Accuracy of a Wrist-Worn Heart Rate Sensing Device during Elective Pediatric Surgical Procedures
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethical Considerations
2.2. Statistical Analysis
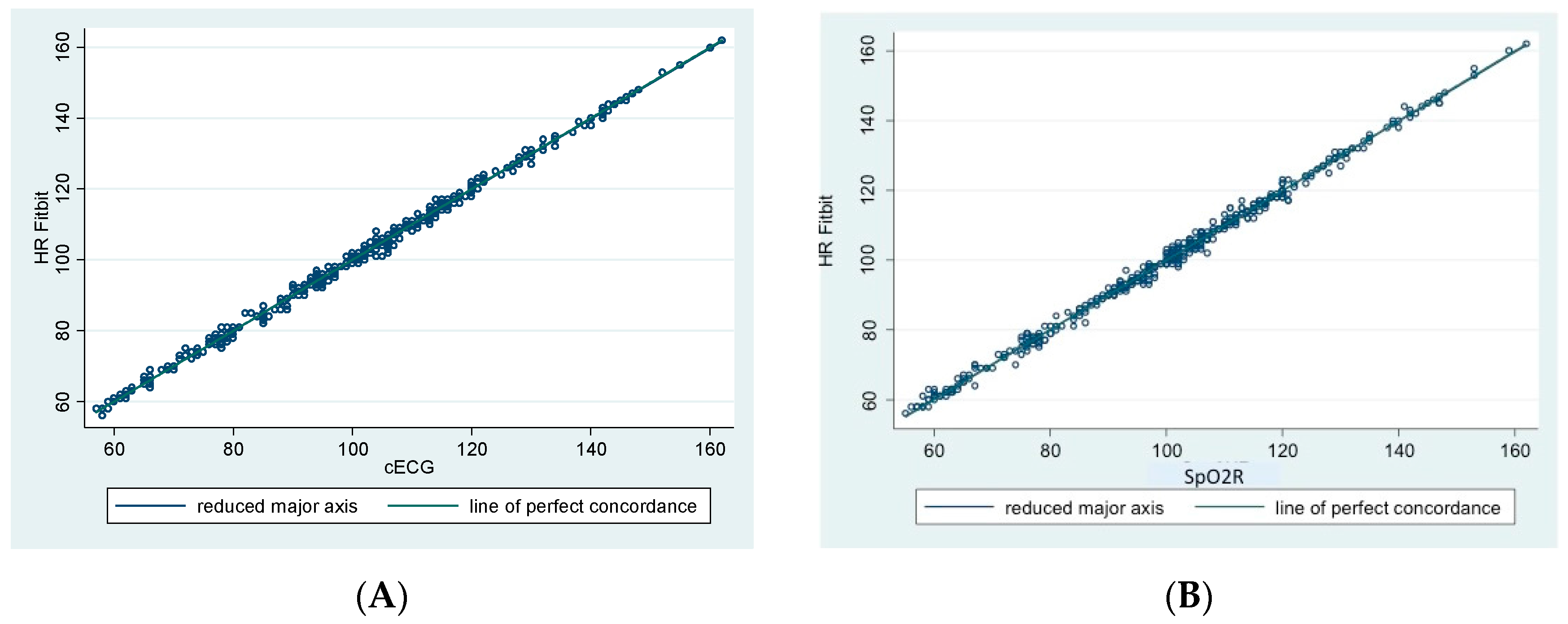
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kelli, H.M.; Witbrodt, B.; Shah, A. The future of mobile health applications and devices in cardiovascular health. Eur. Med. J. Innov. 2017, 2017, 92–97. [Google Scholar]
- Bietz, M.J.; Bloss, C.S.; Calvert, S.; Godino, J.G.; Gregory, J.; Claffey, M.P.; Sheenan, J.; Patrick, K. Opportunities and challenges in the use of personal health data for health research. J. Am. Med. Inform. Assoc. 2016, 23, e42–e48. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, T. Mining the quantified self: Personal knowledge discovery as a challenge for data science. Big Data 2015, 3, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Kostkova, P. Grand challenges in digital health. Front. Public Health 2015, 3, 134. [Google Scholar] [CrossRef] [PubMed]
- Kostkova, P.; Brewer, H.; de Lusignan, S.; Fottrell, E.; Goldacre, B.; Hart, G.; Koczan, P.; Knight, P.; Marsolier, C.; McKendry, R.A.; et al. Who owns the data? Open data for healthcare. Front. Public Health 2016, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.E.; An, H.S.; Dinkel, D.M.; Noble, J.M.; Lee, J.M. How accurate are the wrist-based heart rate monitors during walking and running activities? Are they accurate enough? BMJ Open Sport Exerc. Med. 2016, 2, e000106. [Google Scholar] [CrossRef] [PubMed]
- Spierer, D.K.; Rosen, Z.; Litman, L.L.; Fujii, K. Validation of photoplethysmography as a method to detect heart rate during rest and exercise. J. Med. Eng. Technol. 2015, 39, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Bonato, P. Clinical applications of wearable technology. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, Mi, USA, 3–6 September 2009; pp. 6580–6583. [Google Scholar]
- Chuang, C.; Ye, J.; Lin, W.; Lee, K.T.; Tai, Y.T. Photoplethysmography variability as an alternative approach to obtain heart rate variability information in chronic pain patient. J. Clin. Monit. Comput. 2015, 29, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C. Patient-generated health data: A pathway to enhanced long-term cancer survivorship. J. Am. Med. Inform. Assoc. 2015, 29, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Kroll, R.R.; Boyd, J.G.; Maslove, D.M. Accuracy of a wrist-worn wearable device for monitoring heart rates in hospital inpatients: A prospective observational study. J. Med. Internet Res. 2016, 18, e253. [Google Scholar] [CrossRef] [PubMed]
- Voss, C.; Gardner, R.F.; Dean, P.H.; Harris, K.C. Validity of commercial activity trackers in children with congenital heart disease. Can. J. Cardiol. 2017, 33, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Ridgers, N.D.; McNarry, M.A.; Mackintosh, K.A. Feasibility and effectiveness of using wearable activity trackers in youth: A systematic review. JMIR mHealth uHealth 2016, 4, e129. [Google Scholar] [CrossRef] [PubMed]
