Changes in Gut Microbial Diversity and Correlation with Clinical Outcome in Children with Acute Myeloid Leukemia Receiving Induction Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design
2.3. Clinical Definitions
2.4. Sample Collection
2.5. 16S rRNA Amplicon Sequencing
2.6. Bioinformatics Analysis
2.6.1. Amplicon Processing (16S rRNA)
2.6.2. Definitions
2.7. Statistical Analysis
3. Results
3.1. Patient Clinical Characteristics
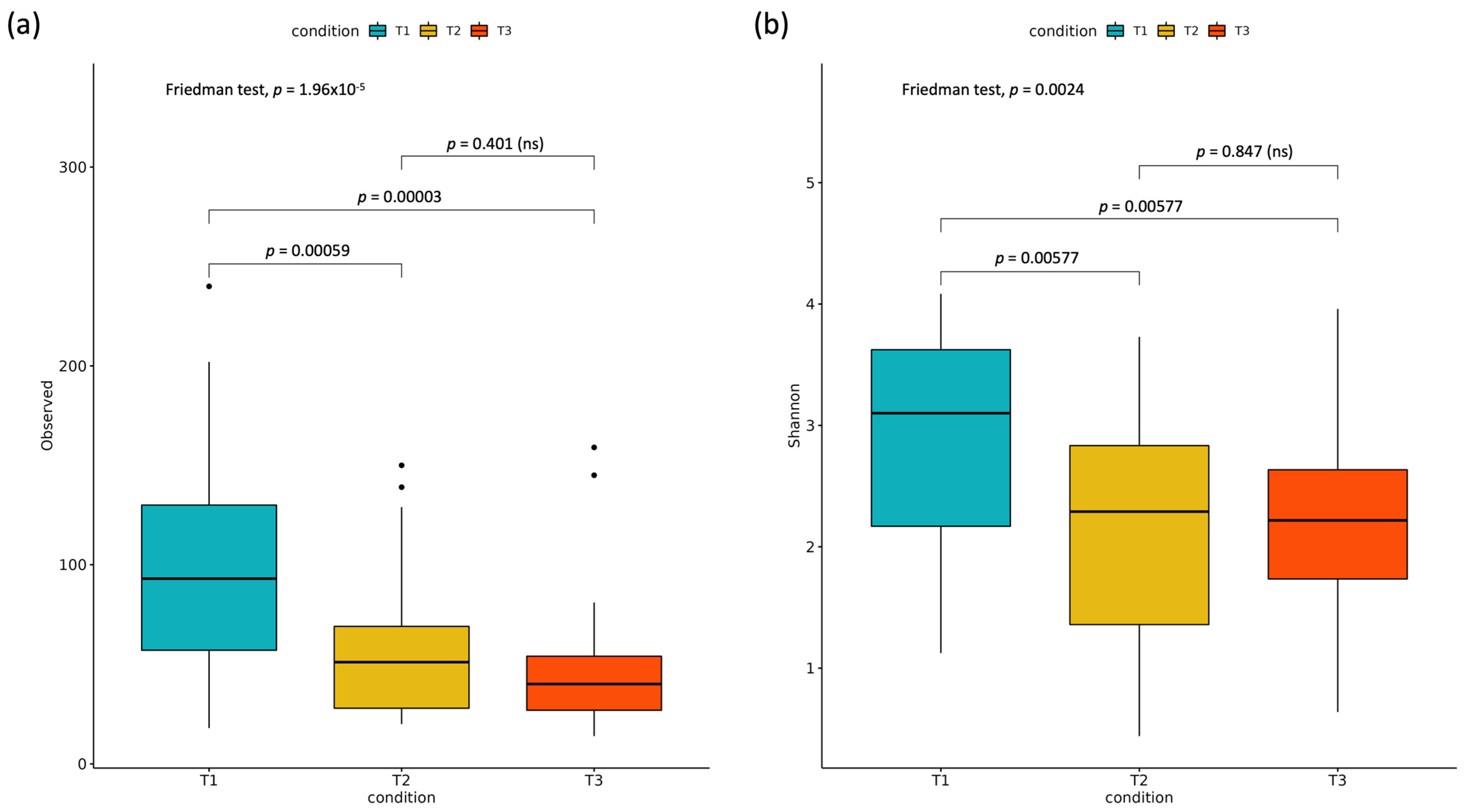
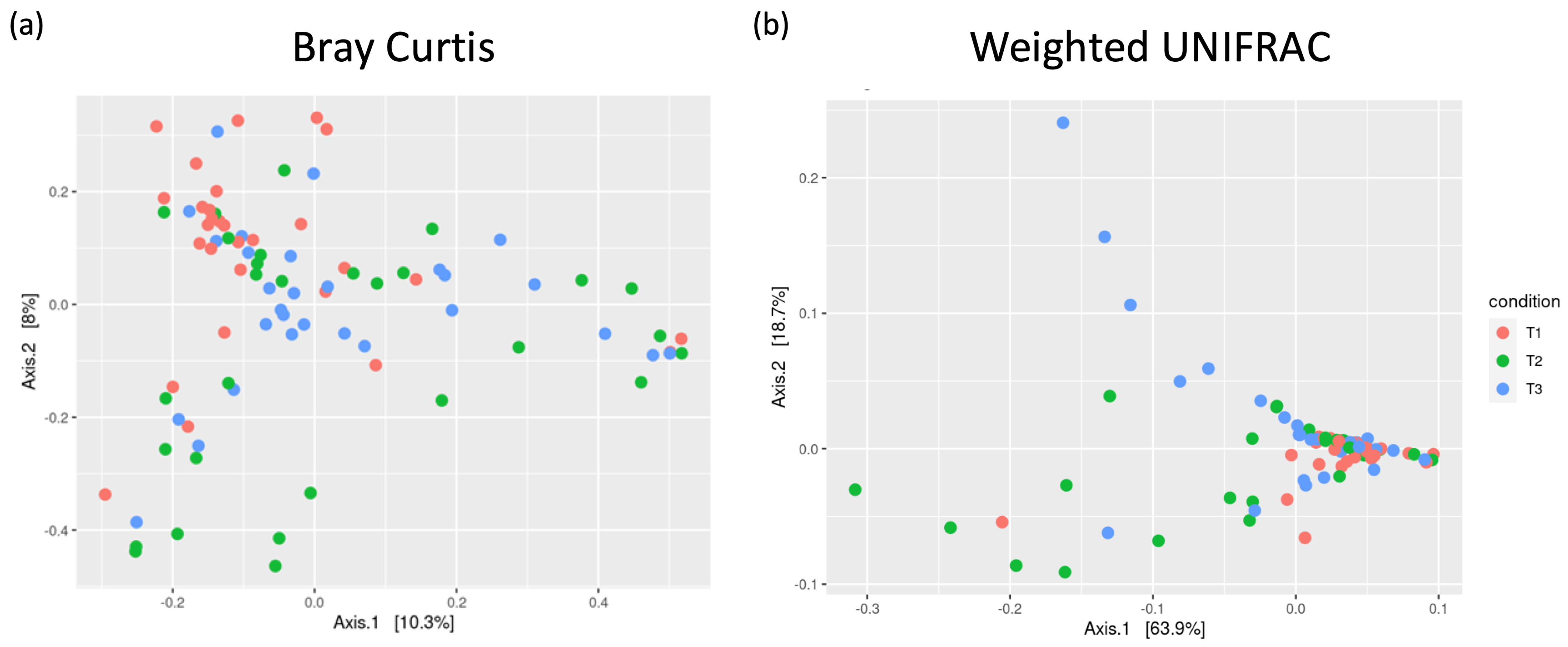
3.2. Changes in Diversity of the Gut Microbiota Among Patients with AML
3.2.1. Alpha Diversity
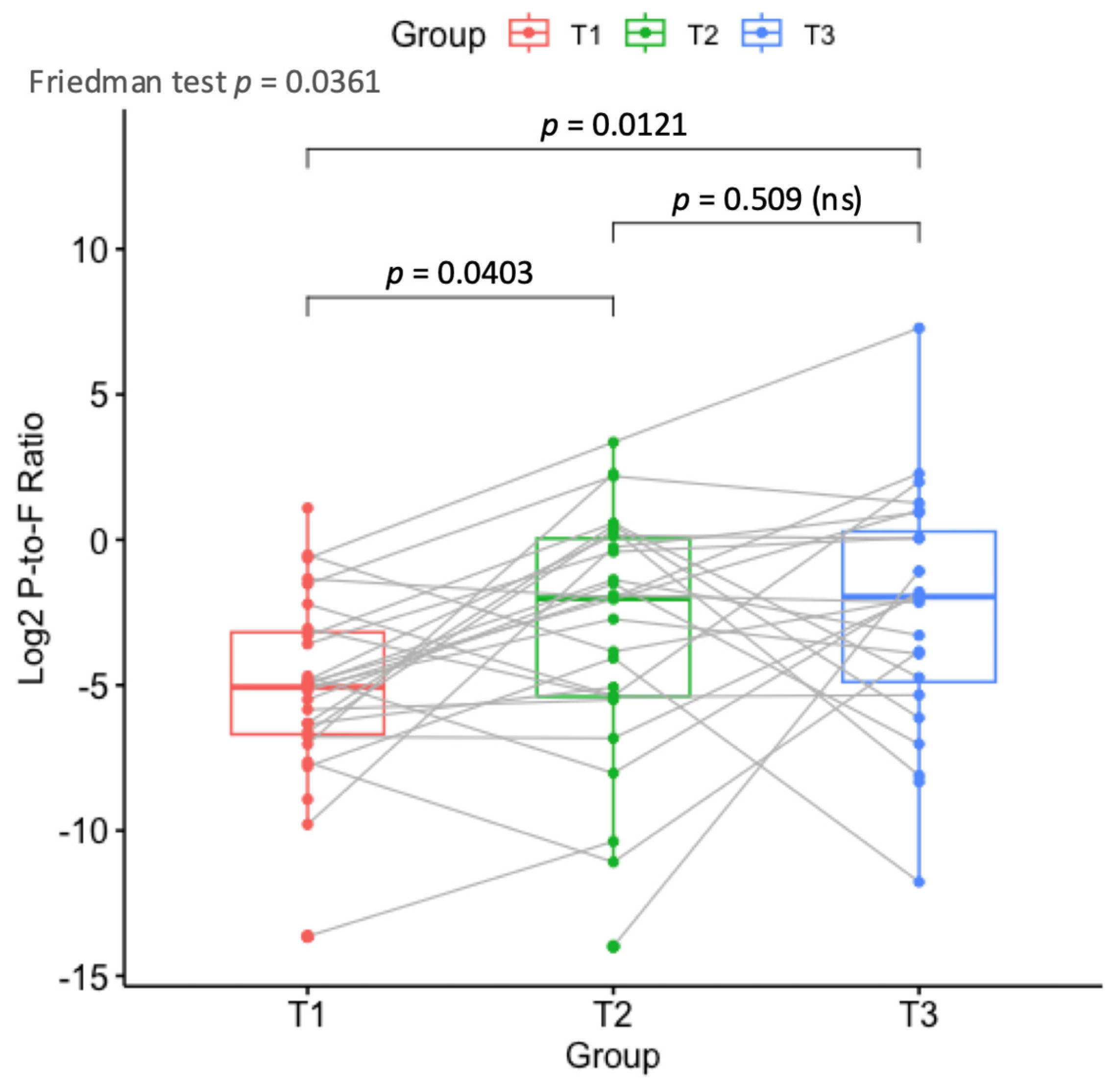
3.2.2. Beta Diversity and Signs of Dysbiosis
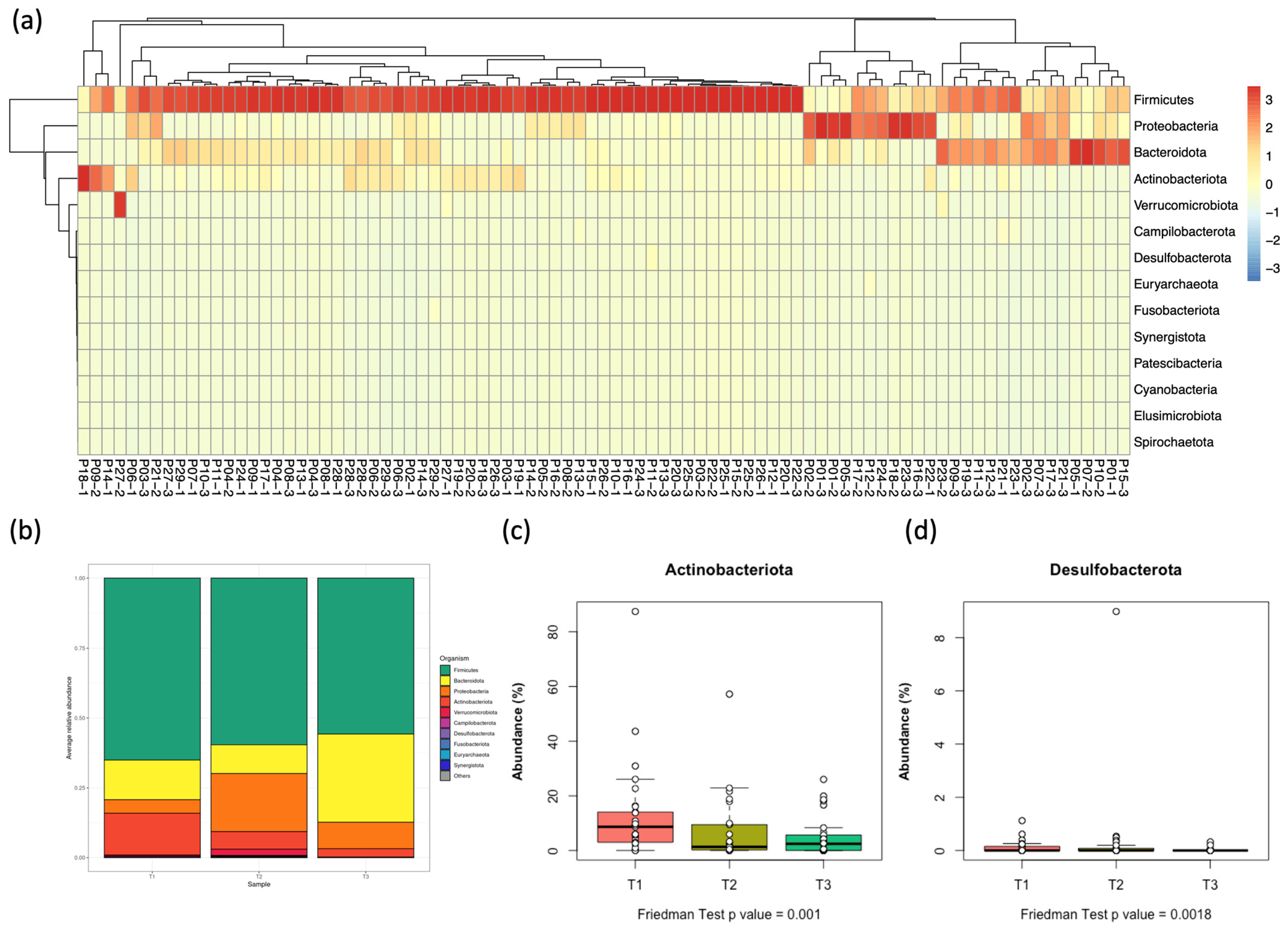
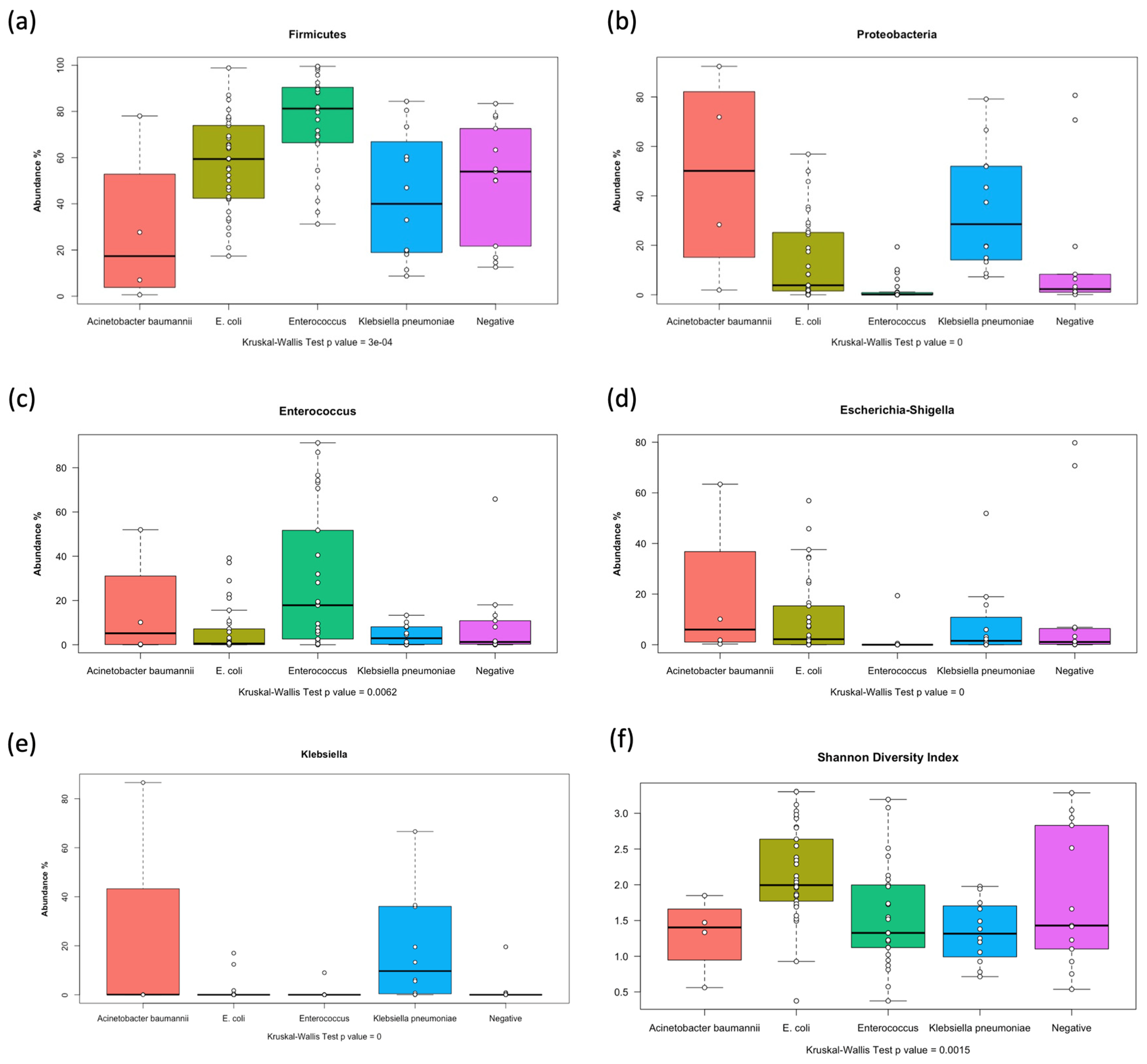
3.3. Distribution of Bacterial Phyla in the Gut Microbiota Among Pediatric Patients with AML
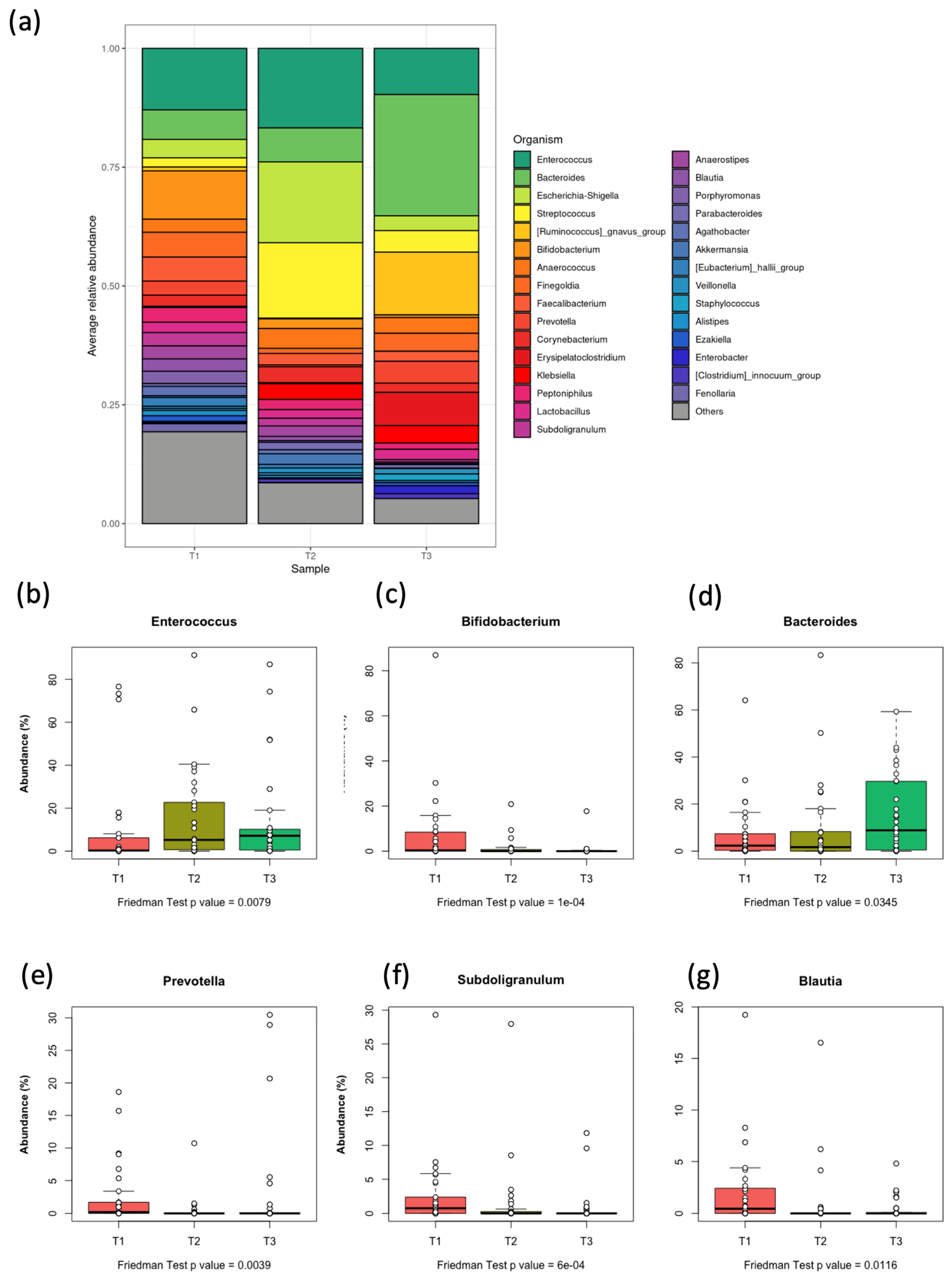
3.4. Distribution of Bacterial Genera in the Gut Microbiota of Pediatric Patients with AML
3.5. Differential Abundance at Phylum and Genus Levels at the Three Time Points
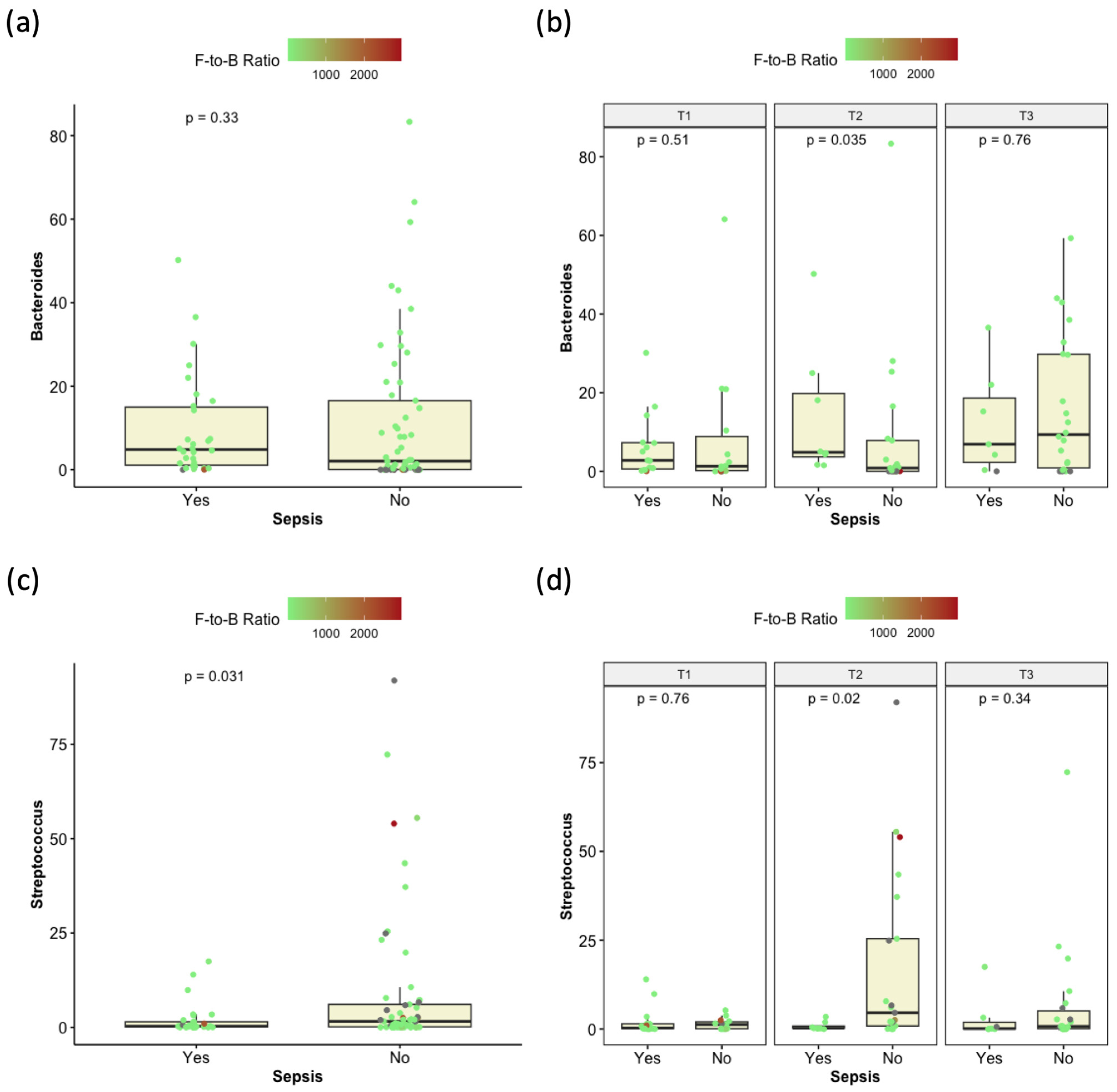
3.6. Differential Abundance of Microbial Taxa in Relation to Stool and Blood Cultures
3.7. Correlation Between Microbiome Composition and Enterocolitis
3.8. Compositional Variation of the Microbiome in Patients Receiving Meropenem and Piperacillin/Tazobactam
3.9. Other Clinical and Phenotypic Associations
3.10. Accounting for Inter-Patient Variability and Assessment of Significant Factors via Linear Mixed Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| ALL | Acute lymphoblastic leukemia |
| HSCT | Hematological stem cell transplant |
| BM | Bone marrow |
| TLR | Toll-like receptors |
| GI | Gastro-intestinal |
| BSI | Bloodstream infection |
| HRFN | High risk fever neutropenia |
