Cognitive and Psychosocial Development in Young Children with Brain Tumors: Observations from a Clinical Sample †
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedures
2.2. Measures
2.3. Analytical Plan
3. Results
3.1. Participants
3.2. Developmental Functioning
3.3. Cognitive and Academic Functioning
3.4. Parent-Reported Adaptive and Psychosocial Functioning
3.5. Predictors of Functioning
3.6. Exploratory Analyses
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Mulhern, R.K.; Merchant, T.E.; Gajjar, A.; Reddick, W.E.; Kun, L.E. Late neurocognitive sequelae in survivors of brain tumors in childhood. Lancet Oncol. 2004, 5, 399–408. [Google Scholar] [CrossRef]
- Mulhern, R.K.; Palmer, S.L.; Reddick, W.E.; Glass, J.O.; Kun, L.E.; Taylor, J.; Langston, J.; Gajjar, A. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J. Clin. Oncol. 2001, 19, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Poggi, G.; Liscio, M.; Galbiati, S.; Adduci, A.; Massimino, M.; Gandola, L.; Spreafico, F.; Clerici, C.A.; Fossati-Bellani, F.; Sommovigo, M.; et al. Brain tumors in children and adolescents: Cognitive and psychological disorders at different ages. Psycho-Oncol. 2005, 14, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, J.E.; Gurney, J.G.; Palmer, S.L.; Bass, J.K.; Wang, M.; Chen, S.; Zhang, H.; Swain, M.; Chapieski, M.L.; Bonner, M.J.; et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro-Oncol. 2014, 16, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Reddick, W.E.; White, H.A.; Glass, J.O.; Wheeler, G.C.; Thompson, S.J.; Gajjar, A.; Leigh, L.; Mulhern, R.K. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer 2003, 97, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Morrall, M.C.H.J.; Reed-Berendt, R.; Moss, K.; Stocks, H.; Houston, A.L.; Siddell, P.; Picton, S.; Grundy, R. Neurocognitive, academic and functional outcomes in survivors of infant ependymoma (UKCCSG CNS 9204). Child’s Nerv. Syst. 2019, 35, 411–420. [Google Scholar]
- Heitzer, A.M.; Ashford, J.M.; Hastings, C.; Liu, A.P.Y.; Wu, S.; Bass, J.K.; Vestal, R.; Hoehn, M.; Chiang, J.; Ghazwani, Y.; et al. Neuropsychological outcomes of patients with low-grade glioma diagnosed during the first year of life. J. Neuro-Oncol. 2019, 141, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Fay-McClymont, T.B.; Ploetz, D.M.; Mabbott, D.; Walsh, K.; Smith, A.; Chi, S.N.; Wells, E.; Madden, J.; Margol, A.; Finlay, J.; et al. Long-term neuropsychological follow-up of young children with medulloblastoma treated with sequential high-dose chemotherapy and irradiation sparing approach. J. Neuro-Oncol. 2017, 133, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Mullen, E.M. Mullen Scales of Early Learning; American Guidance Service: Circle Pines, MN, USA, 1995. [Google Scholar]
