Perioperative Management of Paediatric Hypertension
Abstract
1. Introduction and Prevalence
1.1. Definition of Chronic Hypertension in Paediatric Patients
1.2. Measuring Blood Pressure in Paediatric Patients
2. Aetiology of Chronic Hypertension in Paediatric Patients
3. Secondary Causes of Chronic Hypertension in Paediatric Patients
- Renal and Renovascular
- Endocrine
- Neurological
- Pulmonary
- Cardiovascular
- Medications
- Neoplasia
- Genetic factors
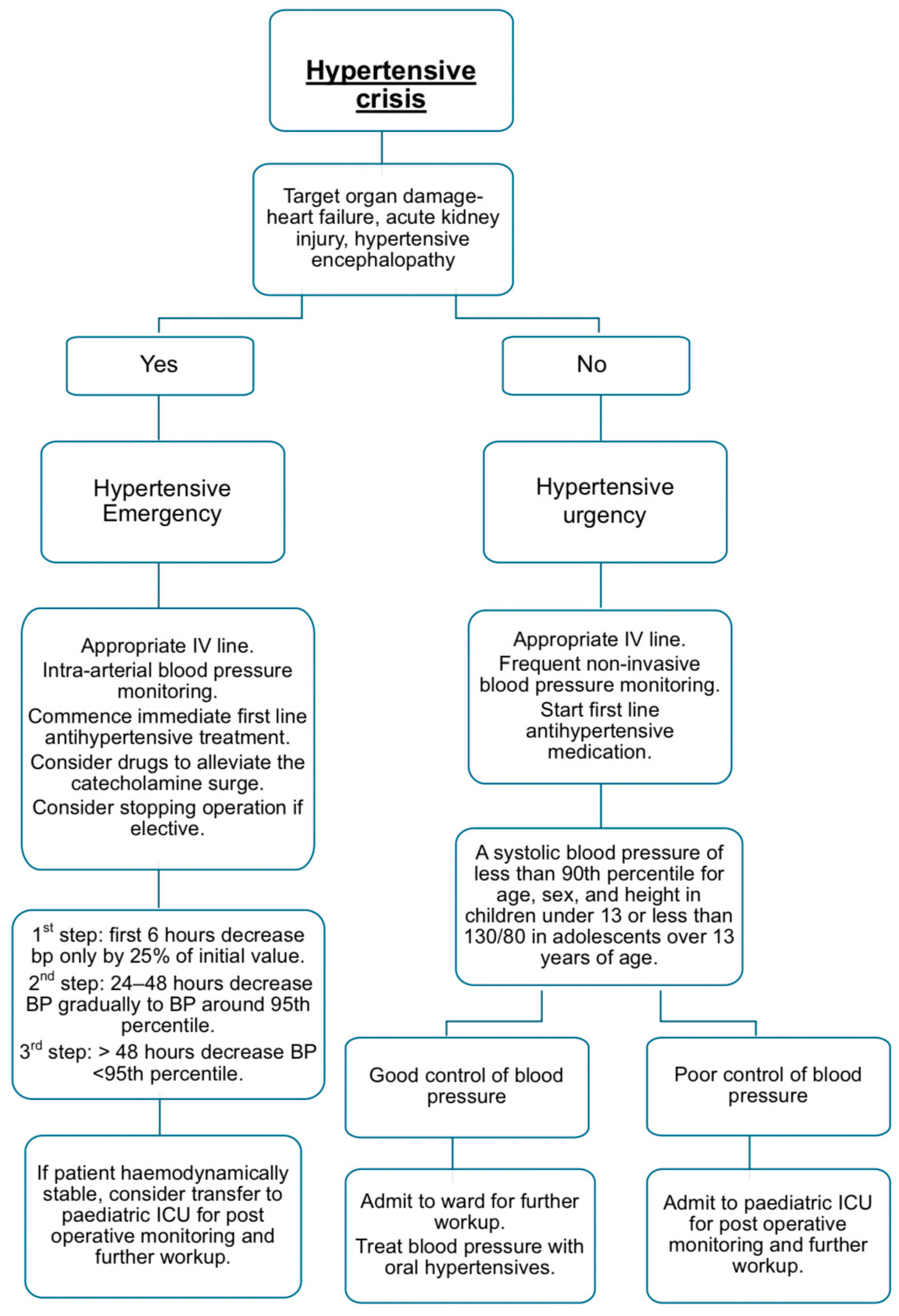
4. Perioperative Management of Severe Hypertension
- Neurological:
- Cardiovascular:
- Renal:
4.1. Preoperative Optimisation for Anaesthesia
4.2. Intraoperative Management of Hypertensive Emergencies
4.3. Pharmacological Management of Intraoperative Hypertensive Crises
4.3.1. First-Line Agents
Vasodilators
Adrenergic Blockers and Agonists
Calcium Channel Blocker
4.3.2. Second-Line Agents
Vasodilators
Adrenergic Blockers and Agonists
Angiotensin-Converting Enzyme (ACE) Inhibitors
Diuretics
4.4. Management of Hypertensive Crisis in Specific Conditions
4.4.1. Phaeochromocytoma
4.4.2. Aortic Coarctation
5. Postoperative Care
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Paediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef]
- Starr, M.C.; Flynn, J.T. Neonatal hypertension: Cases, causes, and clinical approach. Pediatr. Nephrol. 2019, 34, 787–799. [Google Scholar] [CrossRef]
- Kraut, E.J.; Boohaker, L.J.; Askenazi, D.J.; Fletcher, J.; Kent, A.L.; Neonatal Kidney Collaborative (NKC). Correction: Incidence of neonatal hypertension from a large multicentre study [Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates-AWAKEN]. Pediatr. Res. 2018, 84, 314. [Google Scholar] [CrossRef] [PubMed]
- Dionne, J.M.; Abitbol, C.L.; Flynn, J.T. Hypertension in infancy: Diagnosis, management and outcome. Pediatr. Nephrol. 2012, 27, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Batisky, D.L. Neonatal hypertension. Clin. Perinatol. 2014, 41, 529–542. [Google Scholar] [CrossRef]
- Yang, W.C.; Lin, M.J.; Chen, C.Y.; Wu, H.P. Clinical overview of hypertensive crisis in children. World J. Clin. Cases 2015, 3, 510–513. [Google Scholar] [CrossRef]
- de Simone, G.; Mancusi, C.; Hanssen, H.; Genovesi, S.; Lurbe, E.; Parati, G.; Sendzikaite, S.; Valerio, G.; Di Bonito, P.; Di Salvo, G.; et al. Hypertension in children and adolescents. Eur. Heart J. 2022, 43, 3290–3301. [Google Scholar] [CrossRef]
- Dionne, J.M. Determinants of Blood Pressure in Neonates and Infants: Predictable Variability. Hypertension 2021, 77, 781–787. [Google Scholar] [CrossRef]
- Bassareo, P.P.; Calcaterra, G.; Sabatino, J.; Oreto, L.; Ciliberti, P.; Perrone, M.; Martino, F.; D’Alto, M.; Chessa, M.; Salvo, G.D.I.; et al. Primary and secondary paediatric hypertension. J. Cardiovasc. Med. 2023, 24, e77–e85. [Google Scholar] [CrossRef]
- Siddiqui, S.; Malatesta-Muncher, R. Hypertension in Children and Adolescents: A Review of Recent Guidelines. Pediatr. Ann. 2020, 49, e250–e257. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.; Hamdani, G.; Mitsnefes, M. Hypertensive crisis in children and adolescents. Pediatr. Nephrol. 2019, 34, 2523–2537. [Google Scholar] [CrossRef] [PubMed]
