Clinical and Molecular Characterizations of Mitochondrial Disorders: A Tertiary-Care Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
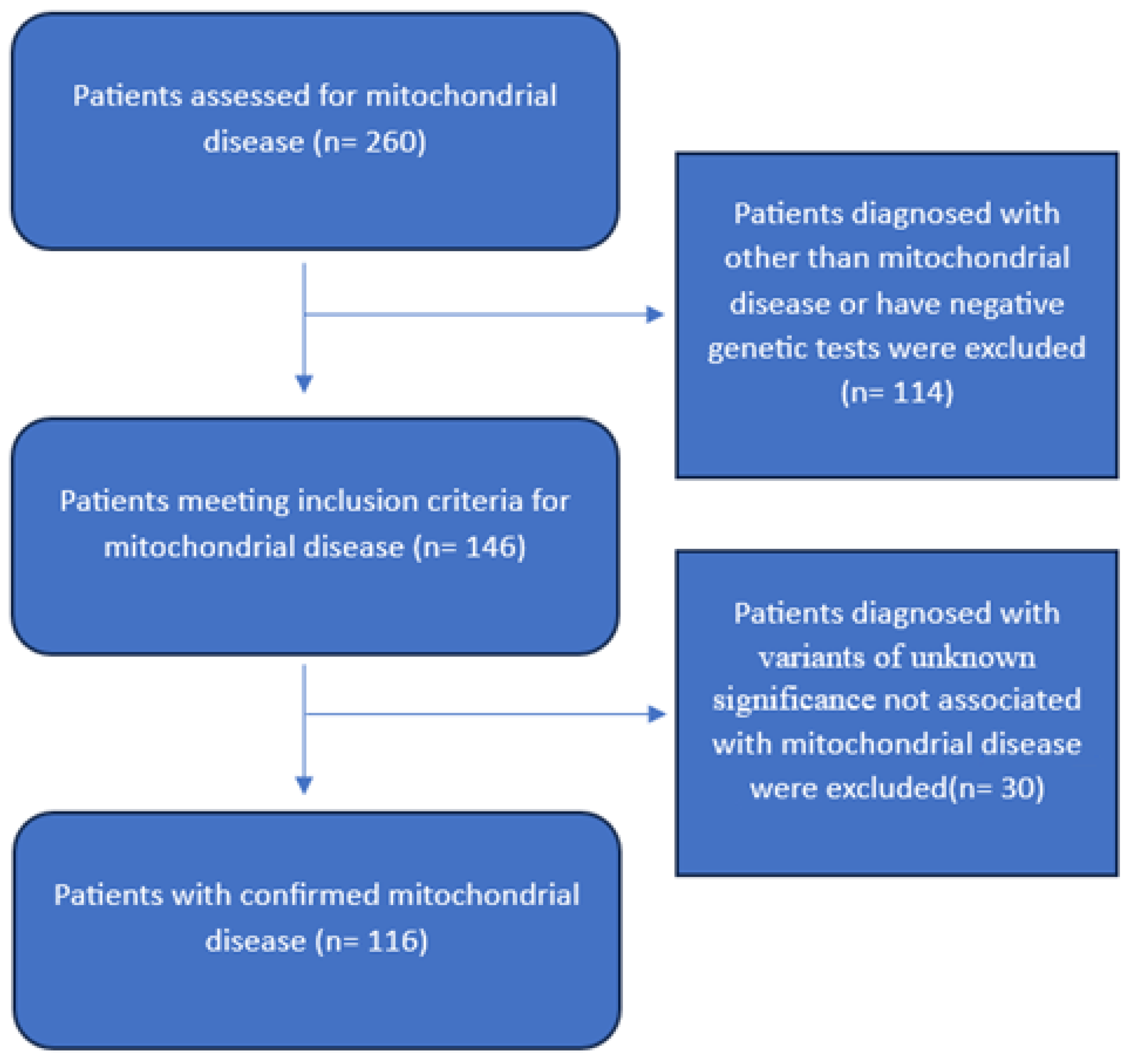
2.2. Study Population and Settings
2.3. Data Collection
2.4. Ethical Approval
2.5. Statistical Analysis
3. Results
3.1. Patients’ Demographic and Clinical Background Characteristics
3.2. Gene Type Stratified by Patients’ Demographic Characteristics
3.3. Factors Associated with Having Mitochondrial Gene Type
3.4. Factors Associated with Survival
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCormick, E.M.; Zolkipli-Cunningham, Z.; Falk, M.J. Mitochondrial disease genetics update: Recent insights into the molecular diagnosis and expanding phenotype of primary mitochondrial disease. Curr. Opin. Pediatr. 2018, 30, 714–724. [Google Scholar] [CrossRef] [PubMed]
- DiMauro, S.; Schon, E.A. Mitochondrial DNA mutations in human disease. Am. J. Med. Genet. 2001, 106, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Alahmad, A.; Muhammad, H.; Pyle, A.; Albash, B.; McFarland, R.; Taylor, R. Mitochondrial disorders in the Arab Middle East Population: The impact of next generation sequencing in the genetic diagnosis. J. Biochem. Clin. Genet. 2019, 2, 54–64. [Google Scholar] [CrossRef]
- Rahman, S. Mitochondrial disease in children. J. Intern. Med. 2020, 287, 609–633. [Google Scholar] [CrossRef]
- Bizzari, S.; Qari, A.; Balobaid, A.; Hana, S.; Deepthi, A.; Nair, P.; El-Hayek, S.; Hamzeh, A.R. Genetic Disorders in Saudi Arabia: A CTGA Perspective. 2018. Available online: https://cags.org.ae/contentfiles/uploads/files/chapter%205.pdf (accessed on 13 April 2025).
- Tucker, E.J.; Compton, A.G.; Thorburn, D.R. Recent advances in the genetics of mitochondrial encephalopathies. Curr. Neurol. Neurosci. Rep. 2010, 10, 277–285. [Google Scholar] [CrossRef]
- Tinker, R.J.; Falk, M.J.; Goldstein, A.; George-Sankoh, I.; Xiao, R.; Adang, L.; Ganetzky, R. Early developmental delay in Leigh syndrome spectrum disorders is associated with poor clinical prognosis. Mol. Genet. Metab. 2022, 135, 342–349. [Google Scholar] [CrossRef]
- Hauser, F. About hereditary and symptomatic congenital thrombopenia. Ann. Paediatr. 1948, 171, 86–102. [Google Scholar]
- Bourgeron, T.; Rustin, P.; Chretien, D.; Birch-Machin, M.; Bourgeois, M.; Viegas-Péquignot, E.; Munnich, A.; Rötig, A. Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat. Genet. 1995, 11, 144–149. [Google Scholar] [CrossRef]
- Holt, I.J.; Harding, A.E.; Morgan-Hughes, J.A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 1988, 331, 717–719. [Google Scholar] [CrossRef]
