Association Between Endotype of Prematurity and Cystic Periventricular Leukomalacia: A Bayesian Model-Averaged Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction, Definitions, and Quality Assessment
2.4. Bayesian Model-Averaged Meta-Analysis
3. Results
3.1. Description of Studies and Risk of Bias Assessment
3.2. Bayesian Meta-Analysis
3.3. Subgroup Analysis and Meta-Regression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volpe, J.J. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr. Res. 2001, 50, 553–562. [Google Scholar] [CrossRef]
- Khwaja, O.; Volpe, J. Pathogenesis of cerebral white matter injury of prematurity. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F153–F161. [Google Scholar] [CrossRef]
- Pierson, C.R.; Volpe, J.J. Encephalopathy of prematurity: Neuropathology. In Volpe’s Neurology of the Newborn; Elsevier: Amsterdam, The Netherlands, 2025; pp. 506–522.e503. [Google Scholar]
- Guillot, M.; Miller, S.P. The dimensions of white matter injury in preterm neonates. Semin. Perinatol. 2021, 45, 151469. [Google Scholar] [CrossRef]
- Agut, T.; Alarcon, A.; Cabañas, F.; Bartocci, M.; Martinez-Biarge, M.; Horsch, S. Preterm white matter injury: Ultrasound diagnosis and classification. Pediatr. Res. 2020, 87, 37–49. [Google Scholar] [CrossRef]
- Abiramalatha, T.; Bandyopadhyay, T.; Ramaswamy, V.V.; Shaik, N.B.; Thanigainathan, S.; Pullattayil, A.K.; Amboiram, P. Risk factors for periventricular leukomalacia in preterm infants: A systematic review, meta-analysis, and GRADE-based assessment of certainty of evidence. Pediatr. Neurol. 2021, 124, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Pierro, M.; Van Mechelen, K.; van Westering-Kroon, E.; Villamor-Martínez, E.; Villamor, E. Endotypes of prematurity and phenotypes of bronchopulmonary dysplasia: Toward personalized neonatology. J. Pers. Med. 2022, 12, 687. [Google Scholar] [CrossRef]
- Pierro, M.; Philip, R.; Renesme, L.; Villamor, E. Endotyping and phenotyping prematurity and its complications. J. Pers. Med. 2023, 11, 1217530. [Google Scholar] [CrossRef]
- Rezaie, P.; Dean, A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology 2002, 22, 106–132. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Park, C.W.; Chaiworapongsa, T. Intrauterine infection and the development of cerebral palsy. BJOG 2003, 110, 124–127. [Google Scholar] [CrossRef]
- Kadhim, H.; Tabarki, B.; Verellen, G.; De Prez, C.; Rona, A.-M.; Sébire, G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology 2001, 56, 1278–1284. [Google Scholar] [CrossRef]
- Parsons, A.; Netsanet, A.; Seedorf, G.; Abman, S.H.; Taglauer, E.S. Understanding the role of placental pathophysiology in the development of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 2022, 323, L651–L658. [Google Scholar] [CrossRef]
- Aparici, S.; Martorell, L.; Codoñer-Franch, P. Role of oxidative stress in preterm infants with bronchopulmonary dysplasia after exposure to chorioamnionitis. J. Pediatr. Biochem. 2013, 3, 143–153. [Google Scholar]
- Kacerovsky, M.; Tothova, L.; Menon, R.; Vlkova, B.; Musilova, I.; Hornychova, H.; Prochazka, M.; Celec, P. Amniotic fluid markers of oxidative stress in pregnancies complicated by preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2015, 28, 1250–1259. [Google Scholar] [CrossRef]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.L. Oxidative stress in placental pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Hung, T.H.; Burton, G.J. Hypoxia and reoxygenation: A possible mechanism for placental oxidative stress in preeclampsia. Taiwan. J. Obstet. Gynecol. 2006, 45, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Gonzalez-Luis, G.E.; Borges-Lujan, M.; Villamor, E. Association between endotypes of prematurity and pharmacological closure of patent ductus arteriosus: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1078506. [Google Scholar] [CrossRef]
- Hundscheid, T.M.; Villamor-Martinez, E.; Villamor, E. Association between endotype of prematurity and mortality: A systematic review, meta-analysis, and meta-regression. Neonatology 2023, 120, 407–416. [Google Scholar] [CrossRef]
- Pierro, M.; Villamor-Martinez, E.; van Westering-Kroon, E.; Alvarez-Fuente, M.; Abman, S.H.; Villamor, E. Association of the dysfunctional placentation endotype of prematurity with bronchopulmonary dysplasia: A systematic review, meta-analysis and meta-regression. Thorax 2022, 77, 268–275. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Cavallaro, G.; Raffaeli, G.; Mohammed Rahim, O.M.; Gulden, S.; Ghazi, A.M.; Mosca, F.; Degraeuwe, P.; Villamor, E. Chorioamnionitis as a risk factor for retinopathy of prematurity: An updated systematic review and meta-analysis. PLoS ONE 2018, 13, e0205838. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Fumagalli, M.; Mohammed Rahim, O.; Passera, S.; Cavallaro, G.; Degraeuwe, P.; Mosca, F.; Villamor, E. Chorioamnionitis is a risk factor for intraventricular hemorrhage in preterm infants: A systematic review and meta-analysis. Front. Physiol. 2018, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Villamor-Martinez, E.; Lubach, G.A.; Rahim, O.M.; Degraeuwe, P.; Zimmermann, L.J.; Kramer, B.W.; Villamor, E. Association of histological and clinical chorioamnionitis with neonatal sepsis among preterm infants: A systematic review, meta-analysis, and meta-regression. Front. Immunol. 2020, 11, 972. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Álvarez-Fuente, M.; Ghazi, A.M.; Degraeuwe, P.; Zimmermann, L.J.; Kramer, B.W.; Villamor, E. Association of chorioamnionitis with bronchopulmonary dysplasia among preterm infants: A systematic review, meta-analysis, and metaregression. JAMA Netw. Open 2019, 2, e1914611. [Google Scholar] [CrossRef] [PubMed]
- Behbodi, E.; Villamor-Martínez, E.; Degraeuwe, P.L.; Villamor, E. Chorioamnionitis appears not to be a risk factor for patent ductus arteriosus in preterm infants: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 37967. [Google Scholar] [CrossRef]
- Borges-Luján, M.; Galán-Henríquez, G.; Rodríguez-Viera, R.I.; Bartoš, F.; González-Luis, G.E.; Villamor, E. Association Between Hypertensive Disorders of Pregnancy and Patent Ductus Arteriosus in Very Preterm Infants: A Bayesian Model-Averaged Meta-Analysis. Children 2025, 12, 762. [Google Scholar] [CrossRef]
- Gordijn, S.; Beune, I.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.; Baker, P.; Silver, R.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Hundscheid, T.M.; Huizing, M.J.; Villamor-Martinez, E.; Bartoš, F.; Villamor, E. Association of funisitis with short-term outcomes of prematurity: A frequentist and Bayesian meta-analysis. Antioxidants 2023, 12, 534. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Scale Cohort Studies; University of Ottawa: Ottawa, ON, Canada, 2014; Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 29 May 2025).