- Hjorten, R.; Flynn, J.T. Neonatal Hypertension. Clin. Perinatol. 2022, 49, 27–42. [Google Scholar] [CrossRef]
- Flynn, J.T. The hypertensive neonate. Semin. Fetal Neonatal Med. 2020, 25, 101138. [Google Scholar] [CrossRef]
- Raina, R.; Mahajan, Z.; Sharma, A.; Chakraborty, R.; Mahajan, S.; Sethi, S.K.; Kapur, G.; Kaelber, D. Hypertensive Crisis in Paediatric Patients: An Overview. Front. Pediatr. 2020, 8, 588911. [Google Scholar] [CrossRef]
- Kilian, K. Hypertension in neonates causes and treatments. J. Perinat. Neonatal Nurs. 2003, 17, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Bholah, R.; Bunchman, T.E. Review of Paediatric Pheochromocytoma and Paraganglioma. Front. Pediatr. 2017, 5, 155. [Google Scholar] [CrossRef]
- Nazari, M.A.; Jha, A.; Kuo, M.J.M.; Patel, M.; Prodanov, T.; Rosenblum, J.S.; Talvacchio, S.; Derkyi, A.; Charles, K.; Pacak, K. Paediatric phaeochromocytoma and paraganglioma: A clinical update. Clin. Endocrinol. 2024, 101, 446–454. [Google Scholar] [CrossRef]
- Dagle, J.M.; Fisher, T.J.; Haynes, S.E.; Berends, S.K.; Brophy, P.D.; Morriss, F.H.; Murray, J.C. Cytochrome P450 (CYP2D6) genotype is associated with elevated systolic blood pressure in preterm infants after discharge from the neonatal intensive care unit. J. Pediatr. 2011, 159, 104–109. [Google Scholar] [CrossRef]
- Anderson, C.; Bannister, C.; Hardy, C.; Goldschneider, K.R.; Cravero, J.P.; Honkanen, A.; Rehman, M.; Tobias, J. The paediatrician’s role in the evaluation and preparation of paediatric patients undergoing anesthesia. Paediatrics 2014, 134, 634–641. [Google Scholar]
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; Davila-Roman, V.G.; Gerhard-Herman, M.D.; Holly, T.A.; Kane, G.C.; et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 130, e278–e333. [Google Scholar]
- Coulthard, M.G. Managing severe hypertension in children. Pediatr. Nephrol. 2023, 38, 3229–3239. [Google Scholar] [CrossRef]
- Hack, H.A. The perioperative management of children with phaeochromocytoma. Paediatr. Anaesth. 2000, 10, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.; Boumphrey, S. Perioperative care of phaeochromocytoma. BJA Educ. 2016, 16, 153–158. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, Y.S.; Min, T.J.; Kim, W.Y.; Kim, J.H.; Park, Y.C. Anaesthetic management of hypertensive crisis in a three-year-old patient with undiagnosed severe renal artery stenosis: A case report. Korean J. Anesthesiol. 2014, 67, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T.; Tullus, K. Severe hypertension in children and adolescents: Pathophysiology and treatment. Pediatr. Nephrol. 2009, 24, 1101–1112. [Google Scholar] [CrossRef]
- Bertazza Partigiani, N.; Spagnol, R.; Di Michele, L.; Santini, M.; Grotto, B.; Sartori, A.; Zamperetti, E.; Nosadini, M.; Meneghesso, D. Management of Hypertensive Crises in Children: A Review of the Recent Literature. Front. Pediatr. 2022, 10, 880678. [Google Scholar] [CrossRef]