- Hooke, M.C.; Gilchrist, L.; Tanner, L.; Hart, N.; Withycombe, J.S. Use of a fitness tracker to promote physical activity in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2016, 63, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in patterns of pubertal changes in boys. Arch. Dis. Child. 1969, 45, 13–23. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in patterns of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of vital signs with flexible and wearable medical devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef] [PubMed]
- Iman, K.A.; Hassan, A.A. Integration of wearable technologies into patients’ electronic medical. Rec. Qual. Prim. Care 2016, 24, 151–155. [Google Scholar]
- Keytel, L.R.; Goedecke, J.H.; Noakes, T.D.; Hiiloskorpi, H.; Laukkanen, R.; van der Merwe, L.; Lambert, E.V. Prediction of energy expenditure from heart rate monitoring during submaximal exercise. J. Sport Sci. 2007, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Luke, A.; Maki, K.C.; Barkey, N.; Cooper, R.; McGee, D. Simultaneous monitoring of heart rate and motion to assess energy expenditure. Med. Sci. Sport Exerc. 1997, 29, 144–148. [Google Scholar] [CrossRef]
- Wallen, M.P.; Gomersall, S.R.; Keating, S.E.; Wisløff, U.; Coombes, J.S. Accuracy of heart rate watches: Implications for weight management. PLoS ONE 2016, 11, e0154420. [Google Scholar] [CrossRef] [PubMed]
- Benzo, R. Activity monitoring in chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. Prev. 2009, 29, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Allet, L.; Knols, R.H.; Shirato, K.; de Bruin, E.D. Wearable systems for monitoring mobility-related activities in chronic disease: A systematic review. Sensors 2010, 10, 9026–9052. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Thompson, J.E.; Prinsen, S.K.; Dearani, J.A.; Deschamps, C. Functional recovery in the elderly after major surgery: Assessment of mobility recovery using wireless technology. Ann. Thorac. Surg. 2013, 96, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Chiauzzi, E.; Rodarte, C.; DasMahapatra, P. Patient-centered activity monitoring in the self-management of chronic health conditions. BMC Med. 2015, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Haegele, J.A.; Brian, A.S.; Wolf, D. Accuracy of the Fitbit Zip for measuring steps for adolescents with visual impairments. Adapt. Phys. Activ. Q. 2017, 34, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Stevens, F.; Conditt, M.A.; Kulkarni, N.; Ismaily, S.K.; Noble, P.C.; Lionberger, D.R. Minimizing electromagnetic interference from surgical instruments on electromagnetic surgical navigation. Clin. Orthop. Relat. Res. 2010, 468, 2244–2250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, M.E.; Chiovaro, J.C.; O’Neil, M.; Kansagara, D.; Quiñones, A.R.; Freeman, M.; Motu’apuaka, M.L.; Slatore, C.G. Early warning system scores for clinical deterioration in hospitalized patients: A systematic review. Ann. Am. Thorac. Soc. 2014, 11, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Yuen, T.C.; Park, S.Y.; Meltzer, D.O.; Hall, J.B.; Edelson, D.P. Derivation of a cardiac arrest prediction model using ward vital signs. Crit. Care Med. 2012, 40, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Yuen, T.C.; Winslow, C.; Meltzer, D.O.; Kattan, M.W.; Edelson, D.P. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit. Care Med. 2016, 44, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Yuen, T.C.; Winslow, C.; Hall, J.; Edelson, D.P. Differences in vital signs between elderly and nonelderly patients prior to ward cardiac arrest. Crit. Care Med. 2015, 43, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Yuen, T.C.; Park, S.Y.; Gibbons, R.; Edelson, D.P. Using electronic health record data to develop and validate a prediction model for adverse outcomes in the wards. Crit. Care Med. 2014, 42, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Appelboom, G.; Yang, A.H.; Christophe, B.R.; Bruce, E.M.; Slomian, J.; Bruyère, O.; Bruce, S.S.; Zacharia, B.E.; Reginster, J.Y.; Conolly, E.S., Jr. The promise of wearable activity sensors to define patient recovery. J. Clin. Neurosci. 2014, 21, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
| Total (N = 30) | Laparoscopy Group (n = 8) | Open Surgery Group (n = 22) | |
|---|---|---|---|
| Sex (Male/Female) | 16/14 | 3/5 | 13/9 |
| Age (years) | 8.21 ± 3.09 | 9.8 ± 3.3 | 7.6 ± 2.7 |
| >8 years | 14 | 4 | 10 |
| <8 years | 16 | 4 | 12 |
| Indication for surgery | |||
| Abdominal-inguinal pathology | 18 | 6 | 12 |
| Gynecological ovarian mass | 2 | 2 | 0 |
| Excision of cutaneous–subcutaneous lesions | 10 | 0 | 10 |
| Weight (kg) | 31.3 ± 15.3 | 43.0 ± 20.1 | 27.0 ± 10.2 |
| <30 kg (n) | 16 | 3 | 13 |
| >30 kg (n) | 14 | 5 | 9 |
| Height (cm) | 132.2 ± 23.3 | 146.0 ± 16.5 | 118.5 ± 20.9 |
| Body Mass Index (kg/m2) | 20.5 ± 5.0 | 23.6 ± 5.2 | 17.0 ± 0.04 |
| Pubertal Stage | |||
| Tanner stage 1 (n) | 22 | 4 | 18 |
| Tanner stage 2–3 (n) | 3 | 0 | 3 |
| Tanner stage 4–5 (n) | 5 | 4 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelizzo, G.; Guddo, A.; Puglisi, A.; De Silvestri, A.; Comparato, C.; Valenza, M.; Bordonaro, E.; Calcaterra, V. Accuracy of a Wrist-Worn Heart Rate Sensing Device during Elective Pediatric Surgical Procedures. Children 2018, 5, 38. https://doi.org/10.3390/children5030038
Pelizzo G, Guddo A, Puglisi A, De Silvestri A, Comparato C, Valenza M, Bordonaro E, Calcaterra V. Accuracy of a Wrist-Worn Heart Rate Sensing Device during Elective Pediatric Surgical Procedures. Children. 2018; 5(3):38. https://doi.org/10.3390/children5030038
Chicago/Turabian StylePelizzo, Gloria, Anna Guddo, Aurora Puglisi, Annalisa De Silvestri, Calogero Comparato, Mario Valenza, Emanuele Bordonaro, and Valeria Calcaterra. 2018. "Accuracy of a Wrist-Worn Heart Rate Sensing Device during Elective Pediatric Surgical Procedures" Children 5, no. 3: 38. https://doi.org/10.3390/children5030038
APA StylePelizzo, G., Guddo, A., Puglisi, A., De Silvestri, A., Comparato, C., Valenza, M., Bordonaro, E., & Calcaterra, V. (2018). Accuracy of a Wrist-Worn Heart Rate Sensing Device during Elective Pediatric Surgical Procedures. Children, 5(3), 38. https://doi.org/10.3390/children5030038