- Bayley, N. Bayley Scales of Infant Development, 2nd ed.; The Psychological Corporation: San Antonio, TX, USA, 1993. [Google Scholar]
- Elliott, C.D. Differential Ability Scales, 2nd ed.; Pearson: San Antonio, TX, USA, 2007. [Google Scholar]
- Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence, 3rd ed.; Pearson: San Antonio, TX, USA, 2002. [Google Scholar]
- Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence, 4th ed.; Pearson: San Antonio, TX, USA, 2012. [Google Scholar]
- Woodcock, R.W.; McGrew, K.S.; Mather, N. Woodcock Johnson III NU Tests of Cognitive Abilities; Riverside Publishing: Rolling Meadows, IL, USA, 2007. [Google Scholar]
- Bracken, B.B. Receptive. In Bracken Basic Concept Scale, 3rd ed.; Pearson: San Antonio, TX, USA, 2006. [Google Scholar]
- Sparrow, S.S.; Cicchetti, D.V.; Balla, D.A. Vineland Adaptive Behavior Scales, 2nd ed.; Pearson: San Antonio, TX, USA, 2005. [Google Scholar]
- Harrison, P.; Oakland, T. Adaptive Behavior Assessment System, 2nd ed.; The Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA School-Age Forms & Profiles; University of Vermont, Research Center for Children, Youth & Families: Burlington, VT, USA, 2001. [Google Scholar]
- Reynolds, C.R.; Kamphaus, R.W. Behavior Assessment Scale for Children, 2nd ed.; AGS: Circle Pines, MN, USA, 2004. [Google Scholar]
- Fay, T.B.; Yeates, K.O.; Wade, S.L.; Drotar, D.; Stancin, T.; Taylor, H.G. Predicting longitudinal patterns of functional defiicts in children with traumatic brain injury. Neuropsychology 2009, 23, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Burgess, L.; Pulsifer, M.B.; Grieco, J.A.; Weinstein, E.R.; Gallotto, S.; Weyman, E.; MacDonald, S.M.; Tarbell, N.J.; Yeap, B.Y.; Yock, T.I. Estimated IQ systematically underestimates neurocognitive sequelae in irradiated pediatric brain tumor survivors. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Kahalley, L.S.; Winter-Greenberg, A.; Stancel, H.; Ris, M.D.; Gragert, M. Utility of the General Ability Index (GAI) and Cognitive Proficiency Index (CPI) with survivors of pediatric brain tumors: Comparison to Full Scale IQ and premorbid IQ estimates. J. Clin. Exp. Neuropsychol. 2016, 38, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Bonner, M.J.; Hardy, K.K.; Willard, V.W.; Gururangan, S. Additional evidence of a nonverbal learning disability in survivors of pediatric brain tumors. Child. Health Care 2009, 38, 49–63. [Google Scholar] [CrossRef]