- Blair, J.M.; Hill, D.A.; Wilson, C.M.; Fee, J.P.H. Assessment of tracheal intubation in children after induction with propofol and different doses of remifentanil. Anaesthesia 2004, 59, 27–33. [Google Scholar] [CrossRef]
- Milner, A.; Welch, E. Applied Pharmacology in Anaesthesiology and Critcal Care, 2nd ed.; Medpharm Publications (Pty) Ltd.: Centurion, South Africa, 2019; pp. 118–650. [Google Scholar]
| Postmenstrual Age (Weeks) | 50th Percentile SBP/DBP (MAP) | 95th Percentile SBP/DBP (MAP) | 99th Percentile SBP/DBP (MAP) |
|---|---|---|---|
| 26 | 55/38 (30) | 72/57 (50) | 77/63 (56) |
| 28 | 60/45 (38) | 75/58 (50) | 80/63 (54) |
| 30 | 65/48 (40) | 80/63 (55) | 85/68 (60) |
| 32 | 65/48 (40) | 83/64 (55) | 88/69 (60) |
| 34 | 70/50 (40) | 85/65 (55) | 90/70 (60) |
| 36 | 72/57 (50) | 87/72 (65) | 92/77 (70) |
| 38 | 77/59 (50) | 92/74 (65) | 97/79 (70) |
| 40 | 80/60 (50) | 95/75 (65) | 100/80 (70) |
| 42 | 85/62 (50) | 98/76 (65) | 102/81 (70) |
| 44 | 88/63 (50) | 105/80 (68) | 110/85 (73) |
| Neonate and Infants | Children 1–13 Years Old | Children ≥ 13 Years Old | |
|---|---|---|---|
| Normal | <90th percentile | <120/<80 mm Hg | |
| Elevated BP | ≥95th percentile for postmenstrual age | ≥90th percentile to <95th percentile or 120/80 mm Hg * to <95th percentile | BP: 120/<80 to 129/<80 mm Hg |
| Stage 1 HTN: | ≥95th percentile to <95th percentile + 12 mmHg, or 130–139/80–89 mm Hg * | 130–139/80–89 mm Hg | |
| Stage 2 HTN: | ≥95th percentile + 12 mm Hg, or ≥140/90 mm Hg * | ≥140/90 mm Hg |
| Systems | Neonates and Infants | Children | Adolescents |
|---|---|---|---|
| Pulmonary | Bronchopulmonary dysplasia | Obstructed sleep apnoea | Obstructed sleep apnoea |
| Cardiac | Congenital heart disease Coarctation of aorta | Coarctation of aorta | Coarctation of aorta |
| Neurological | Pain Intracranial hypertension Seizure Subdural haematoma | Increased intracranial pressure | Increased intracranial pressure |
| Reno-vascular | Thromboembolism Fibromuscular Dysplasia Renal artery stenosis Renal venous thrombosis Compression of renal artery Idiopathic arterial calcification | Fibromuscular Dysplasia Midaortic Syndrome Takayasu Arteritis Renal artery hypoplasia | Fibromuscular Dysplasia Midaortic Syndrome Takayasu Arteritis |
| Renal parenchymal disease | Congenital Polycystic kidney disease Ureteropelvic junction obstruction Acquired Acute tubular injury Cortical necrosis Obstruction (stones, tumours) | Glomerulonephritis Obstructive uropathy | Glomerulonephritis Obstructive uropathy |
| Endocrine | Congenital adrenal hyperplasia Hyperaldosteronism Hyperthyroidism Pseudo hypoaldosteronism type II | Cushing syndrome Hyperaldosteronism Hyperthyroidism | Cushing syndrome Hyperaldosteronism Hyperthyroidism |
| Neoplasia |
Wilms tumour Mesoblastic nephroma Neuroblastoma Pheochromocytoma |
Wilms tumour Neuroblastoma Pheochromocytoma | Pheochromocytoma |
