1999, 82, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.D.; Zubieta, J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 2005, 9, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Schreckenberger, M.; Siessmeier, T.; Viertmann, A.; Landvogt, C.; Buchholz, H.G.; Rolke, R.; Treede, R.D.; Bartenstein, P.; Birklein, F. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology 2005, 64, 1175. [Google Scholar] [CrossRef] [PubMed]
- Bräscher, A.K.; Becker, S.; Hoeppli, M.E.; Schweinhardt, P. Different brain circuitries mediating controllable and uncontrollable pain. J. Neurosci. 2016, 36, 5013. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Hoeft, F.; Sheau, K.E.; Mackey, S.C. Strategy-dependent dissociation of the neural correlates involved in pain modulation. Anesthesiology 2011, 115, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, F.; Martucci, K.T.; Kraft, R.A.; Gordon, N.S.; McHaffie, J.G.; Coghill, R.C. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J. Neurosci. 2011, 31, 5540. [Google Scholar] [CrossRef]
- Kupers, R.; Faymonville, M.-E.; Laureys, S. The cognitive modulation of pain: Hypnosis- and placebo-induced analgesia. Prog. Brain Res. 2005, 150, 251–269. [Google Scholar]
- Lui, F.; Colloca, L.; Duzzi, D.; Anchisi, D.; Benedetti, F.; Porro, C. Neural bases of conditioned placebo analgesia. Pain 2010, 151, 816–824. [Google Scholar] [CrossRef]
- Salomons, T.V.; Moayedi, M.; Weissman-Fogel, I.; Goldberg, M.B.; Freeman, B.V.; Tenenbaum, H.C.; Davis, K.D. Perceived helplessness is associated with individual differences in the central motor output system. Eur. J. Neurosci. 2012, 35, 1481–1487. [Google Scholar] [CrossRef]
- Neugebauer, V. Amygdala pain mechanisms. Handb. Exp. Pharmacol. 2015, 227, 261–284. [Google Scholar]
- Schmidt, K.; Forkmann, K.; Sinke, C.; Gratz, M.; Bitz, A.; Bingel, U. The differential effect of trigeminal vs. Peripheral pain stimulation on visual processing and memory encoding is influenced by pain-related fear. NeuroImage 2016, 134, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Starr, C.J.; Sawaki, L.; Wittenberg, G.F.; Burdette, J.H.; Oshiro, Y.; Quevedo, A.S.; McHaffie, J.G.; Coghill, R.C. The contribution of the putamen to sensory aspects of pain: Insights from structural connectivity and brain lesions. Brain A J. Neurol. 2011, 134, 1987–2004. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Fields, H.L. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984, 7, 309–338. [Google Scholar] [CrossRef]
- Fields, H.L.; Basbaum, A.I. Central nervous system mechanisms of pain modulation. In Textbook of Pain; Churchill Livingstone: London, UK, 1999; pp. 309–329. [Google Scholar]
- Rainville, P. Brain mechanisms of pain affect and pain modulation. Curr. Opin. Neurobiol. 2002, 12, 195–204. [Google Scholar] [CrossRef]
- Wiech, K.; Ploner, M.; Tracey, I. Neurocognitive aspects of pain perception. Trends Cogn. Sci. 2008, 12, 306–313. [Google Scholar] [CrossRef]
- Seminowicz, D.A.; Davis, K.D. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain 2006, 120, 297–306. [Google Scholar] [CrossRef]
- Liossi, C.; Howard, R.F. Pediatric chronic pain: Biopsychosocial assessment and formulation. Pediatrics 2016, 138, e20160331. [Google Scholar] [CrossRef] [Green Version]
- Frith, C.D. The social brain? Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 671–678. [Google Scholar] [CrossRef]
- Davis, K.D.; Kucyi, A.; Moayedi, M. The pain switch: An “ouch” detector. Pain 2015, 156, 2164–2166. [Google Scholar] [CrossRef]
- Vervoort, T.; Karos, K.; Trost, Z.; Prkachin, K.M. Social and Interpersonal Dynamics in Pain: We don’t Suffer Alone; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Connelly, M.; Neville, K. Comparative prospective evaluation of the responsiveness of single-item pediatric pain-intensity self-report scales and their uniqueness from negative affect in a hospital setting. J. Pain 2010, 11, 1451–1460. [Google Scholar] [CrossRef]