- Bartoš, F.; Gronau, Q.F.; Timmers, B.; Otte, W.M.; Ly, A.; Wagenmakers, E.J. Bayesian model-averaged meta-analysis in medicine. Stat. Med. 2021, 40, 6743–6761. [Google Scholar] [CrossRef]
- Gronau, Q.F.; Heck, D.W.; Berkhout, S.W.; Haaf, J.M.; Wagenmakers, E.J. A primer on Bayesian model-averaged meta-analysis. Adv. Methods Pract. Psychol. Sci. 2021, 4, 25152459211031256. [Google Scholar] [CrossRef]
- van Doorn, J.; van den Bergh, D.; Böhm, U.; Dablander, F.; Derks, K.; Draws, T.; Etz, A.; Evans, N.J.; Gronau, Q.F.; Haaf, J.M. The JASP guidelines for conducting and reporting a Bayesian analysis. Psychon. Bull. Rev. 2021, 28, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Bartoš, F.; Maier, M. RoBMA: An R Package for Robust Bayesian Meta-Analyses. Version 3.5.1. 2025R. Available online: https://cran.r-project.org/web/packages/RoBMA/index.html (accessed on 29 May 2025).
- Lee, M.; Wagenmakers, E.J. Bayesian Cognitive Modeling: A Practical Course; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Bartoš, F.; Maier, M.; Wagenmakers, E.J.; Doucouliagos, H.; Stanley, T. Robust Bayesian meta-analysis: Model-averaging across complementary publication bias adjustment methods. Res. Synth. Methods 2023, 14, 99–116. [Google Scholar] [CrossRef]
- Bartoš, F.; Maier, M.; Stanley, T.; Wagenmakers, E.J. Robust Bayesian meta-regression—Model-averaged moderation analysis in the presence of publication bias. Psychol. Methods 2023. online ahead of print. [Google Scholar] [CrossRef]
- Bartoš, F.; Otte, W.M.; Gronau, Q.F.; Timmers, B.; Ly, A.; Wagenmakers, E.J. Empirical prior distributions for Bayesian meta-analyses of binary and time to event outcomes. arXiv 2023, arXiv:2306.11468. [Google Scholar] [CrossRef]
- Ahn, H.M.; Park, E.A.; Cho, S.J.; Kim, Y.J.; Park, H.S. The association of histological chorioamnionitis and antenatal steroids on neonatal outcome in preterm infants born at less than thirty-four weeks’ gestation. Neonatology 2012, 102, 259–264. [Google Scholar] [CrossRef]
- Alexander, J.M.; Gilstrap, L.C.; Cox, S.M.; Leveno, K.J. Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet. Gynecol. 1998, 91, 725–729. [Google Scholar] [CrossRef]
- Altman, M.; Vanpée, M.; Cnattingius, S.; Norman, M. Neonatal morbidity in moderately preterm infants: A Swedish national population-based study. J. Pediatr. 2011, 158, 239–244.e1. [Google Scholar] [CrossRef]
- Ancel, P.Y.; Marret, S.; Larroque, B.; Arnaud, C.; Zupan-Simunek, V.; Voyer, M.; Rozé, J.C.; Matis, J.; Burguet, A.; Ledésert, B. Are maternal hypertension and small-for-gestational age risk factors for severe intraventricular hemorrhage and cystic periventricular leukomalacia? Results of the EPIPAGE cohort study. Am. J. Obstet. Gynecol. 2005, 193, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Baud, O.; Zupan, V.; Lacaze-Masmonteil, T.; Audibert, F.; Shojaei, T.; Thebaud, B.; Ville, Y.; Frydman, R.; Dehan, M. The relationships between antenatal management, the cause of delivery and neonatal outcome in a large cohort of very preterm singleton infants. BJOG 2000, 107, 877–884. [Google Scholar] [CrossRef]
- Bauer, M.; Fast, C.; Haas, J.; Resch, B.; Lang, U.; Pertl, B. Cystic periventricular leukomalacia in preterm infants: An analysis of obstetric risk factors. Early Hum. Dev. 2009, 85, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Been, J.V.; Rours, I.G.; Kornelisse, R.F.; Passos, V.L.; Kramer, B.W.; Schneider, T.A.; de Krijger, R.R.; Zimmermann, L.J. Histologic chorioamnionitis, fetal involvement, and antenatal steroids: Effects on neonatal outcome in preterm infants. Am. J. Obstet. Gynecol. 2009, 201, 587.e1–587.e8. [Google Scholar] [CrossRef]
- Bossung, V.; Fortmann, M.I.; Fusch, C.; Rausch, T.; Herting, E.; Swoboda, I.; Rody, A.; Härtel, C.; Göpel, W.; Humberg, A. Neonatal outcome after preeclampsia and HELLP syndrome: A population-based cohort study in Germany. Front. Pediatr. 2020, 8, 579293. [Google Scholar] [CrossRef]
- Botet, F.; Figueras, J.; Carbonell-Estrany, X.; Narbona, E. The impact of clinical maternal chorioamnionitis on neurological and psychological sequelae in very-low-birth weight infants: A case-control study. J. Perinat. Med. 2011, 39, 203–208. [Google Scholar] [CrossRef]
- Claas, M.; Bruinse, H.; Van der Heide-Jalving, M.; Termote, J.; De Vries, L. Changes in survival and neonatal morbidity in infants with a birth weight of 750 g or less. Neonatology 2010, 98, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martinez, R.; Tenorio, V.; Padilla, N.; Crispi, F.; Figueras, F.; Gratacos, E. Risk of ultrasound-detected neonatal brain abnormalities in intrauterine growth-restricted fetuses born between 28 and 34 weeks’ gestation: Relationship with gestational age at birth and fetal Doppler parameters. Ultrasound Obstet. Gynecol. 2015, 46, 452–459. [Google Scholar] [CrossRef]
- De Jesus, L.C.; Pappas, A.; Shankaran, S.; Li, L.; Das, A.; Bell, E.F.; Stoll, B.J.; Laptook, A.R.; Walsh, M.C.; Hale, E.C. Outcomes of small for gestational age infants born at <27 weeks’ gestation. J. Pediatr. 2013, 163, 55–60.e3. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Chen, M.F.; Kokottis, T.; Vallerand, D.; Usher, R. Outcome of neonates less than 30 weeks gestation with histologic chorioamnionitis. Am. J. Perinatol. 2005, 22, 155–159. [Google Scholar] [CrossRef]