| CRE | Carbapenem-resistant Enterobacteriaceae |
| ANC | Absolute neutrophilic count |
| ASV | Amplicon sequence variant |
| QIIME2 | Quantitative Insights in Microbial Ecology 2 |
| ADE | Adriamycin, Ara-C, and Etoposide |
| AML-M3 | Acute promyelocytic leukemia |
| ATRA | All-trans retinoic acid |
| MDR | Multi-drug-resistant |
| ICU | The intensive care unit |
| PCoA | Principal coordinate analysis |
References
- Smith, M.A.; Altekruse, S.F.; Adamson, P.C.; Reaman, G.H.; Seibel, N.L. Declining childhood and adolescent cancer mortality. Cancer 2014, 120, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.E.S.; Sauer, M.G.; Amrolia, P. Acute Myeloid Leukemia in Children. In The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies; Carreras, E., Dufour, C., Mohty, M., Kröger, N., Eds.; Springer: Cham, Switzerland, 2019; pp. 523–530. [Google Scholar]
- Elsherif, M.; Hammad, M.; Hafez, H.; Yassin, D.; Ashraf, M.; Yasser, N.; Lehmann, L.; Elhaddad, A. MECOM gene overexpression in pediatric patients with acute myeloid leukemia. Acta Oncol. 2022, 61, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Bow, E.J.; Loewen, R.; Cheang, M.S.; Shore, T.B.; Rubinger, M.; Schacter, B. Cytotoxic therapy-induced D-xylose malabsorption and invasive infection during remission-induction therapy for acute myeloid leukemia in adults. J. Clin. Oncol. 1997, 15, 2254–2261. [Google Scholar] [CrossRef]
- Rattanathammethee, T.; Tuitemwong, P.; Thiennimitr, P.; Sarichai, P.; Na Pombejra, S.; Piriyakhuntorn, P.; Hantrakool, S.; Chai-Adisaksopha, C.; Rattarittamrong, E.; Tantiworawit, A.; et al. Gut microbiota profiles of treatment-naïve adult acute myeloid leukemia patients with neutropenic fever during intensive chemotherapy. PLoS ONE 2020, 15, e0236460. [Google Scholar] [CrossRef]