- Goodenough, B.; Thomas, W.; Champion, G.D.; Perrott, D.; Taplin, J.E.; von Baeyer, C.L.; Ziegler, J.B. Unravelling age effects and sex differences in needle pain: Ratings of sensory intensity and unpleasantness of venipuncture pain by children and their parents. Pain 1999, 80, 179–190. [Google Scholar] [CrossRef]
- Goodenough, B.; van Dongen, K.; Brouwer, N.; Abu-Saad, H.; David Champion, G. A comparison of the faces pain scale and the facial affective scale for children’s estimates of the intensity and unpleasantness of needle pain during blood sampling. Eur. J. Pain 1999, 3, 301–315. [Google Scholar] [CrossRef]
- Perrott, D.A.; Goodenough, B.; Champion, G.D. Children’s ratings of the intensity and unpleasantness of post-operative pain using facial expression scales. Eur. J. Pain 2004, 8, 119–127. [Google Scholar] [CrossRef]
- St-Laurent-Gagnon, T.; Bernard-Bonnin, A.C.; Villeneuve, E. Pain evaluation in preschool children and by their parents. Acta Paediatr. 1999, 88, 422–427. [Google Scholar] [CrossRef]
- Pope, N.; Tallon, M.; McConigley, R.; Wilson, S. The experiences of acute non-surgical pain of children who present to a healthcare facility for treatment: A systematic review protocol. JBI Database Syst. Rev. Implement. Rep. 2015, 13, 12–20. [Google Scholar] [CrossRef]
- Hedén, L.; von Essen, L.; Ljungman, G. The relationship between fear and pain levels during needle procedures in children from the parents’ perspective. Eur. J. Pain 2016, 20, 223–230. [Google Scholar] [CrossRef]
- McMurtry, C.M.; Noel, M.; Chambers, C.T.; McGrath, P.J. Children’s fear during procedural pain: Preliminary investigation of the children’s fear scale. Health Psychol. 2011, 30, 780–788. [Google Scholar] [CrossRef]
- Lu, Q.; Tsao, J.C.I.; Myers, C.D.; Kim, S.C.; Zeltzer, L.K. Coping predictors of children’s laboratory-induced pain tolerance, intensity, and unpleasantness. J. Pain 2007, 8, 708–717. [Google Scholar] [CrossRef]
- Esteve, R.; Marquina-Aponte, V.; Ramírez-Maestre, C. Postoperative pain in children: Association between anxiety sensitivity, pain catastrophizing, and female caregivers’ responses to children’s pain. J. Pain 2014, 15, 157–168.e151. [Google Scholar] [CrossRef]
- Vervoort, T.; Craig, K.D.; Goubert, L.; Dehoorne, J.; Joos, R.; Matthys, D.; Buysse, A.; Crombez, G. Expressive dimensions of pain catastrophizing: A comparative analysis of school children and children with clinical pain. Pain 2008, 134, 59–68. [Google Scholar] [CrossRef]
- Palermo, T.M.; Chambers, C.T. Parent and family factors in pediatric chronic pain and disability: An integrative approach. Pain 2005, 119, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Valrie, C.R.; Karlson, C.W. Family and parent influences on pediatric chronic pain. Am. Psychol. 2014, 69, 142–152. [Google Scholar] [CrossRef]
- Goubert, L.; Vlaeyen, J.W.S.; Crombez, G.; Craig, K.D. Learning about pain from others: An observational learning account. J. Pain 2011, 12, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjistavropoulos, T.; Craig, K.D.; Duck, S.; Cano, A.; Goubert, L.; Jackson, P.L.; Mogil, J.S.; Rainville, P.; Sullivan, M.J.; Williams, A.C.D.C.; et al. A biopsychosocial formulation of pain communication. Psychol. Bull. 2011, 137, 910–939. [Google Scholar] [CrossRef] [Green Version]
- Logan, D.E.; Rose, J.B. Is postoperative pain a self-fulfilling prophecy? Expectancy effects on postoperative pain and patient-controlled analgesia use among adolescent surgical patients. J. Pediatric Psychol. 2005, 30, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Michalska, K.J.; Feldman, J.S.; Abend, R.; Gold, A.L.; Dildine, T.C.; Palacios-Barrios, E.E.; Leibenluft, E.; Towbin, K.E.; Pine, D.S.; Atlas, L.Y. Anticipatory effects on perceived pain: Associations with development and anxiety. Psychosom. Med. 2018, 80, 853–860. [Google Scholar] [CrossRef]