- Denzler, A.; Burkhardt, T.; Natalucci, G.; Zimmermann, R. Latency after preterm prelabor rupture of the membranes: Increased risk for periventricular leukomalacia. J. Pregnancy 2014, 2014, 874984. [Google Scholar] [CrossRef] [PubMed]
- Ferdynus, C.; Quantin, C.; Abrahamowicz, M.; Platt, R.; Burguet, A.; Sagot, P.; Binquet, C.; Gouyon, J.B. Can birth weight standards based on healthy populations improve the identification of small-for-gestational-age newborns at risk of adverse neonatal outcomes? Pediatrics 2009, 123, 723–730. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, B.; de Alba, C.; Villalain, C.; Pallás, C.R.; Galindo, A.; Herraiz, I. Obstetric and pediatric growth charts for the detection of fetal growth restriction and neonatal adverse outcomes in preterm newborns before 34 weeks of gestation. J. Matern. Fetal Neonatal Med. 2021, 34, 1112–1119. [Google Scholar] [CrossRef]
- Gagliardi, L.; Bellù, R.; Zanini, R.; Dammann, O. Bronchopulmonary dysplasia and brain white matter damage in the preterm infant: A complex relationship. Paediatr. Perinat. Epidemiol. 2009, 23, 582–590. [Google Scholar] [CrossRef]
- Gagliardi, L.; Rusconi, F.; Bellù, R.; Zanini, R.; Network, I.N. Association of maternal hypertension and chorioamnionitis with preterm outcomes. Pediatrics 2014, 134, e154–e161. [Google Scholar] [CrossRef]
- Gagliardi, L.; Rusconi, F.; Da Frè, M.; Mello, G.; Carnielli, V.; Di Lallo, D.; Macagno, F.; Miniaci, S.; Corchia, C.; Cuttini, M. Pregnancy disorders leading to very preterm birth influence neonatal outcomes: Results of the population-based ACTION cohort study. Pediatr. Res. 2013, 73, 794–801. [Google Scholar] [CrossRef]
- Grisaru-Granovsky, S.; Reichman, B.; Lerner-Geva, L.; Boyko, V.; Hammerman, C.; Samueloff, A.; Schimmel, M.S.; Network, I.N. Mortality and morbidity in preterm small-for-gestational-age infants: A population-based study. Am. J. Obstet. Gynecol. 2012, 206, 150.e1–150.e7. [Google Scholar] [CrossRef] [PubMed]
- Hendson, L.; Russell, L.; Robertson, C.M.; Liang, Y.; Chen, Y.; Abdalla, A.; Lacaze-Masmonteil, T. Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. J. Pediatr. 2011, 158, 397–402. [Google Scholar] [CrossRef]
- Kakita, H.; Hussein, M.H.; Yamada, Y.; Henmi, H.; Kato, S.; Kobayashi, S.; Ito, T.; Kato, I.; Fukuda, S.; Suzuki, S. High postnatal oxidative stress in neonatal cystic periventricular leukomalacia. Brain Dev. 2009, 31, 641–648. [Google Scholar] [CrossRef]
- Khazardoost, S.; Ghotbizadeh, F.; Sahebdel, B.; Amiri, F.N.; Shafaat, M.; Akbarian-Rad, Z.; Pahlavan, Z. Predictors of cranial ultrasound abnormalities in intrauterine growth-restricted fetuses born between 28 and 34 weeks of gestation: A prospective cohort study. Fetal Diagn. Ther. 2019, 45, 238–247. [Google Scholar] [CrossRef]
- Kobayashi, S.; Fujimoto, S.; Fukuda, S.; Hattori, A.; Iwaki, T.; Koyama, N.; Tanaka, T.; Kokubo, M.; Okanishi, T.; Togari, H. Periventricular leukomalacia with late-onset circulatory dysfunction of premature infants: Correlation with severity of magnetic resonance imaging findings and neurological outcomes. Tohoku J. Exp. Med. 2006, 210, 333–339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kubota, H.; Ohsone, Y.; Oka, F.; Sueyoshi, T.; Takanashi, J.; Kohno, Y. Significance of clinical risk factors of cystic periventricular leukomalacia in infants with different birthweights. Acta Paediatr. 2001, 90, 302–308. [Google Scholar] [CrossRef]
- Kumazaki, K.; Nakayama, M.; Sumida, Y.; Ozono, K.; Mushiake, S.; Suehara, N.; Wada, Y.; Fujimura, M. Placental features in preterm infants with periventricular leukomalacia. Pediatrics 2002, 109, 650–655. [Google Scholar] [CrossRef]
- Kurahashi, H.; Okumura, A.; Kubota, T.; Kidokoro, H.; Maruyama, K.; Hayakawa, M.; Itakura, A.; Matsuzawa, K.; Yamamoto, H.; Kato, T.; et al. Increased fetal heart rate variability in periventricular leukomalacia. Brain Dev. 2016, 38, 196–203. [Google Scholar] [CrossRef]
- Lee, D.K.; Kwon, B.S.; Lee, Y.S.; Chang, Y.P. Risk factors for cystic periventricular leukomalacia and neurologic outcomes according to cranial ultrasonography in preterm infants. J. Korean Soc. Neonatol. 2002, 9, 90–98. [Google Scholar]
- Lee, E.Y.; Yeom, J.M.; Lee, S.H.; So, C.H.; Oh, Y.K. Risk factors and effect of early hypocarbia on the development of cystic periventricular leukomalacia in very low birth weight infants. Perinatology 2019, 30, 229–235. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, S.Y. Histological chorioamnionitis, antenatal steroids, and neonatal outcomes in very low birth weight infants: A nationwide study. PLoS ONE 2019, 14, e0224450. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, S.H.; Lee, K.H.; You, D.K.; Choi, S.J.; Hwang, J.H.; Choi, C.W.; Shim, J.W.; Ko, S.Y.; Yang, S.H.; et al. A study on the incidence and risk factors of cystic periventricular leukomalacia in very low birth weight infants. J. Korean Soc. Neonatol. 2003, 10, 61–66. [Google Scholar]
- Leviton, A.; Paneth, N.; Susser, M.; Reuss, M.L.; Allred, E.N.; Kuban, K.; Sanocka, U.; Hegyi, T.; Hiatt, M.; Shahrivar, F.; et al. Maternal receipt of magnesium sulfate does not seem to reduce the risk of neonatal white matter damage. Pediatrics 1997, 99, e2. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, E.; Lorthe, E.; Torchin, H.; Subtil, D.; Marret, S.; Ancel, P.Y.; Kayem, G. Impact of chorioamnionitis on neonatal neurological outcomes and mortality in premature infants born after spontaneous preterm labour or rupture of membranes. Am. J. Obstet. Gynecol. 2017, 216, S202–S203. [Google Scholar] [CrossRef][Green Version]