- Wallace, D.C.; Singh, G.; Lott, M.T.; Hodge, J.A.; Schurr, T.G.; Lezza, A.M.; Elsas, L.J.; Nikoskelainen, E.K. Mitochondrial DNA mutation associated with Leber′s hereditary optic neuropathy. Science 1988, 242, 1427–1430. [Google Scholar] [CrossRef]
- Morava, E.; van den Heuvel, L.P.W.J.; Hol, F.; De Vries, M.C.; Hogeveen, M.; Rodenburg, R.J.; Smeitink, J.A.M. Mitochondrial disease criteria: Diagnostic applications in children. Neurology 2006, 67, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Schlieben, L.D.; Prokisch, H. The Dimensions of Primary Mitochondrial Disorders. Front. Cell Dev. Biol. 2020, 8, 600079. [Google Scholar] [CrossRef] [PubMed]
- Al-Kafaji, G.; Alwehaidah, M.S.; Alsabbagh, M.M.; Alharbi, M.A.; Bakhiet, M. Mitochondrial DNA haplogroup analysis in Saudi Arab patients with multiple sclerosis. PLoS ONE 2022, 17, e0279237. [Google Scholar] [CrossRef]
- Alharbi, K.K.; Alsaikhan, A.S.; Alshammary, A.F.; Al-Hakeem, M.M.; Khan, I.A. Screening of mitochondrial mutations in Saudi women diagnosed with gestational diabetes mellitus: A non-replicative case-control study. Saudi J. Biol. Sci. 2022, 29, 360–365. [Google Scholar] [CrossRef]
- Modell, B.; Darr, A. Science and society: Genetic counselling and customary consanguineous marriage. Nat. Rev. Genet. 2002, 3, 225–259. [Google Scholar] [CrossRef]
- Paiva Coelho, M.; Martins, E.; Vilarinho, L. Diagnosis, management, and follow-up of mitochondrial disorders in childhood: A personalized medicine in the new era of genome sequence. Eur. J. Pediatr. 2019, 178, 21–32. [Google Scholar] [CrossRef]
- Keshavan, N.; Rahman, S. Natural history of mitochondrial disorders: A systematic review. Essays Biochem. 2018, 62, 423–442. [Google Scholar] [CrossRef]
- Nguyen, T.; Alzahrani, T.; Krepp, J.; Panjrath, G. Cardiovascular Outcomes in Patients with Mitochondrial Disease in the United States: A Propensity Score Analysis. Tex. Heart Inst. J. 2021, 48, e207243. [Google Scholar] [CrossRef]
- Al-Gazali, L.; Hamamy, H. Consanguinity and dysmorphology in Arabs. Hum. Hered. 2014, 77, 93–107. [Google Scholar] [CrossRef]
- Whittaker, R.G.; Devine, H.E.; Gorman, G.S.; Schaefer, A.M.; Horvath, R.; Ng, Y.; Nesbitt, V.; Lax, N.Z.; McFarland, R.; Cunningham, M.O.; et al. Epilepsy in adults with mitochondrial disease: A cohort study. Ann. Neurol. 2015, 78, 949–957. [Google Scholar] [CrossRef]
- Lopriore, P.; Gomes, F.; Montano, V.; Siciliano, G.; Mancuso, M. Mitochondrial Epilepsy, a Challenge for Neurologists. Int. J. Mol. Sci. 2022, 23, 13216. [Google Scholar] [CrossRef]
- Falk, M.J. Neurodevelopmental manifestations of mitochondrial disease. J. Dev. Behav. Pediatr. 2010, 31, 610–621. [Google Scholar] [CrossRef]
- Angelini, C.; Bello, L.; Spinazzi, M.; Ferrati, C. Mitochondrial disorders of the nuclear genome. Acta Myol. 2009, 28, 16–23. [Google Scholar] [PubMed]
