Ventricular Subgaleal Shunt in Children Under Three Months of Age, from Diagnosis to Outcome: A Review After 11 Years of Experience in a French University Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. General Characteristics of the Study
2.2. Data Collection
2.3. Statistical Analysis
3. Results
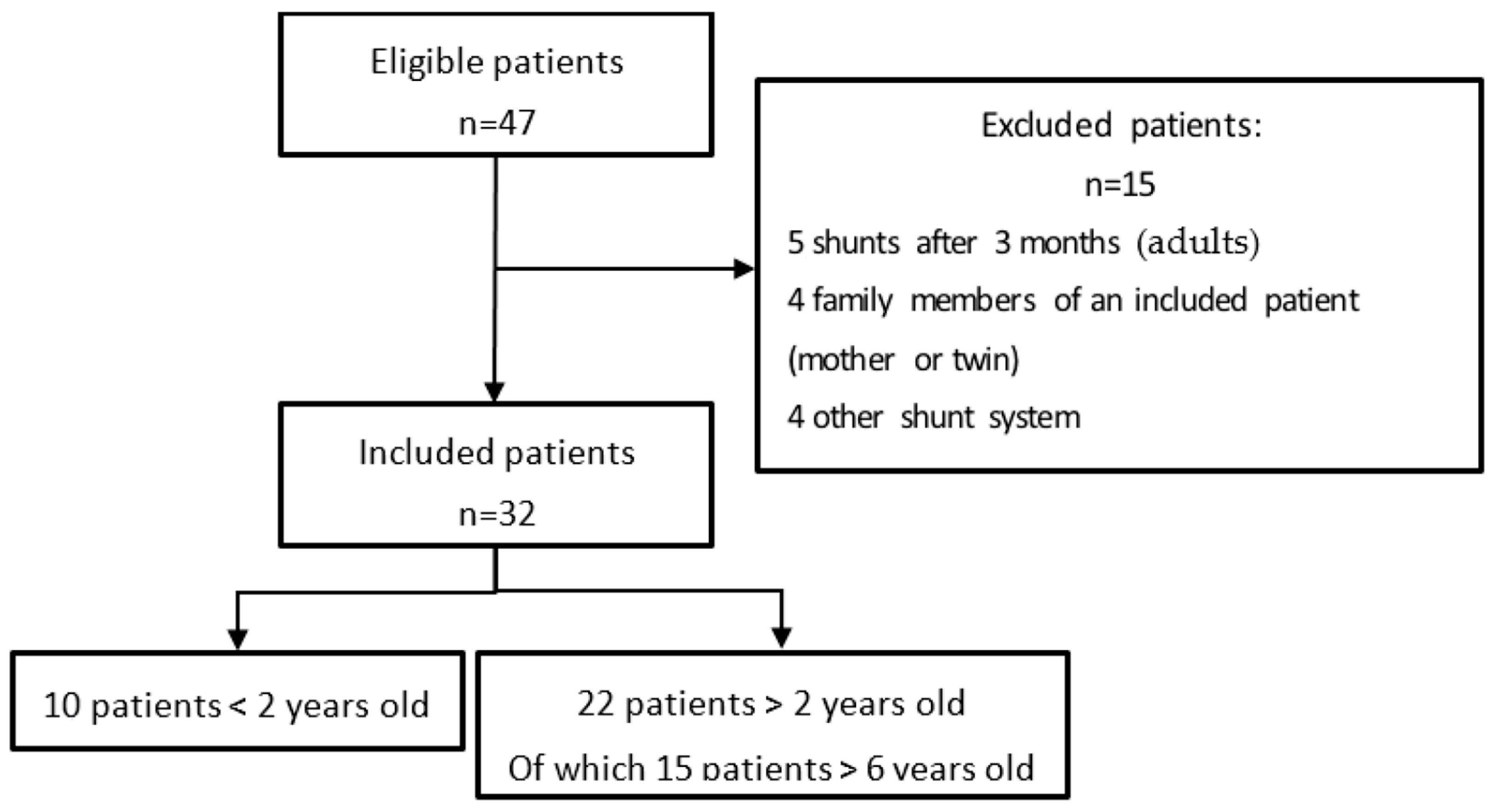
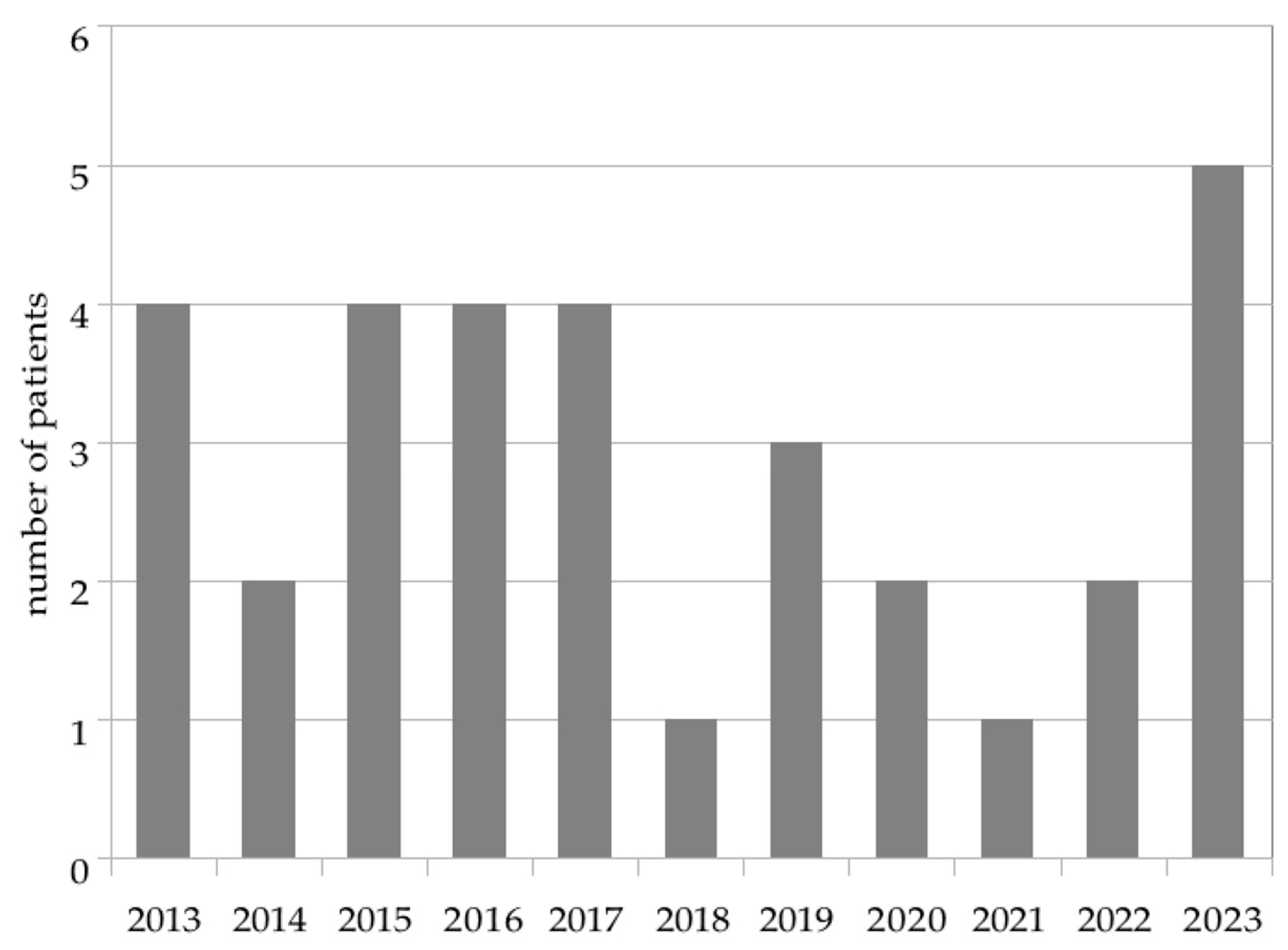
3.1. Study Population
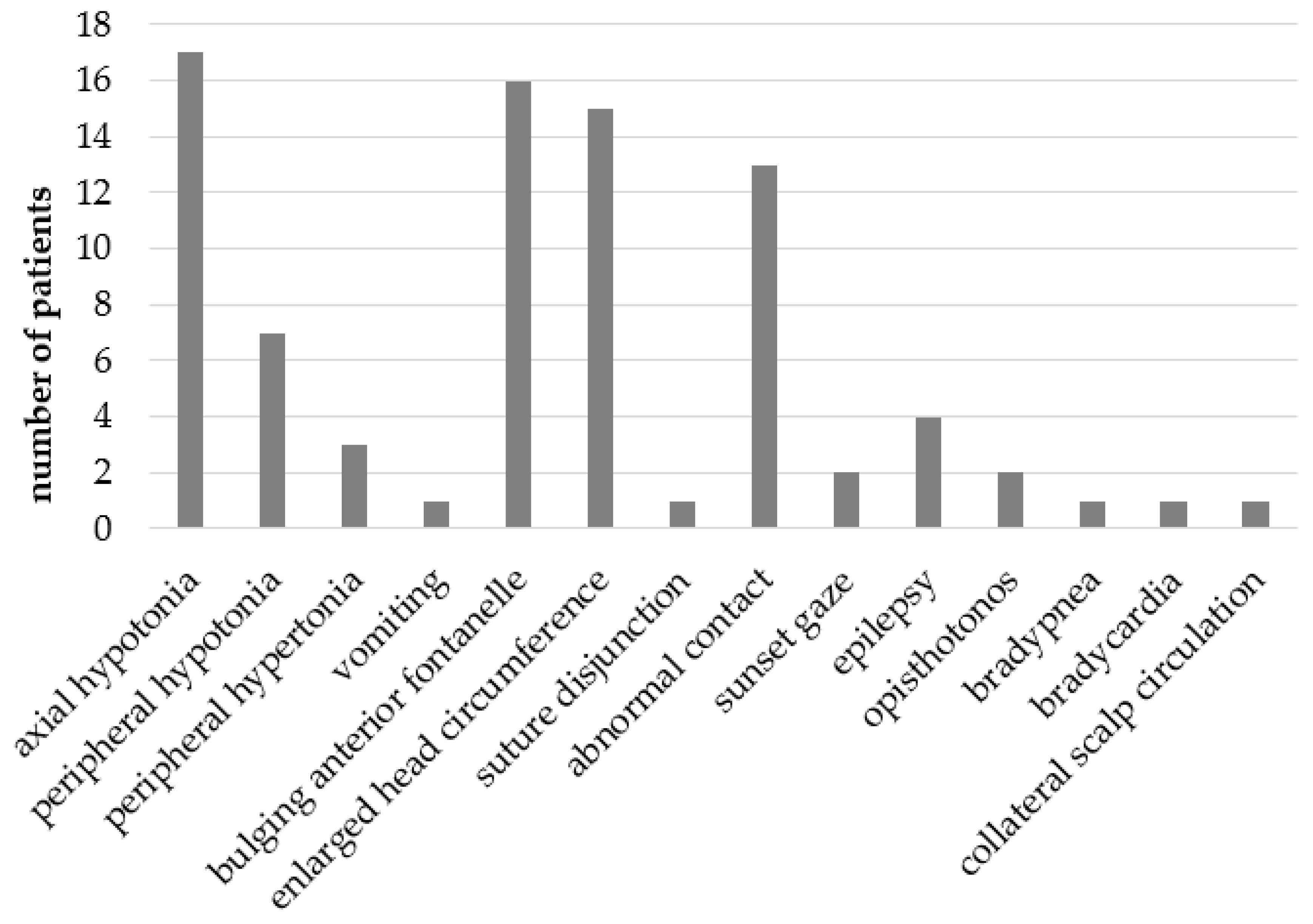
3.2. Symptoms and Signs
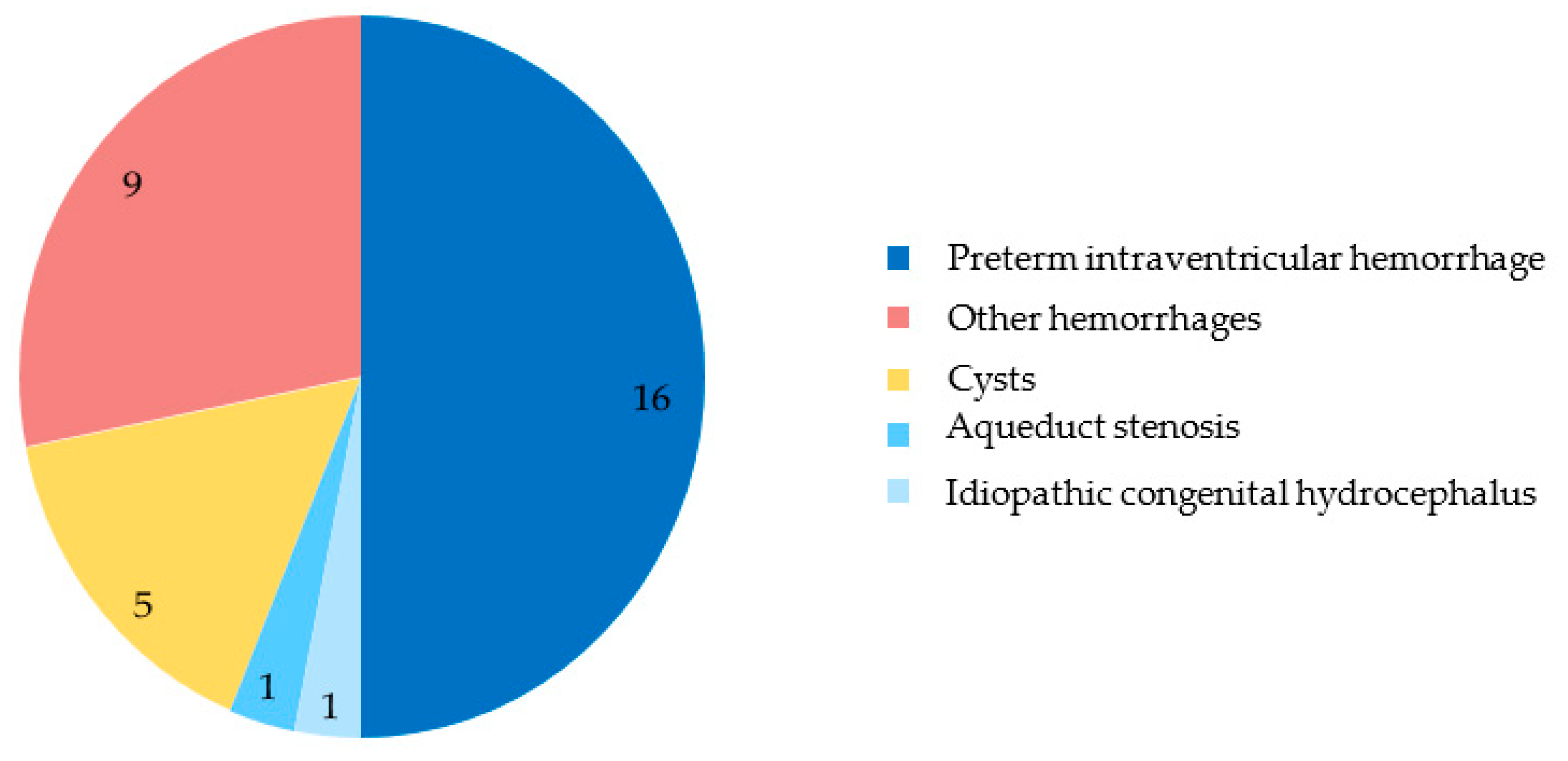
3.3. Diagnosis and Etiology
3.4. Treatment
3.5. Outcome
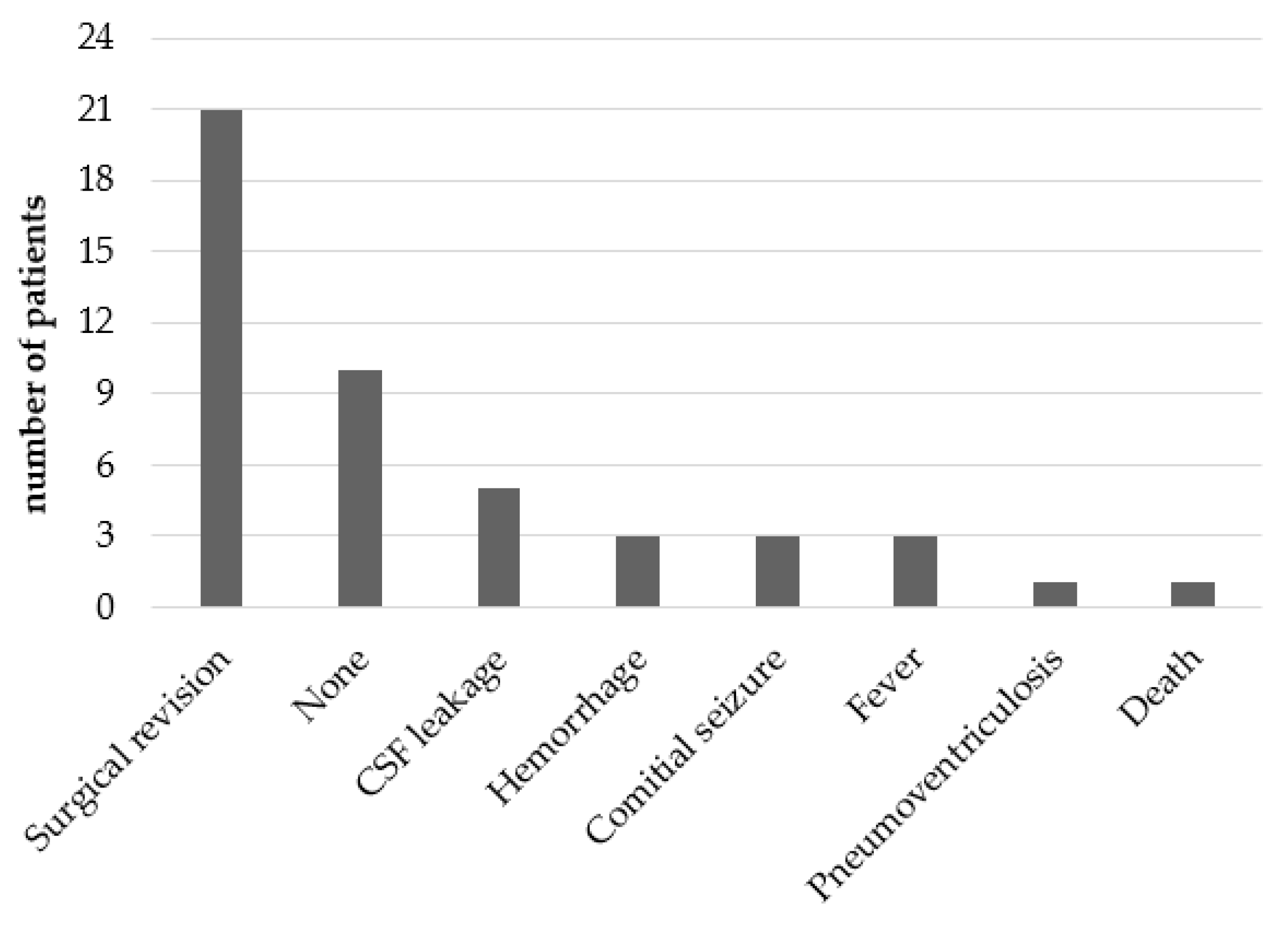
3.5.1. Acute Complications
3.5.2. Neurological Outcome at 2 and 6 Years Old
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NDD | neurodevelopmental disorders |