- Malhotra, A.; Yahya, Z.; Sasi, A.; Jenkin, G.; Ditchfield, M.; Polglase, G.R.; Miller, S.L. Does fetal growth restriction lead to increased brain injury as detected by neonatal cranial ultrasound in premature infants? J. Paediatr. Child. Health 2015, 51, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Monier, I.; Ancel, P.Y.; Ego, A.; Jarreau, P.H.; Lebeaux, C.; Kaminski, M.; Goffinet, F.; Zeitlin, J.; EPIPAGE 2 Study Group. Fetal and neonatal outcomes of preterm infants born before 32 weeks of gestation according to antenatal vs postnatal assessments of restricted growth. Am. J. Obstet. Gynecol. 2017, 216, 516.e1–516.e10. [Google Scholar] [CrossRef] [PubMed]
- Morsing, E.; Maršál, K.; Ley, D. Reduced prevalence of severe intraventricular hemorrhage in very preterm infants delivered after maternal preeclampsia. Neonatology 2018, 114, 205–211. [Google Scholar] [CrossRef]
- Murata, Y.; Itakura, A.; Matsuzawa, K.; Okumura, A.; Wakai, K.; Mizutani, S. Possible antenatal and perinatal related factors in development of cystic periventricular leukomalacia. Brain Dev. 2005, 27, 17–21. [Google Scholar] [CrossRef]
- Oda, N.; Takeuchi, K.; Tanaka, A.; Maruo, T. Obstetric risk factors associated with the development of periventricular leukomalacia in preterm infants born to mothers complicated by placenta previa. Fetal Diagn. Ther. 2008, 24, 345–348. [Google Scholar] [CrossRef]
- Pappas, A.; Kendrick, D.E.; Shankaran, S.; Stoll, B.J.; Bell, E.F.; Laptook, A.R.; Walsh, M.C.; Das, A.; Hale, E.C.; Newman, N.S.; et al. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. 2014, 168, 137–147. [Google Scholar] [CrossRef]
- Park, J.M.; Choi, B.S.; Sohn, I.A.; Seol, I.J.; Kim, C.R.; Park, H.K.; Lee, H.J. Risk factors for cystic periventricular leukomalacia in very low birth weight infants. Neonatal. Med. 2014, 21, 172–178. [Google Scholar] [CrossRef]
- Paul, D.A.; Kepler, J.; Leef, K.H.; Siscione, A.; Palmer, C.; Stefano, J.L. Effect of preeclampsia on mortality, intraventricular hemorrhage, and need for mechanical ventilation in very low-birth-weight infants. Am. J. Perinatol. 1998, 15, 381–386. [Google Scholar] [CrossRef]
- Paul, D.A.; Pearlman, S.A.; Finkelstein, M.S.; Stefano, J.L. Cranial sonography in very-low-birth-weight infants: Do all infants need to be screened? Clin. Pediatr 1999, 38, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Perlman, J.M.; Risser, R.; Broyles, R.S. Bilateral cystic periventricular leukomalacia in the premature infant: Associated risk factors. Pediatrics 1996, 97, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Pladys, P.; Beuchee, A.; Wodey, E.; Treguier, C.; Lassel, L.; Betremieux, P. Patent ductus arteriosus and cystic periventricular leucomalacia in preterm infants. Acta Paediatr. 2001, 90, 309–315. [Google Scholar] [CrossRef]
- Regev, R.H.; Arnon, S.; Litmanovitz, I.; Bauer-Rusek, S.; Boyko, V.; Lerner-Geva, L.; Reichman, B.; Israel Neonatal Network. Outcome of singleton preterm small for gestational age infants born to mothers with pregnancy-induced hypertension: A population-based study. J. Matern. Fetal Neonatal Med. 2015, 28, 666–673. [Google Scholar] [CrossRef]
- Regev, R.H.; Lusky, A.; Dolfin, T.; Litmanovitz, I.; Arnon, S.; Reichman, B.; Network, I.N. Excess mortality and morbidity among small-for-gestational-age premature infants: A population-based study. J. Pediatr. 2003, 143, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Rijken, M.; Wit, J.M.; Veen, S. Similar growth in preterm infants with intra- or extrauterine growth restriction. In A Regional Follow-Up Study at Two Years of Age in Extremely Preterm and Very Preterm Infants; Leiden University Medical Center: Leiden, The Netherlands, 2007; p. 73. [Google Scholar]
- Rocha, G.; de Lima, F.F.; Machado, A.P.; Guimaraes, H. Preeclampsia predicts higher incidence of bronchopulmonary dysplasia. J. Perinatol. 2018, 38, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.; De Lima, F.F.; Machado, A.P.; Guimarães, H.; Proença, E.; Carvalho, C.; Martins, L.G.; Martins, T.; Freitas, A.; Dias, C.P.; et al. Small for gestational age very preterm infants present a higher risk of developing bronchopulmonary dysplasia. J. Neonatal Perinat. Med. 2019, 12, 419–427. [Google Scholar] [CrossRef]
- Rocha, G.; Proença, E.; Quintas, C.; Rodrigues, T.; Guimarães, H. Chorioamnionitis and neonatal morbidity. Acta Med. Port. 2006, 19, 207–212. [Google Scholar]
- Ryan, E.; Eves, D.; Menon, P.J.; Alnafisee, S.; Mooney, E.E.; Downey, P.; Culliton, M.; Murphy, J.F.; Vavasseur, C.; Molloy, E.J. Histological chorioamnionitis is predicted by early infant C-reactive protein in preterm infants and correlates with neonatal outcomes. Acta Paediatr. 2020, 109, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.; Duyme, M.; Rousseau, A.; Audibert, F.; Paupe, A.; Zupan, V.; Ville, Y. Periventricular leukomalacia and mode of delivery in twins under 1500 g. J. Matern. Fetal Neonatal Med. 2003, 13, 224–229. [Google Scholar] [CrossRef]
- Shankaran, S.; Langer, J.C.; Kazzi, S.N.; Laptook, A.R.; Walsh, M.; National Institute of Child Health; Human Development Neonatal Research Network. Cumulative index of exposure to hypocarbia and hyperoxia as risk factors for periventricular leukomalacia in low birth weight infants. Pediatrics 2006, 118, 1654–1659. [Google Scholar] [CrossRef]