- Song, Y.; Himmel, B.; Öhrmalm, L.; Gyarmati, P. The microbiota in hematologic malignancies. Curr. Treat. Options Oncol. 2020, 21, 2. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Little, M.P.; Wakeford, R.; Borrego, D.; French, B.; Zablotska, L.B.; Adams, M.J.; Allodji, R.; de Vathaire, F.; Lee, C.; Brenner, A.V.; et al. Leukaemia and myeloid malignancy among people exposed to low doses (<100 mSv) of ionising radiation during childhood: A pooled analysis of nine historical cohort studies. Lancet Haematol. 2018, 5, e346–e358. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Masetti, R.; Zama, D.; Leardini, D.; Muratore, E.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A. The gut microbiome in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Pediatr. Blood Cancer 2020, 67, e28711. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Phillips, R.; Alexander, S.; Alvaro, F.; Carlesse, F.; Fisher, B.; Hakim, H.; Santolaya, M.; Castagnola, E.; Davis, B.L.; et al. Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. J. Clin. Oncol. 2012, 30, 4427–4438. [Google Scholar] [CrossRef]
- Derrien, M.; Alvarez, A.S.; de Vos, W.M. The gut microbiota in the first decade of life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef]
- Hakim, H.; Dallas, R.; Wolf, J.; Tang, L.; Schultz-Cherry, S.; Darling, V.; Johnson, C.; Karlsson, E.A.; Chang, T.C.; Jeha, S.; et al. Gut microbiome composition predicts infection risk during chemotherapy in children with acute lymphoblastic leukemia. Clin. Infect. Dis. 2018, 67, 541–548. [Google Scholar] [CrossRef]
- Li, Y.; Hall, M.; Fisher, B.T.; Seif, A.E.; Huang, Y.S.; Bagatell, R.; Getz, K.D.; Alonzo, T.A.; Gerbing, R.B.; Sung, L.; et al. Merging Children’s Oncology Group data with an external administrative database using indirect patient identifiers: A report from the Children’s Oncology Group. PLoS ONE 2015, 10, e0143480. [Google Scholar] [CrossRef]