| VSGS | ventricular subgaleal shunt |
| IVH | intraventricular hemorrhage |
| CSF | cerebrospinal fluid |
| MRI | magnetic resonance imaging |
| ICP | intracranial pressure |
| HC | head circumference |
| ELVIS | Early vs. Late Ventricular Intervention Study |
| VPS | ventriculoperitoneal shunt |
| ASD | autism spectrum disorder |
| ADHD | attention deficit disorder with or without hyperactivity |
| WA | weeks of amenorrhea |
| LP | lumbar punction |
| ETV | endoscopic third ventriculostomy |
| GMFCS | Gross Motor Function Classification System |
| IQ | inter quartile |
| CP | cerebral palsy |
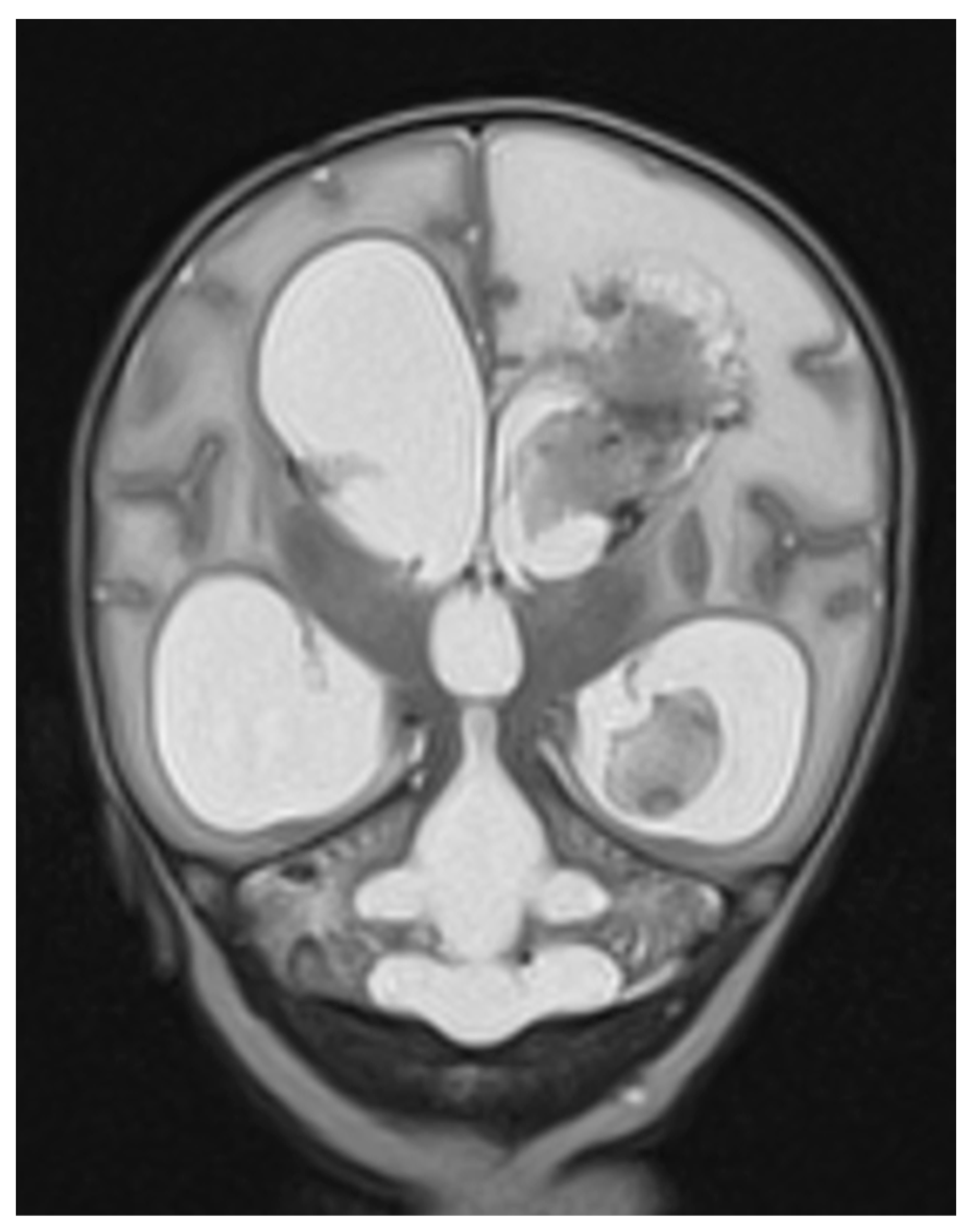
Appendix A
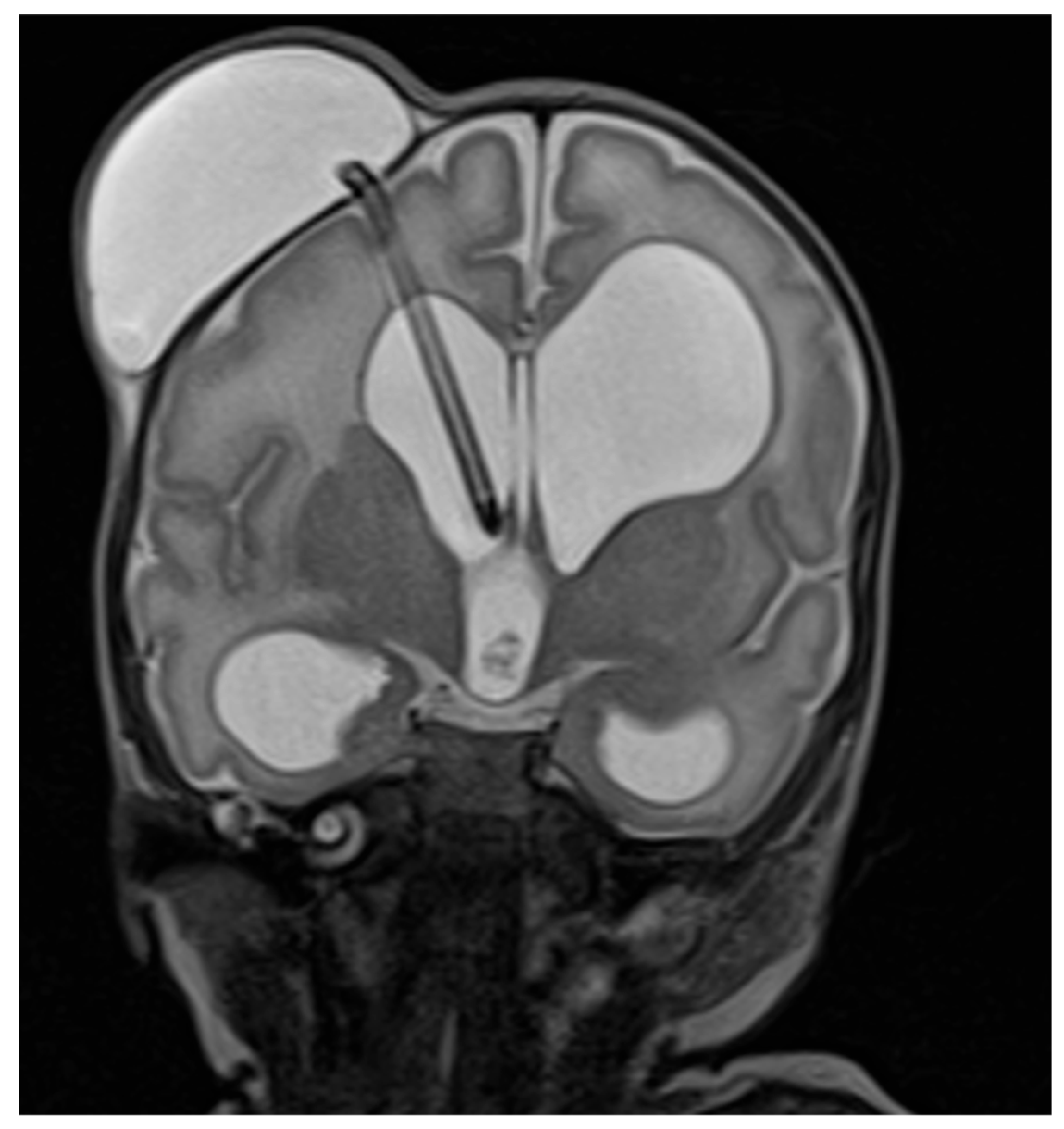