- Trask, C.L.; Peterson, C.C. Educational issues: The impact of cancer in the classroom. In Pediatric Psychosocial Oncology: Textbook for Multidisciplinary Care; Abrams, A.N., Muriel, A.C., Wiener, L., Eds.; Springer: New York, NY, USA, 2016; pp. 175–198. [Google Scholar]
- Legal Information Institute. Individuals with Disabilities Education Act, 20 U.S.C. § 1400. Available online: https://sites.ed.gov/idea/statuteregulations/ (accessed on 1 May 2019).
- Harman, J.L.; Wise, J.; Willard, V.W. Early Intervention for infants and toddlers: Applications for pediatric oncology. Pediatr. Blood Cancer 2018, 65, e26921. [Google Scholar] [CrossRef] [PubMed]
- National Scientific Council on the Developing Child. Early Experiences can Alter Gene Expression and Affect Long-Term Development: Working Paper No. 10; National Scientific Council on the Developing Child: Cambridge, MA, USA, 2010. [Google Scholar]
- National Research Council and Institute of Medicine. From Neurons to Neighborhoods: The Science of Early Childhood Development; Shonkoff, J.P., Phillips, D.A., Eds.; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Annett, R.D.; Patel, S.K.; Phipps, S. Monitoring and assessment of neuropsychological outcomes as a standard of care in pediatric oncology. Pediatr. Blood Cancer 2015, 62, S460–S513. [Google Scholar] [CrossRef] [PubMed]
| N (%) | M ± SD, Range (Years) | ||
|---|---|---|---|
| Age | 4.52 ± 1.20, 1.48–5.98 | ||
| Gender | |||
| Male | 43 (54.4) | ||
| Female | 36 (45.6) | ||
| Race | |||
| White | 54 (68.4) | ||
| Black | 16 (20.3) | ||
| Other | 9 (11.4) | ||
| Common Diagnoses | |||
| Medulloblastoma | 13 (16.5) | ||
| Astrocytoma | 12 (15.2) | ||
| Ependymoma | 11 (13.9) | ||
| Optic Glioma | 8 (10.1) | ||
| Age at Diagnosis | 2.39 ± 1.46, 0.00–5.75 | ||
| Treatment Status | |||
| On | 20 (25.3) | ||
| Off | 59 (74.7) | ||
| Time off-treatment | 1.58 ± 1.28, 0.08–5.17 | ||
| Treatment | |||
| Surgery | 65 (82.3) | ||
| Chemotherapy | 56 (70.9) | ||
| Radiation Therapy | 48 (60.8) | ||
| Parameters | Focal | 28 (58.3) | |
| CSI + Focal | 20 (41.7) | ||
| Type | Proton | 9 (18.8) | |
| Photon | 39 (81.2) | ||
| Relapse/Progression | 14 (17.7) | ||
| Posterior Fossa Syndrome | 12 (15.2) | ||
| Hearing Loss | 22 (27.8) | ||
| Seizures | 19 (24.1) | ||
| Mean ± SD | Range | t | p | N (%) Impaired | |
|---|---|---|---|---|---|
| Age at Assessment | 2.29 ± 0.82 | 1.48–3.99 | |||
| Developmental Testing | |||||
| Composite a | 68.3 ± 10.02 | 55–85 | −10.01 | <0.001 | 9 (90.0) |
| Gross Motor b | 3.6 ± 2.56 | 1–7 | −7.04 | <0.001 | 6 (75.0) |
| Fine Motor b | 4.1 ± 3.69 | 1–11 | −5.05 | 0.001 | 7 (70.0) |
