Gut Microbiota Composition and Sleep in Preschoolers: The ELFE Birth Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. ELFE Birth Cohort Study
2.2. Maternal and Child Data Collection
2.3. Sleep Characteristics
2.4. Gut Microbiota Analysis
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Study Population Description
3.2. Sleep Clusters Characterization
3.3. Gut Microbiota Diversity, Enterotypes, and Sleep Clusters
3.4. Overall Gut Microbiota Composition and Differential Abundances Testing
3.5. Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALDEx2 | Analysis of Differential Abundance Taking Sample Variation into Account |
| ANCOM-BC | Analysis of Compositions of Microbiomes with Bias Correction |
| BMI | Body Mass Index |
| DAG | Directed Acyclic Graph |
| DMM | Dirichlet Multinomial Mixtures |
| DOHaD | Developmental Origins of Health and Disease |
| ELFE | Étude Longitudinale Française depuis l’Enfance |
| FDR | False Discovery Rate |
| LCA | Latent Class Analysis |
| NRMSE | Normalized Root Mean Squared Error |
| OTU | Operational Taxonomic Unit |
| PAM | Partitioning Around Medoids |
| PCA | Principal Component Analysis |
| PCoA | Principal Coordinate Analysis |
| PERMANOVA | Permutational Multivariate Analysis of Variance |
| PFC | Proportion of Fractional Change |
References
- Licis, A. Sleep Disorders: Assessment and Treatment in Preschool-Aged Children. Child Adolesc. Psychiatr. Clin. N. Am. 2017, 26, 587–595. [Google Scholar] [CrossRef]
- Williams, J.A.; Zimmerman, F.J.; Bell, J.F. Norms and Trends of Sleep Time among Us Children and Adolescents. JAMA Pediatr. 2013, 167, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bathory, E.; Tomopoulos, S. Sleep Regulation, Physiology and Development, Sleep Duration and Patterns, and Sleep Hygiene in Infants, Toddlers, and Preschool-Age Children. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Bruni, O. Sleep disorders in children. BMJ Clin. Evid. 2010, 2010, 2304. [Google Scholar] [PubMed]
- Gruber, R.; Michaelsen, S.; Bergmame, L.; Frenette, S.; Bruni, O.; Fontil, L.; Carrier, J. Short Sleep Duration Is Associated with Teacher-Reported Inattention and Cognitive Problems in Healthy School-Aged Children. Nat. Sci. Sleep 2012, 4, 33–40. [Google Scholar] [CrossRef]
- O’Callaghan, F.V.; Al Mamun, A.; O’Callaghan, M.; Clavarino, A.; Williams, G.M.; Bor, W.; Heussler, H.; Najman, J.M. The Link between Sleep Problems in Infancy and Early Childhood and Attention Problems at 5 and 14 Years: Evidence from a Birth Cohort Study. Early Hum. Dev. 2010, 86, 419–424. [Google Scholar] [CrossRef]