- Shim, G.H.; Chey, M.J. Risk factors of cystic periventricular leukomalacia in preterm infants with gestational ages of less than 32 weeks according to gestational age group. Korean J. Perinatol. 2016, 27, 36–44. [Google Scholar] [CrossRef]
- Soudée, S.; Vuillemin, L.; Alberti, C.; Mohamed, D.; Becquet, O.; Farnoux, C.; Biran, V.; Baud, O. Fetal growth restriction is worse than extreme prematurity for the developing lung. Neonatology 2014, 106, 304–310. [Google Scholar] [CrossRef]
- Spinillo, A.; Capuzzo, E.; Stronati, M.; Ometto, A.; De Santolo, A.; Acciano, S. Obstetric risk factors for periventricular leukomalacia among preterm infants. BJOG 1998, 105, 865–871. [Google Scholar] [CrossRef] [PubMed]
- E Stewart, J.; Allred, E.N.; Collins, M.; Abbott, J.; Leviton, A.; Paneth, N.; Reuss, M.L.; Susser, M.; Hegyi, T.; Hiatt, M.; et al. Risk of cranial ultrasound abnormalities in very-low-birth-weight infants conceived with assisted reproductive techniques. J. Perinatol. 2002, 22, 37–45. [Google Scholar] [CrossRef]
- Tokumasu, H.; Tokumasu, S.; Kawakami, K. Impact of pre-eclampsia in extremely premature infants: Population-based study. Pediatr. Int. 2016, 58, 578–583. [Google Scholar] [CrossRef]
- Tyler, C.P.; Paneth, N.; Allred, E.N.; Hirtz, D.; Kuban, K.; McElrath, T.; O’Shea, T.M.; Miller, C.; Leviton, A.; ELGAN Study Investigators. Brain damage in preterm newborns and maternal medication: The ELGAN Study. Am. J. Obstet. Gynecol. 2012, 207, 192.e1–192.e9. [Google Scholar] [CrossRef]
- Wagenaar, N.; Chau, V.; Groenendaal, F.; Kersbergen, K.J.; Poskitt, K.J.; Grunau, R.E.; Synnes, A.; Duerden, E.G.; de Vries, L.S.; Miller, S.P.; et al. Clinical risk factors for punctate white matter lesions on early magnetic resonance imaging in preterm newborns. J. Pediatr. 2017, 182, 34–40.e1. [Google Scholar] [CrossRef]
- Wang, L.-W.; Lin, Y.-C.; Tu, Y.-F.; Wang, S.-T.; Huang, C.-C.; TPIDCS Group. Isolated cystic periventricular leukomalacia differs from cystic periventricular leukomalacia with intraventricular hemorrhage in prevalence, risk factors and outcomes in preterm infants. Neonatology 2017, 111, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Weindling, A.; Wilkinson, A.; Cook, J.; Calvert, S.; Fok, T.F.; Rochefort, M. Perinatal events which precede periventricular haemorrhage and leukomalacia in the newborn. BJOG 1985, 92, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Wiswell, T.E.; Graziani, L.J.; Kornhauser, M.S.; Stanley, C.; Merton, D.A.; McKee, L.; Spitzer, A.R. Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high-frequency jet ventilation. Pediatrics 1996, 98, 918–924. [Google Scholar] [CrossRef]
- Wu, P.M.; Wu, C.Y.; Li, C.I.; Huang, C.C.; Tu, Y.F. Association of cystic periventricular leukomalacia and postnatal epilepsy in very preterm infants. Neonatology 2023, 120, 500–507. [Google Scholar] [CrossRef]
- Yamakawa, T.; Itabashi, K.; Kusuda, S.; Neonatal Research Network of Japan. Mortality and morbidity risks vary with birth weight standard deviation score in growth restricted extremely preterm infants. Early Hum. Dev. 2016, 92, 7–11. [Google Scholar] [CrossRef]
- Zeitlin, J.; El Ayoubi, M.; Jarreau, P.-H.; Draper, E.S.; Blondel, B.; Künzel, W.; Cuttini, M.; Kaminski, M.; Gortner, L.; Van Reempts, P.; et al. Impact of fetal growth restriction on mortality and morbidity in a very preterm birth cohort. J. Pediatr. 2010, 157, 733–739.e1. [Google Scholar] [CrossRef]
- Szpecht, D.; Wiak, K.; Braszak, A.; Surzyn, E.; Szymankiewicz, M.; Gadzinowski, J. Risk factors of periventricular leukomalacia in singleton infants born from 23rd to 26th weeks of gestation–Retrospective study. Pediatria Polska. 2017, 92, 266–270. [Google Scholar] [CrossRef]
- Smith, R.J. p > 0.05: The incorrect interpretation of “not significant” results is a significant problem. Am. J. Phys. Anthropol. 2020, 172, 521–527. [Google Scholar] [CrossRef]
- Keysers, C.; Gazzola, V.; Wagenmakers, E.J. Using Bayes factor hypothesis testing in neuroscience to establish evidence of absence. Nat. Neurosci. 2020, 23, 788–799. [Google Scholar] [CrossRef]
- Hoekstra, R.; Monden, R.; van Ravenzwaaij, D.; Wagenmakers, E.J. Bayesian reanalysis of null results reported in medicine: Strong yet variable evidence for the absence of treatment effects. PLoS ONE 2018, 13, e0195474. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Sapantzoglou, I.; Rodolaki, K.; Varthaliti, A.; Theodora, M.; Antsaklis, P.; Thomakos, N.; Stavros, S.; Daskalakis, G.; Papapanagiotou, A. Maternal and neonatal outcomes following magnesium sulfate in the setting of chorioamnionitis: A meta-analysis. Arch. Gynecol. Obs. 2024, 309, 917–927. [Google Scholar] [CrossRef]
- De Oliveira, L.; Korkes, H.; de Rizzo, M.; Siaulys, M.M.; Cordioli, E. Magnesium sulfate in preeclampsia: Broad indications, not only in neurological symptoms. Pregnancy Hypertens. 2024, 36, 101126. [Google Scholar] [CrossRef]