- Fouad, E.R.; Morsy, A.M.; Kamel, H.E.M.; Ali, A.M. Neutropenic enterocolitis in pediatric leukemia patients treated with intensive chemotherapy in Upper Egypt. Pediatr. Investig. 2020, 4, 5–10. [Google Scholar] [CrossRef]
- Fiedorová, K.; Radvanský, M.; Němcová, E.; Grombiříková, H.; Bosák, J.; Černochová, M.; Lexa, M.; Šmajs, D.; Freiberger, T. The impact of DNA extraction methods on stool bacterial and fungal microbiota community recovery. Front. Microbiol. 2019, 10, 821. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, M.J.; Tissing, W.J.; Dun, C.A.; Meessen, N.E.; Kamps, W.A.; de Bont, E.S.; Harmsen, H.J. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin. Infect. Dis. 2009, 49, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kennes, Y.M.; Lepp, D.; Yin, X.; Wang, Q.; Yu, H.; Yang, C.; Gong, J.; Diarra, M.S. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult. Sci. 2020, 99, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Rizkallah, M.R.; Saad, R.; Aziz, R.K. The Human Microbiome Project, personalized medicine and the birth of pharmacomicrobiomics. Curr. Pharmacogenomics Person. Med. 2010, 8, 182–193. [Google Scholar] [CrossRef]
- Galloway-Peña, J.R.; Shi, Y.; Peterson, C.B.; Sahasrabhojane, P.; Gopalakrishnan, V.; Brumlow, C.E.; Daver, N.G.; Alfayez, M.; Boddu, P.C.; Khan, M.A.W.; et al. Gut microbiome signatures are predictive of infectious risk following induction therapy for acute myeloid leukemia. Clin. Infect. Dis. 2020, 71, 63–71. [Google Scholar] [CrossRef]