- Claar, R.L.; Walker, L.S.; Barnard, J.A. Children’s knowledge, anticipatory anxiety, procedural distress, and recall of esophagogastroduodenoscopy. J. Pediatric Gastroenterol. Nutr. 2002, 34, 68–72. [Google Scholar] [CrossRef]
- Tsao, J.C.I.; Myers, C.D.; Craske, M.G.; Bursch, B.; Kim, S.C.; Zeltzer, L.K. Role of anticipatory anxiety and anxiety sensitivity in children’s and adolescents’ laboratory pain responses. J. Pediatric Psychol. 2004, 29, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Palermo, T.M.; Drotar, D. Prediction of children’s postoperative pain: The role of presurgical expectations and anticipatory emotions1. J. Pediatric Psychol. 1996, 21, 683–698. [Google Scholar] [CrossRef]
- Caes, L.; Goubert, L.; Devos, P.; Verlooy, J.; Benoit, Y.; Vervoort, T. Personal distress and sympathy differentially influence health care professional and parents’ estimation of child procedure-related pain. Pain Med. 2017, 18, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Noel, M.; Pavlova, M.; McCallum, L.; Vinall, J. Remembering the hurt of childhood: A psychological review and call for future research. Can. Psychol. Psychol. Can. 2017, 58, 58–68. [Google Scholar] [CrossRef]
- Fischer, S.; Vinall, J.; Pavlova, M.; Graham, S.; Jordan, A.; Chorney, J.; Rasic, N.; Brookes, J.T.; Hoy, M.; Yunker, W.K.; et al. Role of anxiety in young children’s pain memory development after surgery. Pain 2019, 160, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Lander, J.; Hodgins, M.; Fowler-Kerry, S. Children’s pain predictions and memories. Behav. Res. Ther. 1992, 30, 117–124. [Google Scholar] [CrossRef]
- Ornstein, P.; Manning, E.; Pelphrey, K. Children’s memory for pain. J. Dev. Behav. Pediatr. 1999, 20, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.; Rabbitts, J.A.; Tai, G.G.; Palermo, T.M. Remembering pain after surgery: A longitudinal examination of the role of pain catastrophizing in children’s and parents’ recall. Pain 2015, 156, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.A.E.F.; Silva, T.D.C.R.D.; Siqueira, H.B.D.O.M.; Saltareli, S.; Gomez, R.R.F.; Hortense, P. Pain from the life cycle perspective: Evaluation and measurement through psychophysical methods of category estimation and magnitude estimation. Rev. Lat. Am. Enferm. 2016, 24, e2769. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.Y.; von Baeyer, C.L. Cognitive developmental influences on the ability of preschool-aged children to self-report their pain intensity. Pain 2016, 157, 997–1001. [Google Scholar] [CrossRef]
- Gaffney, A.; Dunne, E.A. Children’s understanding of the causality of pain. Pain 1987, 29, 91–104. [Google Scholar] [CrossRef]
- Myers, C.D.; Tsao, J.C.; Glover, D.A.; Kim, S.C.; Turk, N.; Zeltzer, L.K. Sex, gender, and age: Contributions to laboratory pain responding in children and adolescents. J. Pain 2006, 7, 556–564. [Google Scholar] [CrossRef]
- LeResche, L.; Dworkin, S.F. Facial expressions of pain and emotions in chronic tmd patients. Pain 1988, 35, 71–78. [Google Scholar] [CrossRef]
- Hadjistavropoulos, T.; Craig, K.D. A theoretical framework for understanding self-report and observational measures of pain: A communications model. Behav. Res. Ther. 2002, 40, 551–570. [Google Scholar] [CrossRef]
- Blount, R.L.; Loiselle, K.A. Behavioural assessment of pediatric pain. Pain Res. Manag. 2009, 14, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Blount, R.L. Commentary: Acute pediatric procedural pain, distress, and coping. J. Pediatric Psychol. 