- Wu, Y.W.; Colford, J.M., Jr. Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA 2000, 284, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.D.; Saade, G.; Polin, R.A.; Grobman, W.A.; Buhimschi, I.A.; Watterberg, K.; Silver, R.M.; Raju, T.N. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: Summary of a workshop. Obstet. Gynecol. 2016, 127, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Randis, T.M.; Rice, M.M.; Myatt, L.; Tita, A.T.; Leveno, K.J.; Reddy, U.M.; Varner, M.W.; Thorp, J.M.; Mercer, B.M.; Dinsmoor, M.J.; et al. Incidence of early-onset sepsis in infants born to women with clinical chorioamnionitis. J. Perinat. Med. 2018, 46, 926–933. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Romero, R.; Jung, E.J.; Sánchez, Á.J.G. Management of clinical chorioamnionitis: An evidence-based approach. Am. J. Obs. Gynecol. 2020, 223, 848–869. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Baergen, R.N.; Boyd, T.K.; Carreon, C.K.; Duncan, V.E.; Ernst, L.M.; Faye-Petersen, O.M.; Folkins, A.K.; Hecht, J.L.; Heerema-McKenney, A.; et al. Criteria for placental examination for obstetrical and neonatal providers. Am. J. Obs. Gynecol. 2023, 228, 497–508.e4. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Diaz-Primera, R.; Marin-Concha, J.; Para, R.; Lopez, A.M.; Pacora, P.; Gomez-Lopez, N.; Yoon, B.H.; et al. The fetal inflammatory response syndrome: The origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin. Fetal Neonatal Med. 2020, 25, 101146. [Google Scholar] [CrossRef]
- Pacora, P.; Chaiworapongsa, T.; Maymon, E.; Kim, Y.M.; Gomez, R.; Yoon, B.H.; Ghezzi, F.; Berry, S.M.; Qureshi, F.; Jacques, S.M.; et al. Funisitis and chorionic vasculitis: The histological counterpart of the fetal inflammatory response syndrome. J. Matern. Fetal Neonatal Med. 2002, 11, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Nohr, E.A.; Bech, B.H.; Vestergaard, M.; Catov, J.M.; Olsen, J. Health of children born to mothers who had preeclampsia: A population-based cohort study. Am. J. Obstet. Gynecol. 2009, 201, 269.e1–269.e10. [Google Scholar] [CrossRef]
- Jain, S.; Barnes-Davis, M.E.; Fu, T.T.; Sahay, R.D.; Ehrlich, S.R.; Liu, C.; Kline-Fath, B.; Habli, M.; Parikh, N.A. Hypertensive disorders of pregnancy and risk of early brain abnormalities on magnetic resonance imaging at term among infants born at ≤32 weeks’ gestational age. J. Pediatr. 2024, 273, 114133. [Google Scholar] [CrossRef]
- Matić, M.; Inati, V.; Abdel-Latif, M.E.; Kent, A.L.; Neonatal Admissions Network. Maternal hypertensive disorders are associated with increased use of respiratory support but not chronic lung disease or poorer neurodevelopmental outcomes in preterm neonates at <29 weeks of gestation. J. Paediatr. Child. Health 2017, 53, 391–398. [Google Scholar]
- Sloane, A.J.; Flannery, D.D.; Lafferty, M.; Jensen, E.A.; Dysart, K.; Cook, A.; Greenspan, J.; Aghai, Z.H. Hypertensive disorders during pregnancy are associated with reduced severe intraventricular hemorrhage in very-low-birth-weight infants. J. Perinatol. 2019, 39, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Brandt, J.S. A principled approach to mediation analysis in perinatal epidemiology. Am. J. Obs. Gynecol. 2022, 226, 24–32.e6. [Google Scholar] [CrossRef]
- Wilcox, A.J.; Weinberg, C.R.; Basso, O. On the pitfalls of adjusting for gestational age at birth. Am. J. Epidemiol. 2011, 174, 1062–1068. [Google Scholar] [CrossRef]
- Correia, L.C.L.; Mascarenhas, R.F.; De Menezes, F.S.C.; Oliveira, J.S.; Vaccarino, V.; Ross, J.S.; Wallach, J.D. Confounder Selection in Observational Studies in High-Impact Medical and Epidemiological Journals. JAMA Netw. Open 2025, 8, e2524176. [Google Scholar] [CrossRef]
- Di Martino, D.D.; Avagliano, L.; Ferrazzi, E.; Fusè, F.; Sterpi, V.; Parasiliti, M.; Stampalija, T.; Zullino, S.; Farina, A.; Bulfamante, G.P.; et al. Hypertensive disorders of pregnancy and fetal growth restriction: Clinical characteristics and placental lesions and possible preventive nutritional targets. Nutrients 2022, 14, 3276. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.G.; Julian, C.G.; Lorca, R.A.; Cioffi-Ragan, D.; Gumina, D.; Hobbins, J.C. Does hypoxia prompt fetal brain-sparing in the absence of fetal growth restriction? Physiol. Res. 2024, 73, S487. [Google Scholar] [CrossRef]
- de Vries, L.S.; Benders, M.J.; Groenendaal, F. Imaging the premature brain: Ultrasound or MRI? Neuroradiology 2013, 55, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, S.E.; Miller, S.P.; Leonard, C.; Glidden, D.V.; Goldstein, R.; Ramaswamy, V.; Piecuch, R.; Ferriero, D.M. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: The role of cystic periventricular leukomalacia. J. Pediatr. 2004, 145, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Selvanathan, T.; Guo, T.; Ufkes, S.; Chau, V.; Branson, H.M.; Synnes, A.R.; Ly, L.G.; Kelly, E.; Grunau, R.E.; Miller, S.P. Change in volumes and location of preterm white matter injury over a period of 15 years. J. Pediatr. 2024, 272, 114090. [Google Scholar] [CrossRef]
- Martinez-Biarge, M.; Groenendaal, F.; Kersbergen, K.J.; Benders, M.J.; Foti, F.; Cowan, F.M.; de Vries, L.S. MRI based preterm white matter injury classification: The importance of sequential imaging in determining severity of injury. PLoS ONE 2016, 11, e0156245. [Google Scholar] [CrossRef]
- Bramer, W.M.; Milic, J.; Mast, F. Reviewing retrieved references for inclusion in systematic reviews using EndNote. J. Med. Libr. Assoc. 2017, 105, 84–87. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2000. Available online: https://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf (accessed on 29 May 2025).