- Secombe, K.R.; Coller, J.K.; Gibson, R.J.; Wardill, H.R.; Bowen, J.M. The bidirectional interaction of the gut microbiome and the innate immune system: Implications for chemotherapy-induced gastrointestinal toxicity. Int. J. Cancer 2019, 144, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Ervin, S.M.; Ramanan, S.V.; Bhatt, A.P. Relationship between the gut microbiome and systemic chemotherapy. Dig. Dis. Sci. 2020, 65, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.L.; Rajasuriar, R.; Lim, Y.A.L.; Woo, Y.L.; Loke, P.; Ariffin, H. Temporal changes in gut microbiota profile in children with acute lymphoblastic leukemia prior to commencement-, during-, and post-cessation of chemotherapy. BMC Cancer 2020, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Muratore, E.; Leardini, D.; Zama, D.; Turroni, S.; Brigidi, P.; Esposito, S.; Pession, A. Gut microbiome in pediatric acute leukemia: From predisposition to cure. Blood Adv. 2021, 5, 4619–4629. [Google Scholar] [CrossRef]
- Panebianco, C.; Andriulli, A.; Pazienza, V. Pharmacomicrobiomics: Exploiting the drug-microbiota interactions in anticancer therapies. Microbiome 2018, 6, 92. [Google Scholar] [CrossRef]
- Aziz, R.K.; Hegazy, S.M.; Yasser, R.; Rizkallah, M.R.; ElRakaiby, M.T. Drug pharmacomicrobiomics and toxicomicrobiomics: From scattered reports to systematic studies of drug-microbiome interactions. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1043–1055. [Google Scholar] [CrossRef]
- Montassier, E.; Batard, E.; Massart, S.; Gastinne, T.; Carton, T.; Caillon, J.; Le Fresne, S.; Caroff, N.; Hardouin, J.B.; Moreau, P.; et al. 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation. Microb Ecol. 2014, 67, 690–699. [Google Scholar] [CrossRef]
- Oh, B.; Boyle, F.; Pavlakis, N.; Clarke, S.; Guminski, A.; Eade, T.; Lamoury, G.; Carroll, S.; Morgia, M.; Kneebone, A.; et al. Emerging evidence of the gut microbiome in chemotherapy: A clinical review. Front. Oncol. 2021, 11, 706331. [Google Scholar] [CrossRef]