- Reynaud, E.; Vecchierini, M.F.; Heude, B.; Charles, M.A.; Plancoulaine, S. Sleep and Its Relation to Cognition and Behaviour in Preschool-Aged Children of the General Population: A Systematic Review. J. Sleep Res. 2018, 27, e12636. [Google Scholar] [CrossRef]
- Miller, M.A.; Kruisbrink, M.; Wallace, J.; Ji, C.; Cappuccio, F.P. Sleep Duration and Incidence of Obesity in Infants, Children, and Adolescents: A Systematic Review and Meta-Analysis of Prospective Studies. Sleep 2018, 41, zsy018. [Google Scholar] [CrossRef]
- Blair, P.S.; Humphreys, J.S.; Gringras, P.; Taheri, S.; Scott, N.; Emond, A.; Henderson, J.; Fleming, P.J. Childhood Sleep Duration and Associated Demographic Characteristics in an English Cohort. Sleep 2012, 35, 353–360. [Google Scholar] [CrossRef]
- Iglowstein, I.; Jenni, O.G.; Molinari, L.; Largo, R.H. Sleep Duration from Infancy to Adolescence: Reference Values and Generational Trends. Pediatrics 2003, 111, 302–307. [Google Scholar] [CrossRef]
- Camfferman, D.; Kennedy, J.D.; Gold, M.; Martin, A.J.; Winwood, P.; Lushington, K. Eczema, Sleep, and Behavior in Children. J. Clin. Sleep Med. 2010, 6, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Plancoulaine, S.; Reynaud, E.; Forhan, A.; Lioret, S.; Heude, B.; Charles, M.A.; Annesi-Maesano, I.; Bernard, J.; Botton, J.; Dargent-Molina, P.; et al. Night Sleep Duration Trajectories and Associated Factors among Preschool Children from the Eden Cohort. Sleep Med. 2018, 48, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, S.; Giannotti, F.; Cortesi, F.; Bruni, O.; Ottaviano, C. Sleep Characteristics in Healthy Children from Birth to 6 Years of Age in the Urban Area of Rome. Sleep 1996, 19, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Messayke, S.; Franco, P.; Forhan, A.; Dufourg, M.N.; Charles, M.A.; Plancoulaine, S. Sleep Habits and Sleep Characteristics at Age One Year in the Elfe Birth Cohort Study. Sleep Med. 2020, 67, 200–206. [Google Scholar] [CrossRef]
- Kim, M.; Saade, D.; Dufourg, M.N.; Charles, M.A.; Plancoulaine, S. Longitudinal Sleep Multi-Trajectories from Age 1 to 5.5 Years and Their Early Correlates: Results from the Etude Longitudinale Francaise Depuis L’enfance Birth Cohort Study. Sleep 2023, 46, zsad236. [Google Scholar] [CrossRef]