Appendix B
References
- Flanders, T.M.; Billinghurst, L.; Flibotte, J.; Heuer, G.G. Neonatal Hydrocephalus. NeoReviews 2018, 19, e467–e477. [Google Scholar] [CrossRef]
- Pindrik, J.; Schulz, L.; Drapeau, A. Diagnosis and Surgical Management of Neonatal Hydrocephalus. Semin. Pediatr. Neurol. 2022, 42, 100969. [Google Scholar] [CrossRef]
- Tully, H.M.; Dobyns, W.B. Infantile Hydrocephalus: A Review of Epidemiology, Classification and Causes. Eur. J. Med. Genet. 2014, 57, 359–368. [Google Scholar] [CrossRef]
- Whitelaw, A.; Brion, L.P.; Kennedy, C.R.; Odd, D. Diuretic Therapy for Newborn Infants with Posthemorrhagic Ventricular Dilatation. Cochrane Database Syst. Rev. 2001, 2001, CD002270. [Google Scholar] [CrossRef]
- Kennedy, C.R.; Ayers, S.; Campbell, M.J.; Elbourne, D.; Hope, P.; Johnson, A. Randomized, Controlled Trial of Acetazolamide and Furosemide in Posthemorrhagic Ventricular Dilation in Infancy: Follow-up at 1 Year. Pediatrics 2001, 108, 597–607. [Google Scholar] [CrossRef]
- Whitelaw, A.; Lee-Kelland, R. Repeated Lumbar or Ventricular Punctures in Newborns with Intraventricular Haemorrhage. Cochrane Database Syst. Rev. 2017, 4, 4. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Hong, C.J.; Nassiri, F.; Hong, B.Y.; Riva-Cambrin, J.; Kulkarni, A.V. Treatment of Posthemorrhagic Ventricular Dilation in Preterm Infants: A Systematic Review and Meta-Analysis of Outcomes and Complications. J. Neurosurg. Pediatr. 2015, 16, 545–555. [Google Scholar] [CrossRef]