2019, 44, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Fortier, M.A.; Chou, J.; Maurer, E.L.; Kain, Z.N. Acute to chronic postoperative pain in children: Preliminary findings. J. Pediatric Surg. 2011, 46, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Pagé, M.G.; Campbell, F.; Isaac, L.; Stinson, J.; Katz, J. Parental risk factors for the development of pediatric acute and chronic postsurgical pain: A longitudinal study. J. Pain Res. 2013, 6, 727–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagé, M.G.; Stinson, J.; Campbell, F.; Isaac, L.; Katz, J. Identification of pain-related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. J. Pain Res. 2013, 6, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Noel, M.; Rabbitts, J.A.; Fales, J.; Chorney, J.; Palermo, T.M. The influence of pain memories on children’s and adolescents’ post-surgical pain experience: A longitudinal dyadic analysis. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 2017, 36, 987–995. [Google Scholar] [CrossRef]
- Rabbitts, J.A.; Fisher, E.; Rosenbloom, B.N.; Palermo, T.M. Prevalence and predictors of chronic postsurgical pain in children: A systematic review and meta-analysis. J. Pain Off. J. Am. Pain Soc. 2017, 18, 605–614. [Google Scholar] [CrossRef]
- Rathleff, M.S.; Graven-Nielsen, T. Transition from acute to chronic pain in children: Novel pieces of the puzzle. Pain 2017, 158, 767–768. [Google Scholar] [CrossRef]
- Rabbitts, J.A.; Zhou, C.; Groenewald, C.B.; Durkin, L.; Palermo, T.M. Trajectories of postsurgical pain in children: Risk factors and impact of late pain recovery on long-term health outcomes after major surgery. Pain 2015, 156, 2383–2389. [Google Scholar] [CrossRef] [Green Version]
- Noel, M.; Rosenbloom, B.; Pavlova, M.; Campbell, F.; Isaac, L.; Pagé, M.G.; Stinson, J.; Katz, J. Remembering the pain of surgery 1 year later: A longitudinal examination of anxiety in children’s pain memory development. Pain 2019, 160, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Zavos, H.M.S.; Lachance, G.; Hammond, C.J.; Williams, F.M.K. Shared genetic factors underlie chronic pain syndromes. Pain 2014, 155, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Morlion, B.; Coluzzi, F.; Aldington, D.; Kocot-Kepska, M.; Pergolizzi, J.; Mangas, A.C.; Ahlbeck, K.; Kalso, E. Pain chronification: What should a non-pain medicine specialist know? Curr. Med Res. Opin. 2018, 34, 1169–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlaeyen, J.W.S.; Linton, S.J. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain 2012, 153, 1144–1147. [Google Scholar] [CrossRef]
- Todd, J.; Sharpe, L.; Johnson, A.; Nicholson Perry, K.; Colagiuri, B.; Dear, B.F. Towards a new model of attentional biases in the development, maintenance, and management of pain. Pain 2015, 156, 1589–1600. [Google Scholar] [CrossRef]
- Casey, C.Y.; Greenberg, M.A.; Nicassio, P.M.; Harpin, R.E.; Hubbard, D. Transition from acute to chronic pain and disability: A model including cognitive, affective, and trauma factors. Pain 2008, 134, 69–79. [Google Scholar] [CrossRef]
- Asmundson, G.J.; Noel, M.; Petter, M.; Parkerson, H.A. Pediatric fear-avoidance model of chronic pain: Foundation, application and future directions. Pain Res. Manag. 2012, 17, 397–405. [Google Scholar] [CrossRef]
- Goubert, L.; Simons, L. Cognitive styles and processes in paediatric pain. In Oxford Textbook of Pediatric Pain; Oxford University Press: Oxford, UK, 2013; pp. 95–101. [Google Scholar]
- Edwards, R.R.; Dworkin, R.H.; Sullivan, M.D.; Turk, D.C.; Wasan, A.D. The role of psychosocial processes in the development and maintenance of chronic pain. J. Pain 2016, 17, T70–T92. [Google Scholar] [CrossRef] [Green Version]
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2016, 18, 20–30. [Google Scholar] [CrossRef]
- Pasero, C.; Quinlan-Colwell, A.; Rae, D.; Broglio, K.; Drew, D. American society for pain management nursing position statement: Prescribing and administering opioid doses based solely on pain intensity. Pain Manag. Nurs. 2016, 17, 170–180. [Google Scholar] [CrossRef]