- Al Mamun, A.; Lawlor, D.A.; Cramb, S.; O’Callaghan, M.; Williams, G.; Najman, J. Do Childhood Sleeping Problems Predict Obesity in Young Adulthood? Evidence from a Prospective Birth Cohort Study. Am. J. Epidemiol. 2007, 166, 1368–1373. [Google Scholar] [CrossRef]
- Molnar, Z. Thomas Willis (1621–1675), the Founder of Clinical Neuroscience. Nat. Rev. Neurosci. 2004, 5, 329–335. [Google Scholar] [CrossRef]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut Microbiome Diversity Is Associated with Sleep Physiology in Humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, B.; Zhou, S.; Huang, Z.; Xu, Y.; Lu, X.; Zheng, X.; Ouyang, D. Associations between Gut Microbiota and Sleep: A Two-Sample, Bidirectional Mendelian Randomization Study. Front. Microbiol. 2023, 14, 1236847. [Google Scholar] [CrossRef]
- Zimmermann-Rosner, A.; Prehn-Kristensen, A. The Microbiome in Child and Adolescent Psychiatry. Z. Kinder Jugendpsychiatr. Psychother. 2024, 52, 213–226. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Liu, X. The Microbiota-Gut-Brain Axis and Neurodevelopmental Disorders. Protein Cell 2023, 14, 762–775. [Google Scholar] [CrossRef]
- Ringel-Kulka, T.; Cheng, J.; Ringel, Y.; Salojarvi, J.; Carroll, I.; Palva, A.; de Vos, W.M.; Satokari, R. Intestinal Microbiota in Healthy U.S. Young Children and Adults—A High Throughput Microarray Analysis. PLoS ONE 2013, 8, e64315. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Alvarez, A.S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A.; et al. Temporal Development of the Gut Microbiome in Early Childhood from the teddy Study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Virta, L.J.; Kekkonen, R.A.; de Vos, W.M. Association of Early-Life Antibiotic Use and Protective Effects of Breastfeeding: Role of the Intestinal Microbiota. JAMA Pediatr. 2016, 170, 750–757. [Google Scholar] [CrossRef]
- Zhong, H.; Penders, J.; Shi, Z.; Ren, H.; Cai, K.; Fang, C.; Ding, Q.; Thijs, C.; Blaak, E.E.; Stehouwer, C.D.A.; et al. Impact of Early Events and Lifestyle on the Gut Microbiota and Metabolic Phenotypes in Young School-Age Children. Microbiome 2019, 7, 2. [Google Scholar] [CrossRef]
- Suzuki, K. The Developing World of Dohad. J. Dev. Orig. Health Dis. 2018, 9, 266–269. [Google Scholar] [CrossRef]
- Butel, M.J.; Waligora-Dupriet, A.J.; Wydau-Dematteis, S. The Developing Gut Microbiota and Its Consequences for Health. J. Dev. Orig. Health Dis. 2018, 9, 590–597. [Google Scholar] [CrossRef]