- Levene, M.I.; Starte, D.R. A Longitudinal Study of Post-Haemorrhagic Ventricular Dilatation in the Newborn. Arch. Dis. Child. 1981, 56, 905–910. [Google Scholar] [CrossRef]
- de Vries, L.S.; Groenendaal, F.; Liem, K.D.; Heep, A.; Brouwer, A.J.; van ’t Verlaat, E.; Benavente-Fernández, I.; van Straaten, H.L.; van Wezel-Meijler, G.; Smit, B.J.; et al. Treatment Thresholds for Intervention in Posthaemorrhagic Ventricular Dilation: A Randomised Controlled Trial. Arch. Dis. Child.-Fetal Neonatal Ed. 2019, 104, F70–F75. [Google Scholar] [CrossRef]
- Cizmeci, M.N.; Groenendaal, F.; Liem, K.D.; Van Haastert, I.C.; Benavente-Fernández, I.; Van Straaten, H.L.M.; Steggerda, S.; Smit, B.J.; Whitelaw, A.; Woerdeman, P.; et al. Randomized Controlled Early versus Late Ventricular Intervention Study in Posthemorrhagic Ventricular Dilatation: Outcome at 2 Years. J. Pediatr. 2020, 226, 28–35.e3. [Google Scholar] [CrossRef]
- Leijser, L.M.; Miller, S.P.; van Wezel-Meijler, G.; Brouwer, A.J.; Traubici, J.; van Haastert, I.C.; Whyte, H.E.; Groenendaal, F.; Kulkarni, A.V.; Han, K.S.; et al. Posthemorrhagic Ventricular Dilatation in Preterm Infants. Neurology 2018, 90, e698–e706. [Google Scholar] [CrossRef]
- La Stratégie Nationale Autisme et Troubles du Neurodéveloppement (2018–2022)|handicap.gouv.fr. Available online: https://handicap.gouv.fr/la-strategie-nationale-autisme-et-troubles-du-neurodeveloppement-2018-2022 (accessed on 16 March 2021).