- Aitken, S.L.; Sahasrabhojane, P.V.; Kontoyiannis, D.P.; Savidge, T.C.; Arias, C.A.; Ajami, N.J.; Shelburne, S.A.; Galloway-Peña, J.R. Alterations of the oral microbiome and cumulative carbapenem exposure are associated with Stenotrophomonas maltophilia infection in patients with acute myeloid leukemia receiving chemotherapy. Clin. Infect. Dis. 2021, 72, 1507–1513. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, C.; Zhang, A. Gut microbiota in acute leukemia: Current evidence and future directions. Front. Microbiol. 2022, 13, 1045497. [Google Scholar] [CrossRef]
- Liu, F.; Li, J.; Guan, Y.; Lou, Y.; Chen, H.; Xu, M.; Deng, D.; Chen, J.; Ni, B.; Zhao, L.; et al. Dysbiosis of the gut microbiome is associated with tumor biomarkers in lung cancer. Int. J. Biol. Sci. 2019, 15, 2381–2392. [Google Scholar] [CrossRef]
- Singha, B.; Rawat, B.S.; Venkataraman, R.; Nair, T.; Rosenn, E.H.; Soni, V. Gut microbiome associated dysbiosis: Limited regimens and expanding horizons of phage therapy. Asp. Mol. Med. 2023, 2, 100029. [Google Scholar] [CrossRef]
- Caballero-Flores, G.; Pickard, J.M.; Núñez, G. Microbiota-mediated colonization resistance: Mechanisms and regulation. Nat. Rev. Microbiol. 2023, 21, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, T.; Tsergouli, K.; Behzadi, P. Carbapenem-resistant Klebsiella pneumoniae: Virulence factors, molecular epidemiology and latest updates in treatment options. Antibiotics 2023, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Lindner, B.G.; Gerhardt, K.; Feistel, D.J.; Rodriguez, R.L.; Hatt, J.K.; Konstantinidis, K.T. A user’s guide to the bioinformatic analysis of shotgun metagenomic sequence data for bacterial pathogen detection. Int. J. Food Microbiol. 2024, 410, 110488. [Google Scholar] [CrossRef]
| Characteristics | Categories | Number (%) |