- Wang, Y.; van de Wouw, M.; Drogos, L.; Vaghef-Mehrabani, E.; Reimer, R.A.; Tomfohr-Madsen, L.; Giesbrecht, G.F. Sleep and the Gut Microbiota in Preschool-Aged Children. Sleep 2022, 45, zsac020. [Google Scholar] [CrossRef]
- Charles, M.A.; Thierry, X.; Lanoe, J.L.; Bois, C.; Dufourg, M.N.; Popa, R.; Cheminat, M.; Zaros, C.; Geay, B. Cohort Profile: The French National Cohort of Children (Elfe): Birth to 5 Years. Int. J. Epidemiol. 2020, 49, 368–369j. [Google Scholar] [CrossRef]
- Vandentorren, S.; Bois, C.; Pirus, C.; Sarter, H.; Salines, G.; Leridon, H. Rationales, Design and Recruitment for the Elfe Longitudinal Study. BMC Pediatr. 2009, 9, 58. [Google Scholar] [CrossRef]
- Toubon, G.; Butel, M.J.; Roze, J.C.; Nicolis, I.; Delannoy, J.; Zaros, C.; Ancel, P.Y.; Aires, J.; Charles, M.A. Early Life Factors Influencing Children Gut Microbiota at 3.5 Years from Two French Birth Cohorts. Microorganisms 2023, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Methé, B.A.; Nelson, K.E.; Pop, M.; Creasy, H.H.; Giglio, M.G.; Huttenhower, C.; Gevers, D.; Petrosino, J.F.; Abubucker, S.; Mannon, P.J.; et al. A Framework for Human Microbiome Research. Nature 2012, 486, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Tennant, P.W.G.; Murray, E.J.; Arnold, K.F.; Berrie, L.; Fox, M.P.; Gadd, S.C.; Harrison, W.J.; Keeble, C.; Ranker, L.R.; Textor, J.; et al. Use of Directed Acyclic Graphs (Dags) to Identify Confounders in Applied Health Research: Review and Recommendations. Int. J. Epidemiol. 2021, 50, 620–632. [Google Scholar] [CrossRef]
- McCutcheon, A. Latent Class Analysis, 7th ed.; SAGE Publication Inc.: California, CA, USA, 1987. [Google Scholar]
- Fernandes, A.D.; Reid, J.N.; Macklaim, J.M.; McMurrough, T.A.; Edgell, D.R.; Gloor, G.B. Unifying the Analysis of High-Throughput Sequencing Datasets: Characterizing Rna-Seq, 16s Rrna Gene Sequencing and Selective Growth Experiments by Compositional Data Analysis. Microbiome 2014, 2, 15. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Analysis of Compositions of Microbiomes with Bias Correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Stekhoven, D.J.; Buhlmann, P. Missforest–Non-Parametric Missing Value Imputation for Mixed-Type Data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef]