| Medications/intoxications | Dexamethasone Adrenergic agents Vitamin D intoxication Theophylline Caffeine Pancuronium Phenylephrine Maternal Cocaine Heroin | Sympathomimetics: cocaine, amphetamines, pseudoephedrine Corticosteroids | Sympathomimetics: cocaine, amphetamines, pseudoephedrine Corticosteroids Oral contraceptive |
| Miscellaneous | Prematurity Closure of abdominal wall defect Uterine arterial and venous catheters Low birth weight Extracorporeal membrane oygenation | Primary hypertension Preeclampsia/eclampsia | |
| Genetic syndromes | Von Hippel-Lindau (VHL gene) MEN 2 (RET & SDH genes) Neurofibromatosis type 1 (NF1 gene) |
| Drug Class | Drug | Route | Dose | Onset of Action | Adverse Effects |
|---|---|---|---|---|---|
| First Line Agents | |||||
| Direct Vasodilators | Sodium Nitroprusside | Intravenous infusion | 0.5–10 μg/kg/min | 2–10 min | Thiocyanate toxicity, inactivated by light. |
| Hydralazine | Intravenous bolus | 0.2–0.6 mg/kg. Maximum single dose 20 mg | 5–20 min | Reflex tachycardia, headache, fluid retention. | |
| Beta Blockers | Esmolol | Intravenous infusion | 100–500 μg/kg/min, up to 1000 μg/kg/min | 2–10 min | Contraindicated in asthma, may cause bradycardia. |
| Calcium Channel Blockers (CCB) | Nicardipine | Intravenous bolus/infusion | 30 μg/kg up to 2 mg/dose/0.5–4 μg/kg/min | Within minutes | Reflex tachycardia. |
| Second Line Agents | |||||
| Direct Vasodilators | Diazoxide | Intravenous bolus | 1–3 mg/kg every 5–15 min | Within minutes | Risk of hypotension in large doses. |
| Nitroglycerine | Intravenous infusion | 0.1–2 μg/kg/min | 1–2 min | Methemoglobinemia, vasodilating effect primarily on the venous side, efficient in heart failure, limited efficacy in children. | |
| Alpha and Beta Blockers | Labetalol | Intravenous bolus/infusion | 0.2–1 mg/kg/dose, up to 40 mg/dose/0.25–3 mg/kg/h | 2–5 min | Contraindicated in asthma, heart failure, bradycardia. |
| Alpha Blockers | Phentolamine | Intravenous bolus | 0.05–0.1 mg/kg/dose, up to 5 mg | Immediate | Tachycardia. Used only in pheochromocytoma. |
| Angiotensin-Converting Enzyme Inhibitors (ACEIs) | Enalaprilat | Intravenous bolus | 5–10 μg/kg/min, up to 1.2 mg/dose | 15 min | Contraindicated in suspected bilateral renal artery stenosis. |
| Diuretics (Loop) | Furosemide | Intravenous bolus | 0.5–5 mg/kg/dose | Within minutes | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, N.; Redelinghuys, C.; Mogane, P. Perioperative Management of Paediatric Hypertension. Children 2025, 12, 1174. https://doi.org/10.3390/children12091174
Barbosa N, Redelinghuys C, Mogane P. Perioperative Management of Paediatric Hypertension. Children. 2025; 12(9):1174. https://doi.org/10.3390/children12091174
Chicago/Turabian StyleBarbosa, Nicole, Cara Redelinghuys, and Palesa Mogane. 2025. "Perioperative Management of Paediatric Hypertension" Children 12, no. 9: 1174. https://doi.org/10.3390/children12091174
APA StyleBarbosa, N., Redelinghuys, C., & Mogane, P. (2025). Perioperative Management of Paediatric Hypertension. Children, 12(9), 1174. https://doi.org/10.3390/children12091174