| Receptive Language b | 4.1 ± 3.07 | 1–11 | −6.08 | <0.001 | 8 (80.0) |
| Expressive Language b | 5.2 ± 1.87 | 3–8 | −8.10 | <0.001 | 5 (50.0) |
| N | Mean ± SD | Range | t | p | N (%) Impaired | |
|---|---|---|---|---|---|---|
| Age at Assessment | 67 | 4.88 ± 0.81 | 2.52–5.98 | |||
| Cognitive Functioning | ||||||
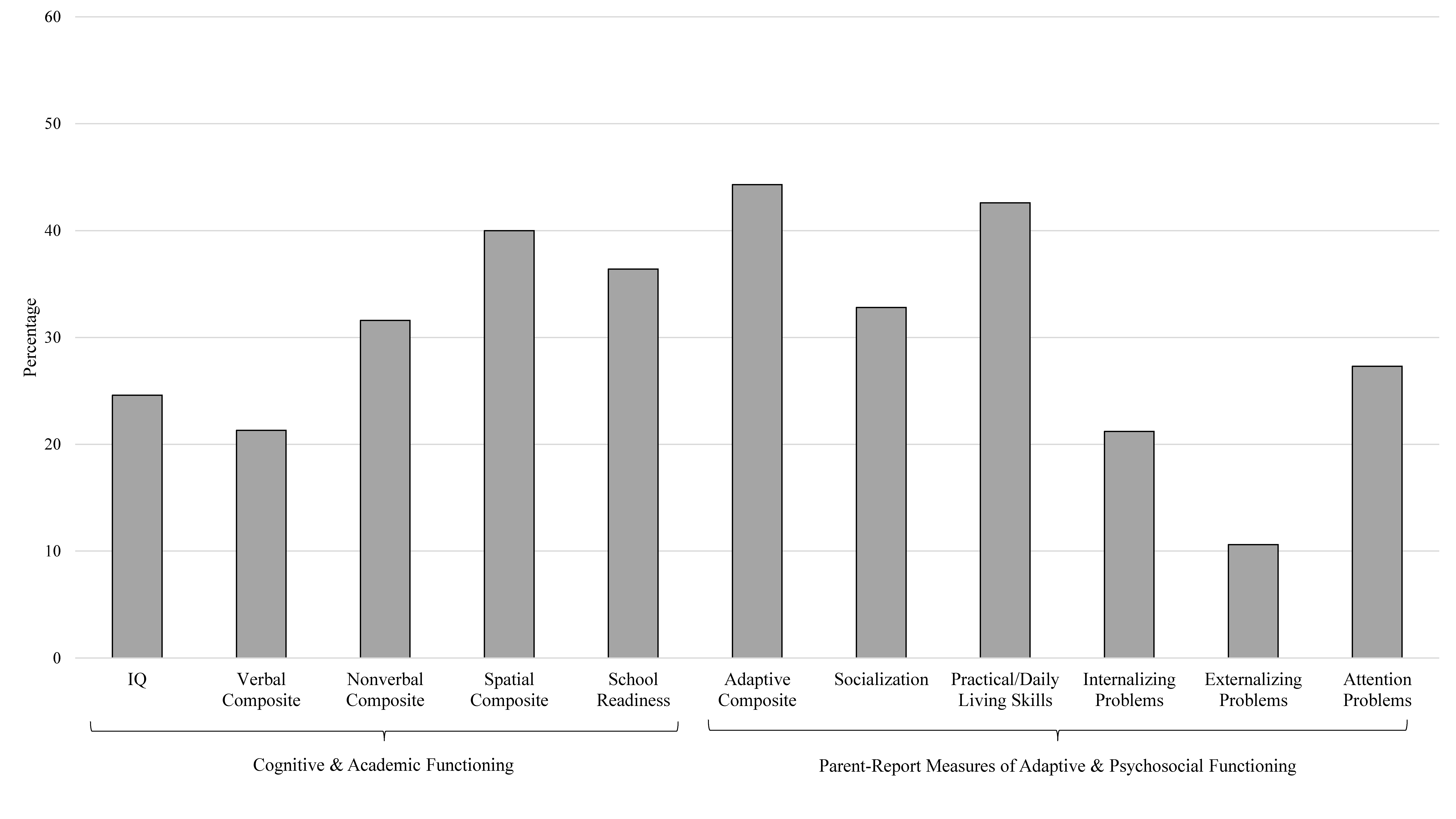
| FSIQ | 57 | 88.09 ± 18.38 | 43–139 | −4.89 | <0.001 | 14 (24.6) |
| Verbal Composite | 61 | 91.43 ± 16.45 | 53–134 | −4.07 | <0.001 | 13 (21.3) |
| Nonverbal Composite | 57 | 89.28 ± 18.87 | 45–140 | −4.29 | <0.001 | 18 (31.6) |
| Spatial Composite | 35 | 83.63 ± 20.33 | 42–121 | −4.76 | <0.001 | 14 (40.0) |
| Academic Functioning | ||||||
| School Readiness Composite | 55 | 86.84 ± 19.75 | 40–126 | −4.94 | <0.001 | 20 (36.4) |
| N | Mean ± SD | Range | t | p | N (%) Impaired | |
|---|---|---|---|---|---|---|
| Adaptive Functioning a | ||||||
| Composite | 70 | 82.10 ± 16.21 | 50–120 | −9.34 | <0.001 | 31 (44.3) |
| Socialization | 67 | 88.09 ± 17.14 | 49–124 | −5.69 | <0.001 | 22 (32.8) |
| Practical/Daily Living Skills | 68 | 82.69 ± 16.04 | 45–114 | −8.90 | <0.001 | 29 (42.6) |
| Psychosocial Functioning b | ||||||
| Internalizing Problems | 66 | 54.02 ± 11.09 | 33–84 | 2.94 | 0.005 | 14 (21.2) |
| Externalizing Problems | 66 | 50.67 ± 11.60 | 32–95 | 0.47 | 0.64 | 7 (10.6) |
| Attention Problems | 66 | 56.29 ± 10.24 | 34–75 | 4.99 | <0.001 | 18 (27.3) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurbergs, N.; Harman, J.L.; Kenney, A.E.; Semenkovich, K.; Molnar, A.E., Jr.; Willard, V.W. Cognitive and Psychosocial Development in Young Children with Brain Tumors: Observations from a Clinical Sample. Children 2019, 6, 128. https://doi.org/10.3390/children6110128
Jurbergs N, Harman JL, Kenney AE, Semenkovich K, Molnar AE Jr., Willard VW. Cognitive and Psychosocial Development in Young Children with Brain Tumors: Observations from a Clinical Sample. Children. 2019; 6(11):128. https://doi.org/10.3390/children6110128
Chicago/Turabian StyleJurbergs, Niki, Jennifer L. Harman, Ansley E. Kenney, Katherine Semenkovich, Andrew E. Molnar, Jr., and Victoria W. Willard. 2019. "Cognitive and Psychosocial Development in Young Children with Brain Tumors: Observations from a Clinical Sample" Children 6, no. 11: 128. https://doi.org/10.3390/children6110128
APA StyleJurbergs, N., Harman, J. L., Kenney, A. E., Semenkovich, K., Molnar, A. E., Jr., & Willard, V. W. (2019). Cognitive and Psychosocial Development in Young Children with Brain Tumors: Observations from a Clinical Sample. Children, 6(11), 128. https://doi.org/10.3390/children6110128