- De Ruddere, L.; Goubert, L.; Stevens, M.A.; Deveugele, M.; Craig, K.D.; Crombez, G. Health care professionals’ reactions to patient pain: Impact of knowledge about medical evidence and psychosocial influences. J. Pain 2014, 15, 262–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsze, D.S.; Hirschfeld, G.; von Baeyer, C.L.; Bulloch, B.; Dayan, P.S. Clinically significant differences in acute pain measured on self-report pain scales in children. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2015, 22, 415–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowbotham, M.C. What is a ‘clinically meaningful’ reduction in pain? Pain 2001, 94, 131–132. [Google Scholar] [CrossRef]
- Olsen, M.F.; Bjerre, E.; Hansen, M.D.; Hilden, J.; Landler, N.E.; Tendal, B.; Hróbjartsson, A. Pain relief that matters to patients: Systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. 2017, 15, 35. [Google Scholar] [CrossRef] [Green Version]
- Sloman, R.; Wruble, A.W.; Rosen, G.; Rom, M. Determination of clinically meaningful levels of pain reduction in patients experiencing acute postoperative pain. Pain Manag. Nurs. 2006, 7, 153–158. [Google Scholar] [CrossRef]
- McGrath, P.; Walco, G.; Turk, D.; Dworkin, R.; Brown, M.; Davidson, K.; Eccleston, C.; Finley, A.; Goldschneider, K.; Haverkos, L.; et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: Pedimmpact recommendations. J. Pain Off. J. Am. Pain Soc. 2008, 9, 771–783. [Google Scholar] [CrossRef]
- Van Boekel, R.L.M.; Vissers, K.C.P.; van der Sande, R.; Bronkhorst, E.; Lerou, J.G.C.; Steegers, M.A.H. Moving beyond pain scores: Multidimensional pain assessment is essential for adequate pain management after surgery. PLoS ONE 2017, 12, e0177345. [Google Scholar] [CrossRef]
- Le-Wendling, L.; Glick, W.; Tighe, P. Goals and objectives to optimize the value of an acute pain service in perioperative pain management. Tech. Orthop. 2017, 32, 200–208. [Google Scholar] [CrossRef]
- Gordon, D.B.; Watt-Watson, J.; Hogans, B.B. Interprofessional pain education-with, from, and about competent, collaborative practice teams to transform pain care. Schmerz 2019, 33, 66–72. [Google Scholar] [CrossRef]
- Hilário, T.S.; dos Santos, S.M.; Kruger, J.; Goes, M.G.; Casco, M.F.; Rabelo-Silva, E.R. Pain assessment and management in patients undergoing endovascular procedures in the catheterization laboratory. Rev. Esc. Enferm. 2017, 51. [Google Scholar] [CrossRef] [Green Version]
- Garland, E.L.; Baker, A.K.; Larsen, P.; Riquino, M.R.; Priddy, S.E.; Thomas, E.; Hanley, A.W.; Galbraith, P.; Wanner, N.; Nakamura, Y. Randomized controlled trial of brief mindfulness training and hypnotic suggestion for acute pain relief in the hospital setting. J. Gen. Intern. Med. 2017, 32, 1106–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, E.A.; Dias, N.; Hendricks-Ferguson, V.; Hellsten, M.; Skeens-Borland, M.; Thornton, C.; Linder, L.A. Perspectives on cancer pain assessment and management in children. Semin. Oncol. Nurs. 2019, 35, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, T.N.; Taenzer, A.H.; Elkassabany, N.M.; Le Wendling, L.; Nin, O.; Kent, M.L. Safety in acute pain medicine—Pharmacologic considerations and the impact of systems-based gaps. Pain Med. 2018, 19, 2296–2315. [Google Scholar] [CrossRef] [PubMed]
- Schiavenato, M.P.R.N.; Craig, K.D.P. Pain assessment as a social transaction: Beyond the “gold standard”. Clin. J. Pain 2010, 26, 667–676. [Google Scholar] [CrossRef]
- Auvray, M.; Myin, E.; Spence, C. The sensory-discriminative and affective-motivational aspects of pain. Neurosci. Biobehav. Rev. 2010, 34, 214–223. [Google Scholar] [CrossRef]
- Scher, C.; Meador, L.; Van Cleave, J.H.; Reid, M.C. Moving beyond pain as the fifth vital sign and patient satisfaction scores to improve pain care in the 21st century. Pain Manag. Nurs. 2018, 19, 125–129. [Google Scholar] [CrossRef]
- McGrath, P.A.; DeVeber, L.L.; Hearn, M.T. Multidimensional pain assessment in children. In Advances in Pain Research and Therapy; Fields, H.L., Cervero, R.D.F., Eds.; Raven Press: New York, NY, USA, 1985; Volume 9, pp. 387–393. [Google Scholar]