- Niknahad, H.; Jamshidzadeh, A.; Heidari, R.; Zarei, M.; Ommati, M.M. Ammonia-induced mitochondrial dysfunction and energy metabolism disturbances in isolated brain and liver mitochondria, and the effect of taurine administration: Relevance to hepatic encephalopathy treatment. Clin. Exp. Hepatol. 2017, 3, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Saneto, R.P.; Friedman, S.D.; Shaw, D.W. Neuroimaging of mitochondrial disease. Mitochondrion 2008, 8, 396–413. [Google Scholar] [CrossRef]
- Gropman, A.L. Neuroimaging in mitochondrial disorders. Neurotherapeutics 2013, 10, 273–285. [Google Scholar] [CrossRef]
- Chevallier, J.A.; Von Allmen, G.K.; Koenig, M.K. Seizure semiology and EEG findings in mitochondrial diseases. Epilepsia 2014, 55, 707–712. [Google Scholar] [CrossRef]
- Horvath, R.; Medina, J.; Reilly, M.M.; Shy, M.E.; Zuchner, S. Peripheral neuropathy in mitochondrial disease. Handb. Clin. Neurol. 2023, 194, 99–116. [Google Scholar]
- Ng, Y.S.; Turnbull, D.M. Mitochondrial disease: Genetics and management. J. Neurol. 2016, 263, 179–191. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial aging and age-related dysfunction of mitochondria. Biomed. Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef]
- Goldstein, A.C.; Bhatia, P.; Vento, J.M. Mitochondrial disease in childhood: Nuclear encoded. Neurotherapeutics 2013, 10, 212–226. [Google Scholar] [CrossRef]
- Wesół-Kucharska, D.; Rokicki, D.; Jezela-Stanek, A. Epilepsy in Mitochondrial Diseases-Current State of Knowledge on Aetiology and Treatment. Children 2021, 8, 532. [Google Scholar] [CrossRef]
- Lopriore, P.; Ricciarini, V.; Siciliano, G.; Mancuso, M.; Montano, V. Mitochondrial Ataxias: Molecular Classification and Clinical Heterogeneity. Neurol. Int. 2022, 14, 337–356. [Google Scholar] [CrossRef]
| Variable | Frequency (Percentage) | |
|---|---|---|
| Sex | ||
| Female | 63 (54.3%) | |
| Male | 53 (45.7%) | |
| Mean ± SD | Range | |
| Current age (Years) | 10 ± 7 | 1–59 |
| Age of Onset (Years) | 2 ± 5.6 | 0–52 |
| Age at Diagnosis (Years) | 5.3 ± 8 | 1–59 |
| Age at Death (Years) | 3 ± 4 | 0–17.6 |
| Mortality | ||
| Yes | 40 (34.5%) | |
| No | 76 (65.5%) | |
| Cause of Death | ||
| Cardiopulmonary arrest | 22 (55.0%) | |
| Respiratory Failure Other | 7 (17.5%) 11 (27.5%) | |
| Family history of mitochondrial disease | 69 (59.5%) | |
| Consanguinity | 95 (81.9%) | |
| Clinical feature | ||
| Developmental delay | 79 (67.9%) | |
| Global | 70 | |
| Language | 5 | |
| Motor | 3 | |
| Social | 1 | |
| Endocrinopathy | 40 (34.5%) | |
| Poor growth | 23 | |
| Short stature | 15 | |
| Hypoglycemia | 9 | |
| Adrenal insufficiency | 9 | |