- Mendez-Salazar, E.O.; Ortiz-Lopez, M.G.; Granados-Silvestre, M.L.A.; Palacios-Gonzalez, B.; Menjivar, M. Altered Gut Microbiota and Compositional Changes in Firmicutes and Proteobacteria in Mexican Undernourished and Obese Children. Front. Microbiol. 2018, 9, 2494. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, J.; Zheng, J.; Li, X.; Zhao, F. Deterministic Transition of Enterotypes Shapes the Infant Gut Microbiome at an Early Age. Genome Biol. 2021, 22, 243. [Google Scholar] [CrossRef]
- Matenchuk, B. Sleep Duration and the Gut Microbiota in Infancy: An Exploration of the Determinants of Sleep and the Association between Sleep and the Gut Microbiota at 3 Months of Age in a Canadian Birth Cohort. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2019. [Google Scholar]
- Schoch, S.F.; Castro-Mejia, J.L.; Krych, L.; Leng, B.; Kot, W.; Kohler, M.; Huber, R.; Rogler, G.; Biedermann, L.; Walser, J.C.; et al. From Alpha Diversity to Zzz: Interactions among Sleep, the Brain, and Gut Microbiota in the First Year of Life. Prog. Neurobiol. 2022, 209, 102208. [Google Scholar] [CrossRef]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schurmann, A.; Cedernaes, J. Gut Microbiota and Glucometabolic Alterations in Response to Recurrent Partial Sleep Deprivation in Normal-Weight Young Individuals. Mol. Metab. 2016, 5, 1175–1186. [Google Scholar] [CrossRef]
- Karl, J.P.; Whitney, C.C.; Wilson, M.A.; Fagnant, H.S.; Radcliffe, P.N.; Chakraborty, N.; Campbell, R.; Hoke, A.; Gautam, A.; Hammamieh, R.; et al. Severe, Short-Term Sleep Restriction Reduces Gut Microbiota Community Richness but Does Not Alter Intestinal Permeability in Healthy Young Men. Sci. Rep. 2023, 13, 213. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Riemann, B.L.; Flatt, A.A.; Valentino, T.; Lustgarten, M.S. Self-Reported Sleep Quality is Associated with Gut Microbiome Composition in Young, Healthy Individuals: A Pilot Study. Sleep Med. 2020, 73, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; So-Ngern, A.; Chirakalwasan, N.; Saetung, S.; Chanprasertyothin, S.; Thakkinstian, A.; Chlipala, G.E. No Changes in Gut Microbiota after Two-Week Sleep Extension in Chronically Sleep-Deprived Individuals. Sleep Med. 2020, 68, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.L.; Douglas, P.S.; Kulasinghe, K.; Whittingham, K.; Hill, P. The Possums Infant Sleep Program: Parents’ Perspectives on a Novel Parent-Infant Sleep Intervention in Australia. Sleep Health 2018, 4, 519–526. [Google Scholar] [CrossRef] [PubMed]
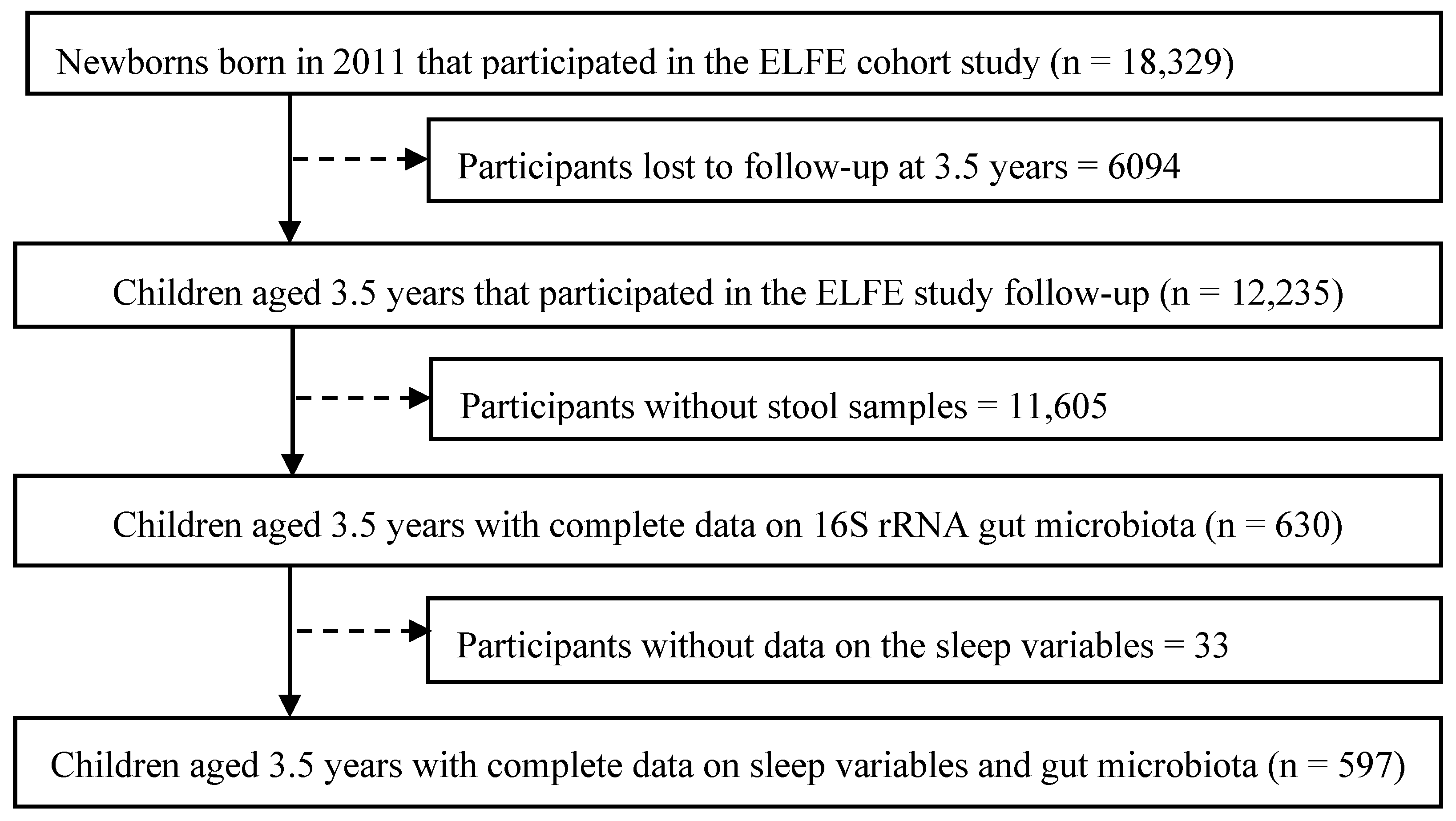
| Suboptimal Sleep (n = 150) | Optimal Sleep (n = 447) | Overall (n = 597) | p-Value | |
|---|---|---|---|---|