- Jacob, E.; Mack, A.K.; Savedra, M.; Van Cleve, L.; Wilkie, D.J. Adolescent pediatric pain tool for multidimensional measurement of pain in children and adolescents. Pain Manag. Nurs. Off. J. Am. Soc. Pain Manag. Nurses 2014, 15, 694–706. [Google Scholar] [CrossRef] [Green Version]
- Abu-Saad, H.H.; Kroonen, E.; Halfens, R. On the development of a multidimensional dutch pain assessment tool for children. Pain 1990, 43, 249–256. [Google Scholar] [CrossRef]
- Kuttner, L.; LePage, T. Face scales for the assessment of pediatric pain: A critical review. Can. J. Behav. Sci. Rev. Can. Des Sci. Du Comport. 1989, 21, 198. [Google Scholar] [CrossRef]
- Twining, J.; Padula, C. Pilot testing the clinically aligned pain assessment (capa) measure. Pain Manag. Nurs. 2019. [Google Scholar] [CrossRef]
- Savedra, M.C.; Holzemer, W.L.; Tesler, M.D.; Wilkie, D.J. Assessment of postoperation pain in children and adolescents using the adolescent pediatric pain tool. Nurs. Res. 1993, 42, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Franck, L.S.; Bruce, E. Putting pain assessment into practice: Why is it so painful? Pain Res. Manag. 2009, 14, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsze, D.S.; von Baeyer, C.L.; Bulloch, B.; Dayan, P.S. Validation of self-report pain scales in children. Pediatrics 2013, 132, e971–e979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castarlenas, E.P.; Jensen, M.P.P.; von Baeyer, C.L.P.; Miro, J.P. Psychometric properties of the numerical rating scale to assess self-reported pain intensity in children and adolescents: A systematic review. Clin. J. Pain 2017, 33, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Zimmermann, M.; Kagerer, S.; Schienle, A.; Walter, B.; Weygandt, M.; Vaitl, D. Hemodynamic brain correlates of disgust and fear ratings. NeuroImage 2007, 37, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Rhudy, J.L.; Meagher, M.W. Fear and anxiety: Divergent effects on human pain thresholds. Pain 2000, 84, 65–75. [Google Scholar] [CrossRef]
- Von Baeyer, C.L.; Pasero, C. What nurses’ work-arounds tell us about pain assessment. Int. J. Nurs. Stud. 2017, 67, A1–A2. [Google Scholar] [CrossRef]
- Deák, G.O.; Ray, S.D.; Pick, A.D. Effects of age, reminders, and task difficulty on young children’s rule-switching flexibility. Cogn. Dev. 2004, 19, 385–400. [Google Scholar] [CrossRef] [Green Version]
- Chajut, E.; Algom, D. Selective attention improves under stress: Implications for theories of social cognition. J. Personal. Soc. Psychol. 2003, 85, 231–248. [Google Scholar] [CrossRef] [Green Version]
- Kochanska, G.; Murray, K.T.; Harlan, E.T. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Dev. Psychol. 2000, 36, 220–232. [Google Scholar] [CrossRef]
- Birnie, K.A.; Hundert, A.S.; Lalloo, C.; Nguyen, C.; Stinson, J.N. Recommendations for selection of self-report pain intensity measures in children and adolescents: A systematic review and quality assessment of measurement properties. Pain 2019, 160, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, H.B.P.; Handberg, G.M.D.; Emmeluth, C.P.; Graven-Nielsen, T.D.P. Preoperative hypoalgesia after cold pressor test and aerobic exercise is associated with pain relief 6 months after total knee replacement. Clin. J. Pain 2017, 33, 475–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.M.; Scott, E.L.; Trost, Z.; Hirsh, A.T. Perceived injustice is associated with pain and functional outcomes in children and adolescents with chronic pain: A preliminary examination. J. Pain 2016, 17, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Eccleston, C.; Fisher, E.A.; Vervoort, T.; Crombez, G. Worry and catastrophizing about pain in youth: A reappraisal. Pain 2012, 153, 1560–1562. [Google Scholar] [CrossRef] [Green Version]