|---|---|---|
| Age (Years) | ≤7 | 15 (51.7%) |
| >7 | 14 (48.3%) | |
| FAB Classification | AML | 27 (39.1%) |
| AML M3 | 2 (6.9%) | |
| Period of Febrile/Neutropenia (Days) | ≤25 | 14 (48.3%) |
| >25 | 15 (51.7%) | |
| ICU Admission | Admission for Septic Shock | 4 (13.7%) |
| Others | 1 (3.5%) | |
| Not Admitted to ICU | 24 (82.8) | |
| Enterocolitis (Typhlitis + Colitis) | No | 18 (62%) |
| Yes | 11 (38%) | |
| CT Abdomen Finding | No | 21 (72.4%) |
| Yes | 8 (27.5%) | |
| Died | No | 25 (86.2%) |
| Yes | 4 (13.8%) | |
| Cause of Death | Pulmonary Hemorrhage | 1 (25%) |
| Septic Shock | 3 (75%) | |
| Total number = 29 |
| Code | Sex | Age (Years) | Time to Neutropenia (Days) | Duration of Neutropenia (Days) | Rectal Swab at Initial Diagnosis (T1) | Microbiological Blood Culture | Fate |
|---|---|---|---|---|---|---|---|
| P06 | Male | 11 | 5 | 16 | E. coli (carbapenem-resistant) | E. coli (carbapenem-resistant) | Alive |
| P14 | Male | 7 | 3 | 13 | Klebsiella pneumoniae (MDR) | Klebsiella pneumoniae (MDR) | Died |
| P13 | Male | 13 | 8 | 15 | E. coli (ESBL) | Klebsiella pneumoniae (MDR) | Alive |
| P7 | Male | 2 | 3 | 20 | Normal fecal microbiota | Klebsiella pneumoniae (MDR) | Died |
| P14 | Male | 7 | 5 | 17 | Normal fecal microbiota | Klebsiella pneumoniae (MDR) | Alive |
| P16 | Female | 3 | 6 | 7 | Klebsiella pneumoniae (MDR) | Klebsiella pneumoniae (MDR) | Alive |
| P26 | Male | 4 | 7 | 15 | Normal fecal microbiota | Klebsiella pneumoniae (MDR) | Alive |
| P1 | Female | 9 | 4 | 20 | Acinetobacter baumannii (MDR) | Acinetobacter baumannii (MDR) | Died |
| P28 | Male | 10 | 7 | 19 | E. coli (ESBL) | Acinetobacter baumannii (MDR) | Died |
| Blood Culture | A. baumannii | E. coli | K. pneumoniae | MRSA | Staphylococcus | S. mitis | Negative | Sum | |