- Surveillance of Cerebral Palsy in Europe. Surveillance of Cerebral Palsy in Europe: A Collaboration of Cerebral Palsy Surveys and Registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev. Med. Child Neurol. 2000, 42, 816–824. [Google Scholar] [CrossRef]
- Gilard, V.; Chadie, A.; Ferracci, F.-X.; Brasseur-Daudruy, M.; Proust, F.; Marret, S.; Curey, S. Post Hemorrhagic Hydrocephalus and Neurodevelopmental Outcomes in a Context of Neonatal Intraventricular Hemorrhage: An Institutional Experience in 122 Preterm Children. BMC Pediatr. 2018, 18, 288. [Google Scholar] [CrossRef]
- Pressat-Laffouilhère, T.; Balayé, P.; Dahamna, B.; Lelong, R.; Billey, K.; Darmoni, S.J.; Grosjean, J. Evaluation of Doc’EDS: A French Semantic Search Tool to Query Health Documents from a Clinical Data Warehouse. BMC Med. Inform. Decis. Mak. 2022, 22, 34. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Anurag, J.; Sandeep, C.; Arunav, S. Ventriculosubgaleal Shunt: An Institutional Experience. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2023, 39, 2131–2137. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chen, W.-C.; Tseng, J.-J.; Ho, E.S.-C.; Chou, M.-M. Fetal Intracranial Hemorrhage (Fetal Stroke): Report of Four Antenatally Diagnosed Cases and Review of the Literature. Taiwan. J. Obstet. Gynecol. 2006, 45, 135–141. [Google Scholar] [CrossRef]
- Cinalli, G.; Spennato, P.; Nastro, A.; Aliberti, F.; Trischitta, V.; Ruggiero, C.; Mirone, G.; Cianciulli, E. Hydrocephalus in Aqueductal Stenosis. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2011, 27, 1621–1642. [Google Scholar] [CrossRef]
- Mancha, G.T.; Kadakia, S.; Muñoz, L.; Seske, L.M. Ten-Year Review of Neonatal Neurosurgical Outcomes and Cost Analysis. Surg. Neurol. Int. 2023, 14, 203. [Google Scholar] [CrossRef]
- Garcia-Navarro, V.; Perez-Vega, C.; Robles-Lomelín, P.; Valdez-Sandoval, P.; Vazquez, P.M.G.; Rodriguez, Y.L.; Cortes, S.G.L.; Naranjo, E.C. Early Intervention and Neurodevelopmental Outcome of Infants with Posthemorrhagic Hydrocephalus: A Case Series and Literature Review. Clin. Neurol. Neurosurg. 2021, 201, 106432. [Google Scholar] [CrossRef]
- Frassanito, P.; Serrao, F.; Gallini, F.; Bianchi, F.; Massimi, L.; Vento, G.; Tamburrini, G. Ventriculosubgaleal Shunt and Neuroendoscopic Lavage: Refining the Treatment Algorithm of Neonatal Post-Hemorrhagic Hydrocephalus. Childs Nerv. Syst. 2021, 37, 3531–3540. [Google Scholar] [CrossRef]
- Lam, H.P.; Heilman, C.B. Ventricular Access Device versus Ventriculosubgaleal Shunt in Post Hemorrhagic Hydrocephalus Associated with Prematurity. J. Matern. Fetal Neonatal Med. 2009, 22, 1097–1101. [Google Scholar] [CrossRef]
- Troubles du Neurodéveloppement-Repérage et Orientation des Enfants à Risque. Haute Autorité de Santé. Available online: https://www.has-sante.fr/jcms/p_3161334/fr/troubles-du-neurodeveloppement-reperage-et-orientation-des-enfants-a-risque (accessed on 19 March 2020).