| Sleep Characteristics | ||||
| n (%) | n (%) | n (%) | ||
| Day sleep duration § | 0.85 | |||
| ≤1 h 30 | 82 (54.7) | 240 (53.7) | 322 (53.9) | |
| >1 h 30 | 68 (45.3) | 207 (46.3) | 275 (46.1) | |
| Night sleep duration § | <0.001 | |||
| ≤10 h 49 | 119 (79.3) | 185 (41.4) | 304 (50.9) | |
| >10 h 49 | 31 (20.7) | 262 (58.6) | 293 (49.1) | |
| Sleep onset difficulty | <0.001 | |||
| No | 52 (34.7) | 399 (89.3) | 451 (75.5) | |
| Yes | 98 (65.3) | 48 (10.7) | 146 (24.5) | |
| Night waking | <0.001 | |||
| No | 62 (41.3%) | 447 (100%) | 509 (85.3%) | |
| Yes | 88 (58.7%) | 0 (0%) | 88 (14.7%) | |
| Maternal Characteristics | ||||
| n (%) | n (%) | n (%) | ||
| Born in France | 139 (92.7) | 417 (93.3) | 556 (93.1) | 0.85 |
| Education at 2 months | 0.90 | |||
| ≤Secondary level | 33 (22.0) | 106 (23.7) | 139 (23.3) | |
| ≤Baccalaureat +2 | 39 (26.0) | 118 (26.4) | 157 (26.3) | |
| >Baccalaureat +2 | 78 (52.0) | 223 (49.9) | 301 (50.4) | |
| Vaginal child delivery | 127 (84.7) | 362 (81.0) | 489 (81.9) | 0.31 |
| Exposure to psychotropic medications during pregnancy | 6 (4.0) | 10 (2.2) | 16 (2.7) | 0.25 |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Maternal age (years) | 31.7 (4.18) | 31.8 (4.27) | 31.8 (4.25) | 0.78 |
| Pre-pregnancy BMI (Kg/m2) | 23.2 (4.58) | 23.4 (4.71) | 23.3 (4.65) | 0.76 |
| Breastfeeding duration (months) | 4.81 (6.20) | 3.87 (5.04) | 4.10 (5.33) | 0.06 |
| Household Characteristics | ||||
| n (%) | n (%) | n (%) | ||
| Income at 2 months (€/month/CPU) | 0.84 | |||
| ≤1500 | 51 (34.0) | 159 (35.6) | 210 (35.2) | |
| 1500–1944 | 49 (32.7) | 151 (33.8) | 200 (33.5) | |
| >1944 | 50 (33.3) | 137 (30.6) | 187 (31.3) | |
| Pet ownership at 2 months | 64 (42.7) | 242 (54.1) | 306 (51.3) | 0.02 |
| Residential setting at 3.5 years | 0.04 | |||
| Rural | 43 (28.7) | 130 (29.1) | 173 (29.0) | |
| Suburban | 42 (28.0) | 169 (37.8) | 211 (35.3) | |
| Urban | 65 (43.3) | 148 (33.1) | 213 (35.7) | |
| Child Characteristics | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Gestational age (weeks) | 39.5 (1.57) | 39.6 (1.27) | 0.0538 (0.918) | 0.31 |
| BMI Z-score at 2 years | 0.10 (1.18) | 0.04 (1.01) | 0.05 (0.91) | 0.56 |
| Diet at 2 years | ||||
| Unhealthy dietary pattern | 0.04 (1.62) | −0.01 (1.55) | 0.00 (1.56) | 0.70 |
| Healthy dietary pattern | −0.13 (1.52) | 0.04 (1.39) | 0.00 (1.42) | 0.23 |
| Age at 3 years (months) | 42.3 (1.78) | 42.3 (1.71) | 42.3 (1.73) | 0.96 |