- Fraenkel, L.; Falzer, P.; Fried, T.; Kohler, M.; Peters, E.; Kerns, R.; Leventhal, H. Measuring pain impact versus pain severity using a numeric rating scale. J. Gen. Intern. Med. 2012, 27, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Rainville, P.; Bao, Q.V.H.; Chretien, P. Pain-related emotions modulate experimental pain perception and autonomic responses. Pain 2005, 118, 306–318. [Google Scholar] [CrossRef]
- Petter, M.; Chambers, C.T.; Chorney, J.M. The effects of mindful attention on cold pressor pain in children. Pain Res. Manag. 2013, 18, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Chen, E.; Zeltzer, L.K.; Craske, M.G.; Katz, E.R. Alteration of memory in the reduction of children’s distress during repeated aversive medical procedures. J. Consult. Clin. Psychol. 1999, 67, 481–490. [Google Scholar] [CrossRef]
- Von Baeyer, C.L.; Piira, T.; Chambers, C.T.; Trapanotto, M.; Zeltzer, L.K. Guidelines for the cold pressor task as an experimental pain stimulus for use with children. J. Pain 2005, 6, 218–227. [Google Scholar] [CrossRef]
- Chapman, C.R.; Gavrin, J. Suffering: The contributions of persistent pain. Lancet 1999, 353, 2233–2237. [Google Scholar] [CrossRef]
- Vervoort, T.; Trost, Z. Examining affective-motivational dynamics and behavioral implications within the interpersonal context of pain. J. Pain 2017, 18, 1174–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racine, N.M.; Pillai Riddell, R.R.; Flora, D.B.; Taddio, A.; Garfield, H.; Greenberg, S. Predicting preschool pain-related anticipatory distress: The relative contribution of longitudinal and concurrent factors. Pain 2016, 157, 1918–1932. [Google Scholar] [CrossRef] [PubMed]
| Reason 1 | Time constraints of acute pain ward rounds. |
| Reason 2 | Misperception that the sensory dimension of pain is the most bothersome aspect of pain. |
| Reason 3 | Misperception that the sensory dimension of pain is primary and the affective and cognitive dimensions occurs as a reaction to the sensory experience. |
| Reason 4 | Healthcare professionals may feel better equipped to manage the sensory dimension of pain and may avoid assessing other dimensions. |
| Reason 5 | Unintended legacy of the ‘5th Vital Sign campaign’, whereby nurses who were trained and mandated to include a pain intensity score with their routine observations may feel that this is sufficient. |
| Reason 6 | Inadequate availability of validated tools to assess other dimensions of pain in children, especially young children. |
| Reason 7 | Lack of clarity of what is meant by affect (e.g., unpleasantness, distress, fear, disgust etc.), consequently making it difficult to assess the affective dimensions of pain. |
| Reason 8 | Healthcare professionals may perceive existing tools to assess pain as clinically ineffectual, conceptually incomplete or administratively too complex. |
| Reason 9 | Belief by health professionals that children lack the cognitive maturity to self-report more than a single dimension of the pain experience. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaaniste, T.; Noel, M.; Yee, R.D.; Bang, J.; Tan, A.C.; Champion, G.D. Why Unidimensional Pain Measurement Prevails in the Pediatric Acute Pain Context and What Multidimensional Self-Report Methods Can Offer. Children 2019, 6, 132. https://doi.org/10.3390/children6120132
Jaaniste T, Noel M, Yee RD, Bang J, Tan AC, Champion GD. Why Unidimensional Pain Measurement Prevails in the Pediatric Acute Pain Context and What Multidimensional Self-Report Methods Can Offer. Children. 2019; 6(12):132. https://doi.org/10.3390/children6120132
Chicago/Turabian StyleJaaniste, Tiina, Melanie Noel, Renee D. Yee, Joseph Bang, Aidan Christopher Tan, and G. David Champion. 2019. "Why Unidimensional Pain Measurement Prevails in the Pediatric Acute Pain Context and What Multidimensional Self-Report Methods Can Offer" Children 6, no. 12: 132. https://doi.org/10.3390/children6120132