- Whitelaw, A.; Jary, S.; Kmita, G.; Wroblewska, J.; Musialik-Swietlinska, E.; Mandera, M.; Hunt, L.; Carter, M.; Pople, I. Randomized Trial of Drainage, Irrigation and Fibrinolytic Therapy for Premature Infants with Posthemorrhagic Ventricular Dilatation: Developmental Outcome at 2 Years. Pediatrics 2010, 125, e852–e858. [Google Scholar] [CrossRef]
- Pierrat, V.; Marchand-Martin, L.; Marret, S.; Arnaud, C.; Benhammou, V.; Cambonie, G.; Debillon, T.; Dufourg, M.-N.; Gire, C.; Goffinet, F.; et al. Neurodevelopmental Outcomes at Age 5 among Children Born Preterm: EPIPAGE-2 Cohort Study. BMJ 2021, 373, n741. [Google Scholar] [CrossRef]
- Thomale, U.-W.; Cinalli, G.; Kulkarni, A.V.; Al-Hakim, S.; Roth, J.; Schaumann, A.; Bührer, C.; Cavalheiro, S.; Sgouros, S.; Constantini, S.; et al. TROPHY Registry Study Design: A Prospective, International Multicenter Study for the Surgical Treatment of Posthemorrhagic Hydrocephalus in Neonates. Childs Nerv. Syst. 2019, 35, 613–619. [Google Scholar] [CrossRef]
| General Population n = 32 | MD | |
|---|---|---|
| Gender, n (%) Female Male | 14 (44) 18 (56) | |
| Term of birth in weeks of amenorrhea, median (IQ) | 33.3 (28.5–36.8) | 1 |
| Preterm infant, n (%) | 22 (69) | |
| Birth measurement, median (1st IQ–3rd IQ) | ||
| Birth weight (g) | 1960 (1285–2440) | 1 |
| Birth weight percentile | 56 (24.5–78) | 1 |
| Birth height (cm) | 43 (39–45) | 3 |
| Birth height percentile | 49 (14–76) | 3 |
| Birth head circumference (cm) | 31 (26.7–34.5) | 1 |
| Birth head circumference percentile | 54.1 (17.3–90.25) | 1 |
| Delivery mode, n (%) | ||
| Vaginal delivery Caesarean section | 11 (34) 18 (56) | 3 |
| Age at first intervention, median (IQ) In days Age-adjusted (WA) | 19.5 (13.0–34.5) 36.0 (33.5–38.6) |
| Shunt side, n (%) Right Left | 23 (72%) 9 (28%) |
| Associated treatment, n (%) Diuretic Subtractive LP Subgaleal puncture | 5 (15%) 1 (3.1%) 4 (12.5%) 2 (6.2%) |
| Surgical revision | 32 (100%) |
| Cause of revision Infection 2nd shunt required 1st shunt revision Permanent shunt Hemorrhage | 3 (12%) 8 (25%) 14 (44%) 5 (15%) 2 (6%) |
| Revision delay, in days, median (IQ) | 27 (11–43) |
| Shunt life, in days, median (IQ) 1st ventriculosubgaleal shunt 2nd ventriculosubgaleal shunt | 50 (29.2–76.2) 29 (25–31) |
| Permanent CSF diversion Ventriculoperitoneal shunt Endoscopic third ventriculostomy None | 24 (75%) 23 (72%) 4 (12.5%) 8 (25%) |
| Age at permanent CSF diversion, median (IQ) | 73 (57–98) |
| Total number of neurosurgical procedures, median (IQ) | 4 (3–6.2) |
| Population n = 32 | MD | |
|---|---|---|
| Follow-up by a pediatrician, n (%) | 24 (75) | |
| Follow-up by a neurosurgeon, n (%) | 27 (84) | |
| Death, n (%) | 3 (13.6) | |
| Patients over 2 years of age, n (%) | n = 22 | |
| Acquired walking | 10 (45) | |
| Cerebral palsy | 10 (45) | |
| Acquired language | 11 (50) | 1 |
| Normal vision | 17 (77) | |
| Normal hearing | 19 (86) | 3 |
| Epilepsy | 2 (9) | |
| Persistent Hydrocephalus | 6 (27) | |
| Patients over 6 years of age, n (%) | n = 15 | |
| Acquired walking | 10 (67%) | 1 |
| Attending schooling | 13 (87) | |
| Educational facilities | 6 (40) | 2 |
| Rehabilitation | 11 (73) | |
| Associated NDD | 3 (20%) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Follin-Arbelet, T.; Chadie, A.; Muller, J.-B.; Curey, S.; Grosjean, J.; Toulemonde, C.; Marret, S. Ventricular Subgaleal Shunt in Children Under Three Months of Age, from Diagnosis to Outcome: A Review After 11 Years of Experience in a French University Hospital. Children 2025, 12, 983. https://doi.org/10.3390/children12080983
Follin-Arbelet T, Chadie A, Muller J-B, Curey S, Grosjean J, Toulemonde C, Marret S. Ventricular Subgaleal Shunt in Children Under Three Months of Age, from Diagnosis to Outcome: A Review After 11 Years of Experience in a French University Hospital. Children. 2025; 12(8):983. https://doi.org/10.3390/children12080983
Chicago/Turabian StyleFollin-Arbelet, Timothée, Alexandra Chadie, Jean-Baptiste Muller, Sophie Curey, Julien Grosjean, Cécile Toulemonde, and Stéphane Marret. 2025. "Ventricular Subgaleal Shunt in Children Under Three Months of Age, from Diagnosis to Outcome: A Review After 11 Years of Experience in a French University Hospital" Children 12, no. 8: 983. https://doi.org/10.3390/children12080983
APA StyleFollin-Arbelet, T., Chadie, A., Muller, J.-B., Curey, S., Grosjean, J., Toulemonde, C., & Marret, S. (2025). Ventricular Subgaleal Shunt in Children Under Three Months of Age, from Diagnosis to Outcome: A Review After 11 Years of Experience in a French University Hospital. Children, 12(8), 983. https://doi.org/10.3390/children12080983