| n (%) | n (%) | n (%) | ||
| Sex; girl | 50 (33.3) | 208 (46.5) | 258 (43.2) | <0.01 |
| Children with sibling | 75 (50.0) | 250 (55.9) | 325 (54.4) | 0.22 |
| Main mode of care at 2 years | 0.07 | |||
| Family | 38 (25.3) | 120 (26.8) | 158 (26.5) | |
| Child sitter | 76 (50.7) | 254 (56.8) | 330 (55.3) | |
| Collective care | 36 (24.0) | 73 (16.3) | 109 (18.3) | |
| Tobacco exposure from pregnancy up to 3.5 years | 53 (35.3) | 159 (35.6) | 212 (35.5) | 1.00 |
| Antibiotics intake between 2 and 3.5 years | 0.65 | |||
| Never | 47 (31.3) | 152 (34.0) | 199 (33.3) | |
| Once | 36 (24.0) | 92 (20.6) | 128 (21.4) | |
| More than once | 67 (44.7) | 203 (45.4) | 270 (45.2) | |
| Crude | Adjusted § | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Alpha diversity metrics | ||||||
| Chao1 estimate † | 0.98 | 0.81, 1.18 | 0.80 | 0.95 | 0.78, 1.17 | 0.64 |
| Shannon index † | 1.01 | 0.84, 1.22 | 0.90 | 0.98 | 0.80, 1.20 | 0.84 |
| Microbiota Enterotypes | ||||||
| B_type ‡ | — | — | 0.88 | — | — | 0.96 |
| P_type | 1.04 | 0.64–1.67 | 0.99 | 0.59, 1.62 | ||
| Crude | Adjusted † | |||||
|---|---|---|---|---|---|---|
| R2 | p-Value | FDR p-Value | R2 | p-Value | FDR p-Value | |
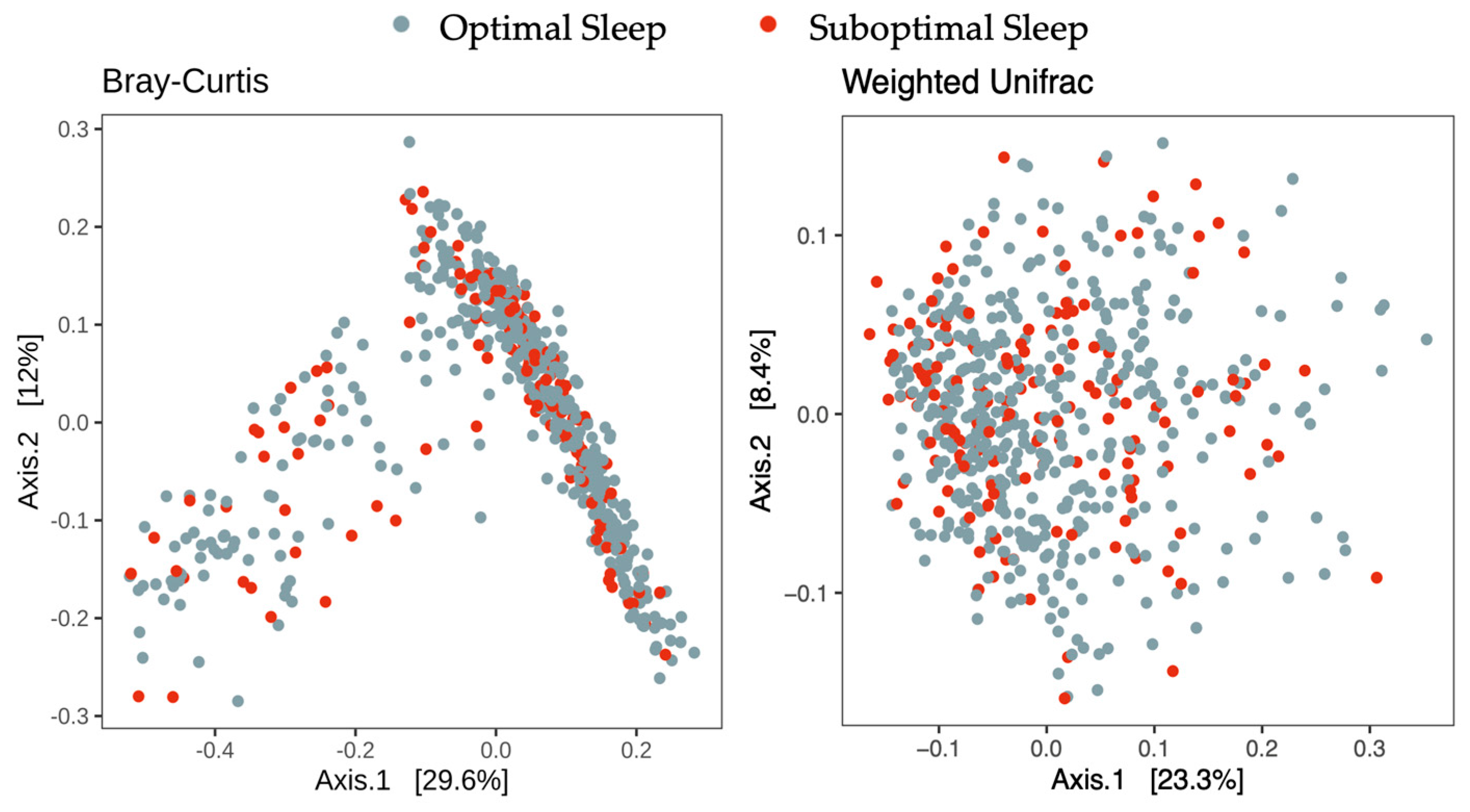
| Beta Diversity distances | ||||||
| Bray–Curtis | 0.0014 | 0.53 | 0.53 | 0.0013 | 0.62 | 0.71 |
| Weighted UniFrac | 0.0024 | 0.19 | 0.19 | 0.0020 | 0.34 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houshialsadat, Z.; Zaros, C.; Butel, M.-J.; Charles, M.-A.; Toubon, G.; Plancoulaine, S. Gut Microbiota Composition and Sleep in Preschoolers: The ELFE Birth Cohort Study. Children 2025, 12, 1240. https://doi.org/10.3390/children12091240
Houshialsadat Z, Zaros C, Butel M-J, Charles M-A, Toubon G, Plancoulaine S. Gut Microbiota Composition and Sleep in Preschoolers: The ELFE Birth Cohort Study. Children. 2025; 12(9):1240. https://doi.org/10.3390/children12091240
Chicago/Turabian StyleHoushialsadat, Zeinab, Cécile Zaros, Marie-José Butel, Marie-Aline Charles, Gaël Toubon, and Sabine Plancoulaine. 2025. "Gut Microbiota Composition and Sleep in Preschoolers: The ELFE Birth Cohort Study" Children 12, no. 9: 1240. https://doi.org/10.3390/children12091240
APA StyleHoushialsadat, Z., Zaros, C., Butel, M.-J., Charles, M.-A., Toubon, G., & Plancoulaine, S. (2025). Gut Microbiota Composition and Sleep in Preschoolers: The ELFE Birth Cohort Study. Children, 12(9), 1240. https://doi.org/10.3390/children12091240