|---|---|---|---|---|---|---|---|---|---|
| Stool Culture | |||||||||
| A. baumannii | 1 | 1 | 2 | 4 | |||||
| E. coli | 1 | 2 | 3 | 4 | 1 | 22 | 33 | ||
| Enterococcus | 2 | 4 | 1 | 2 | 16 | 25 | |||
| K. pneumoniae | 3 | 1 | 8 | 12 | |||||
| Negative | 3 | 1 | 9 | 13 | |||||
| Sum | 2 | 4 | 14 | 1 | 8 | 1 | 57 | 87 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adel, M.; Khedr, R.A.; Sayed, A.A.; Shalaby, L.; Diab, A.A.; Yahia, A.; Elanany, M.; Lehmann, L.E.; Ahmed, S.; Aziz, R.K.; et al. Changes in Gut Microbial Diversity and Correlation with Clinical Outcome in Children with Acute Myeloid Leukemia Receiving Induction Chemotherapy. Children 2025, 12, 1176. https://doi.org/10.3390/children12091176
Adel M, Khedr RA, Sayed AA, Shalaby L, Diab AA, Yahia A, Elanany M, Lehmann LE, Ahmed S, Aziz RK, et al. Changes in Gut Microbial Diversity and Correlation with Clinical Outcome in Children with Acute Myeloid Leukemia Receiving Induction Chemotherapy. Children. 2025; 12(9):1176. https://doi.org/10.3390/children12091176
Chicago/Turabian StyleAdel, Mai, Reham Abdelaziz Khedr, Ahmed A. Sayed, Lobna Shalaby, Aya A. Diab, Abdelrahman Yahia, Mervat Elanany, Leslie E. Lehmann, Sonia Ahmed, Ramy K. Aziz, and et al. 2025. "Changes in Gut Microbial Diversity and Correlation with Clinical Outcome in Children with Acute Myeloid Leukemia Receiving Induction Chemotherapy" Children 12, no. 9: 1176. https://doi.org/10.3390/children12091176
APA StyleAdel, M., Khedr, R. A., Sayed, A. A., Shalaby, L., Diab, A. A., Yahia, A., Elanany, M., Lehmann, L. E., Ahmed, S., Aziz, R. K., & Elhaddad, A. (2025). Changes in Gut Microbial Diversity and Correlation with Clinical Outcome in Children with Acute Myeloid Leukemia Receiving Induction Chemotherapy. Children, 12(9), 1176. https://doi.org/10.3390/children12091176