| Diabetes mellitus | 2 | |
| Growth hormone deficiency | 2 | |
| Hypoparathyroidism | 1 | |
| Hypothyroidism | 1 | |
| Pancreatic insufficiency | 1 | |
| Hypogonadism | 1 | |
| Syndrome of Inappropriate Anti-Diuretic Hormone Release | 1 | |
| Epilepsy | 33 (28.4%) | |
| Spasticity | 32 (27.8%) | |
| Cardiomyopathy | 25 (21.6%) | |
| Optic atrophy | 18 (15.5%) | |
| Encephalopathy | 18 (15.5%) | |
| Myopathy | 13 (11.2%) | |
| Ophthalmoplegia | 9 (7.8%) | |
| Ataxia | 8 (7.0%) | |
| Chorea | 8 (7.0%) | |
| Sensorineural deafness | 8 (7.0%) | |
| Exercise intolerance | 6 (5.2%) | |
| Ptosis | 5 (4.3%) | |
| Migraine | 3 (2.6%) | |
| Stroke-like episodes | 2 (1.7%) | |
| Pigmentary retinopathy | 1 (0.9%) | |
| Frequency (Percentage) | |
|---|---|
| Seizure Type | |
| Generalized tonic clonic | 15 (12.9%) |
| Focal, motor, clonic | 11 (9.5%) |
| Focal, motor, tonic | 5 (4.3%) |
| Myoclonic | 3 (2.6%) |
| Infantile spasms | 3 (2.6%) |
| Focal to bilateral tonic clonic | 2 (1.7%) |
| Anti-seizure Medications | |
| Multiple | 21 (18.1%) |
| Single | 11 (9.5%) |
| Frequency (Percentage) | |
|---|---|
| Type of test | |
| Whole Exome Sequencing (WES) | 66 (56.9%) |
| Single Gene | 27 (23.3%) |
| Whole Genome Sequencing (WGS) | 12 (10.3%) |
| Mitochondrial Panel | 9 (7.8%) |
| Gene Panel | 2 (1.7%) |
| Demographic Characteristics | Type of Gene | p-Value | |
|---|---|---|---|
| Nuclear | Mitochondrial | ||
| Frequency Percentage | Frequency (Percentage) | ||
| Sex | |||
| Male | 50 (94.3%) | 3 (5.7%) | 0.725 |
| Female | 58 (92.1%) | 5 (7.9%) | |
| Mortality | |||
| Yes | 39 (97.5%) | 1 (2.5%) | 0.260 |
| No | 69 (90.8%) | 7 (9.2%) | |
| Cause of Death | |||
| Respiratory Failure | 7 (100.0%) | 0 (0.0%) | 0.450 |
| Cardiopulmonary arrest due to an unknown cause | 22 (100.0%) | 0 (0.0%) | |
| Others | 10 (90.9%) | 1 (9.1%) | |
| Family history of mitochondrial disease | |||
| Yes | 66 (95.7%) | 3 (4.3%) | 0.266 |
| No | 42 (89.4%) | 5 (10.6%) | |
| Consanguinity | |||
| Yes | 88 (92.6%) | 7 (7.4%) | 1.000 |
| No | 20 (95.2%) | 1 (4.8%) | |
| Median age differences stratified by gene type | |||
| Median (IQR) | Median (IQR) | p-Value | |
| Current age (Years) | 8 (4–13) | 17 (15–22) | <0.001 * |
| Age of Onset (Years) | 0.5 (0–1) | 1.5 (0–10) | 0.675 |
| Age at Diagnosis (Years) | 2 (0.8–6) | 11.5 (7–16) | <0.001 * |
| Age at Death (Years) | 1.3 (0.8–3) | 17.6 (17.6–17.6) | 0.091 |
| Frequency (Percentage) | ||
|---|---|---|
| Ammonia | ||
| Normal | 55 (47.4%) | |
| Abnormal | 39 (33.6%) | |
| Not Available | 22 (19.0%) | |
| Lactic Acid | ||
| Normal | 44 (37.9%) | |
| Increased | 58 (50.0%) | |
| Not Available | 14 (12.1%) | |
| Thyroid-Stimulating Hormone | ||
| Normal | 55 (47.4%) | |
| High | 9 (7.8%) | |
| Low | 3 (2.6%) | |
| Not Available | 49 (42.2%) | |
| T4 | ||
| Normal | 43 (37.1%) | |
| High | 1 (0.9%) | |
| Low | 5 (4.3%) | |
| Not Available | 67 (57.8%) | |
| Urine Organic Acids | ||
| Normal | 35 (30.2%) | |
| Abnormal | 14 (12.1%) | |
| Not Available | 67(57.8%) | |
| Plasma Amino Acids | ||
| Normal | 33 (28.4%) | |
| Abnormal | ||
| Not Available | ||
| Variable | Adjusted Odds Ratio of Having Mitochondrial Gene Type 95% Confidence Interval § | p-Value |
|---|---|---|
| Sex | ||
| Male (Reference category) | 1.00 | |
| Female | 1.63 (0.33–8.06) | 0.550 |
| Mortality | ||
| No (Reference category) | 1.00 | |
| Yes | 0.39 (0.04–3.70) | 0.411 |
| Epilepsy | ||
| No (Reference category) | 1.00 | |
| Yes | 0.98 (0.16–5.94) | 0.981 |
| Family history of mitochondrial disease | ||
| No (Reference category) | 1.00 | |
| Yes | 0.42 (0.09–2.01) | 0.275 |
| Variable | Adjusted Odds Ratio for Patients’ Survival 95% Confidence Interval | p-Value |
|---|---|---|
| Sex | ||
| Male (Reference category) | 1.00 | |
| Female | 1.01 (0.19–5.40) | 0.996 |
| Consanguinity | ||
| No (Reference category) | 1.00 | |
| Yes | 0.77 (0.09–6.96) | 0.815 |
| Family history of mitochondrial disease | ||
| No (Reference category) | 1.00 | |
| Yes | 0.18 (0.04–0.87) | 0.033 * |
| Anti-seizure medication patterns | ||
| Single anti-seizure medication (Reference category) | 1.00 | |
| Muliple anti-seizure medication | 1.19 (0.27–5.29) | 0.821 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuqbil, M.; Binsabbar, N.; Alsaif, S.; Almasoud, S.; Albasry, T.; Baarmah, D.; Altwaijri, W.; Alrumayyan, A. Clinical and Molecular Characterizations of Mitochondrial Disorders: A Tertiary-Care Center Experience. Children 2025, 12, 1102. https://doi.org/10.3390/children12081102
Almuqbil M, Binsabbar N, Alsaif S, Almasoud S, Albasry T, Baarmah D, Altwaijri W, Alrumayyan A. Clinical and Molecular Characterizations of Mitochondrial Disorders: A Tertiary-Care Center Experience. Children. 2025; 12(8):1102. https://doi.org/10.3390/children12081102
Chicago/Turabian StyleAlmuqbil, Mohammed, Najla Binsabbar, Shahad Alsaif, Sulaiman Almasoud, Talah Albasry, Duaa Baarmah, Waleed Altwaijri, and Ahmed Alrumayyan. 2025. "Clinical and Molecular Characterizations of Mitochondrial Disorders: A Tertiary-Care Center Experience" Children 12, no. 8: 1102. https://doi.org/10.3390/children12081102
APA StyleAlmuqbil, M., Binsabbar, N., Alsaif, S., Almasoud, S., Albasry, T., Baarmah, D., Altwaijri, W., & Alrumayyan, A. (2025). Clinical and Molecular Characterizations of Mitochondrial Disorders: A Tertiary-Care Center Experience. Children, 12(8), 1102. https://doi.org/10.3390/children12081102