- Stanley, T.D.; Doucouliagos, H. Meta-regression approximations to reduce publication selection bias. Res. Synth. Methods 2014, 5, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Wagenmakers, E.-J. Bayesian Data Analysis for Cognitive Science: A Practical Course; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
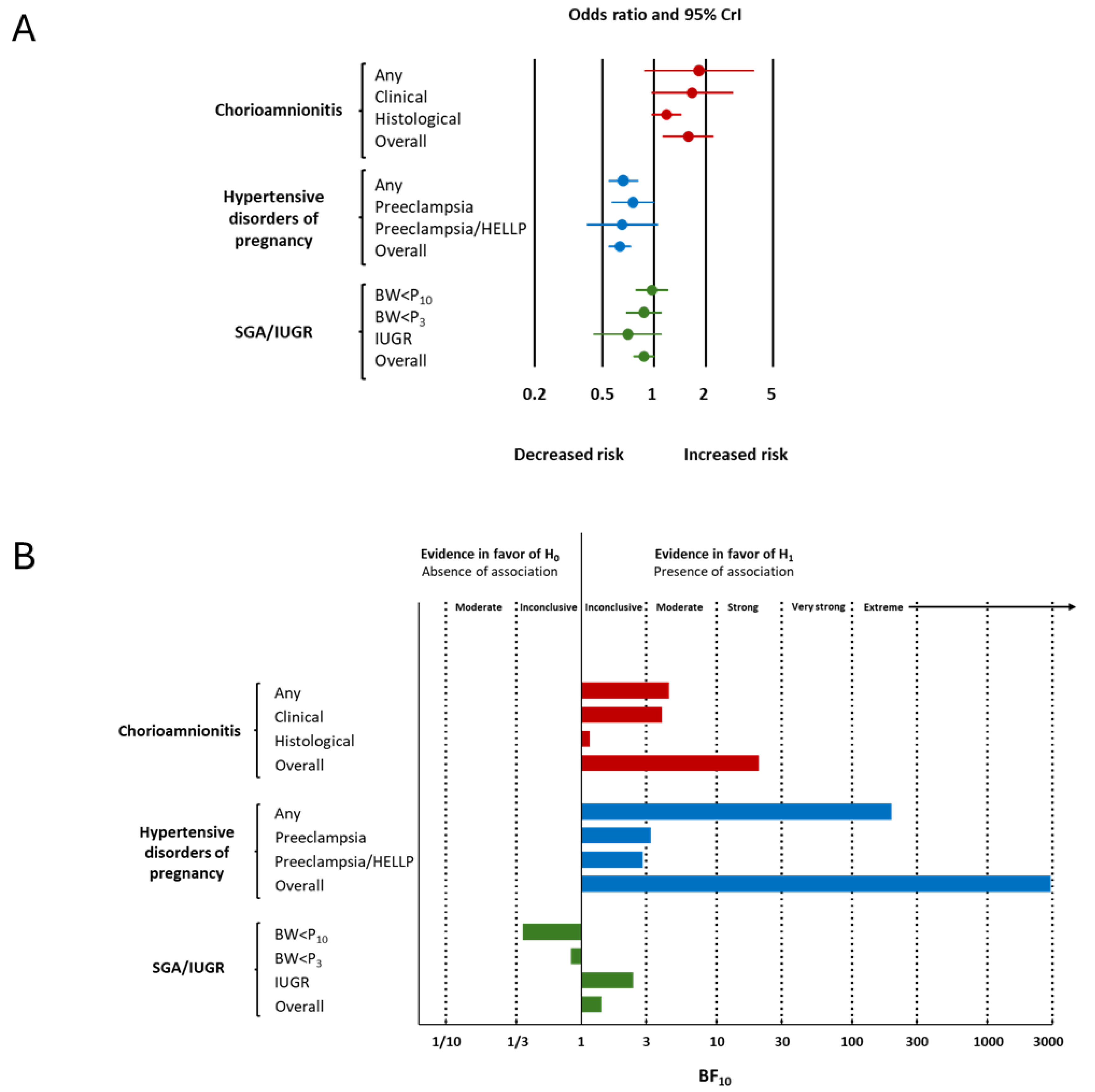
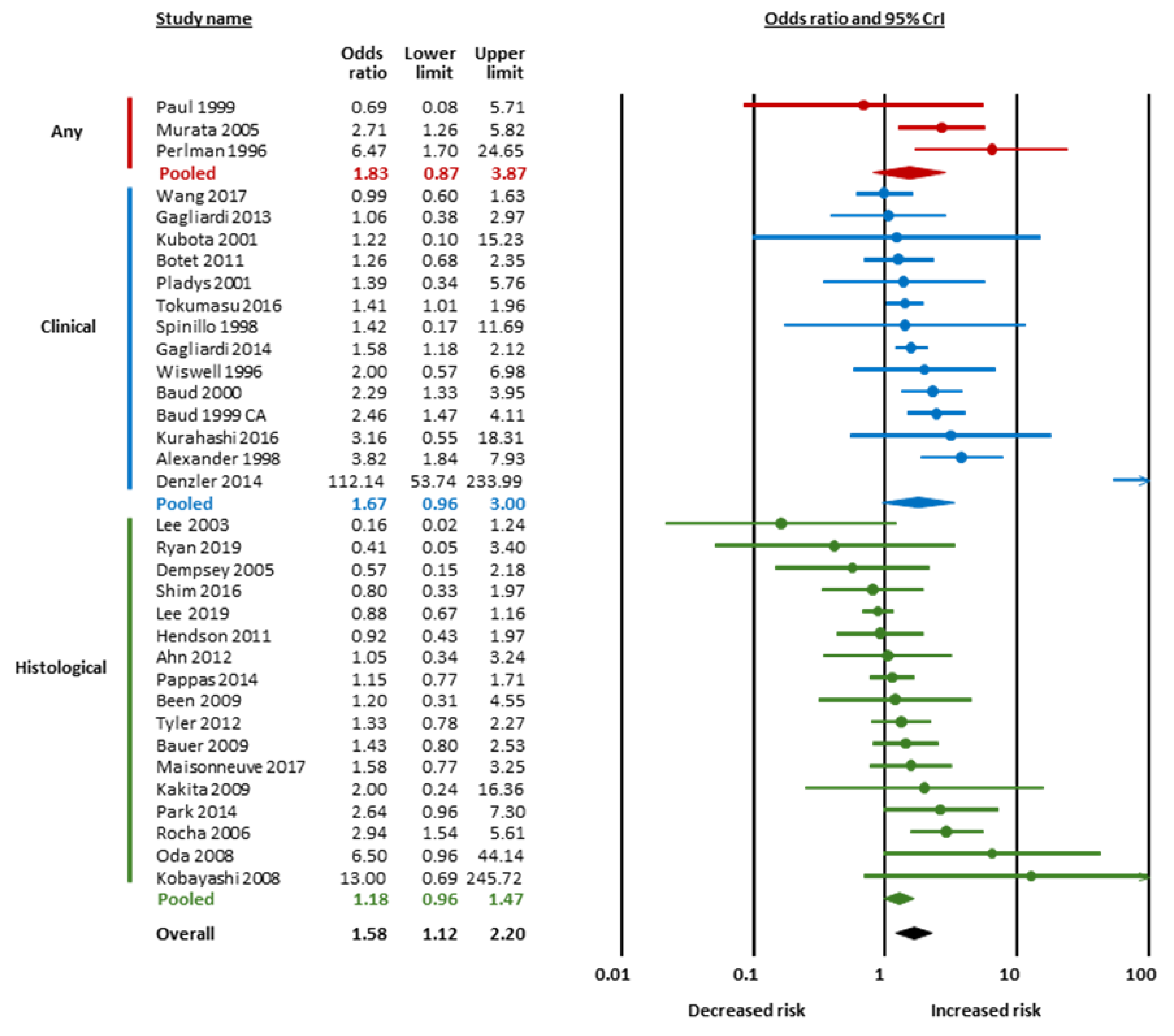
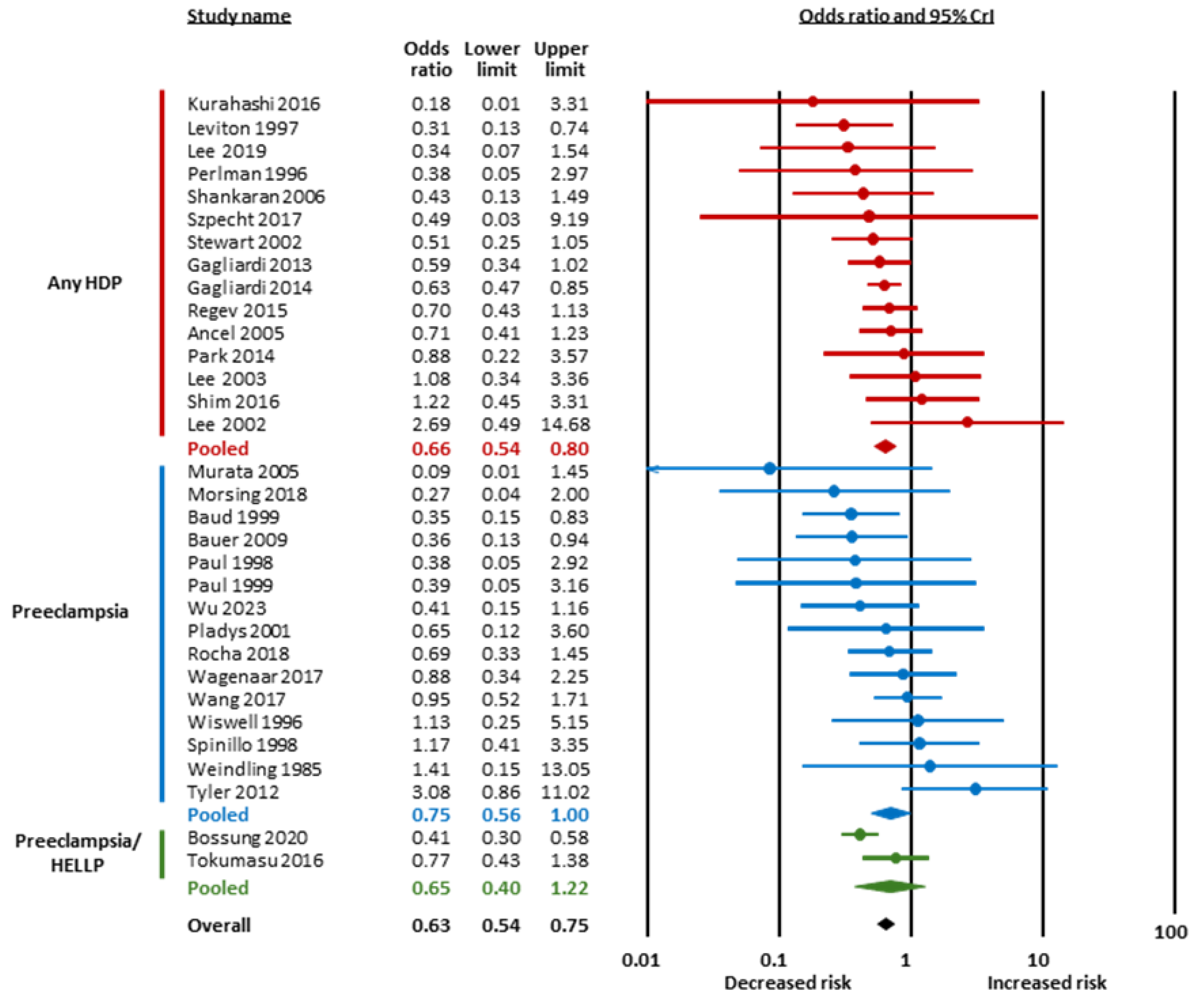
| Condition | Subgroup | k | OR | 95% CrI | Tau | 95% CrI | BF10 | BFrf | Evidence for Effect | Evidence for Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | |||||||||
| Chorioamnionitis | Any | 3 | 1.83 | 0.87 | 3.87 | 1.75 | 1.10 | 5.77 | 4.44 | 1.69 | moderate for | undecided for |
| Clinical | 14 | 1.67 | 0.96 | 3.00 | 2.97 | 2.00 | 5.46 | 3.93 | >106 | moderate for | extreme for | |
| Histological | 17 | 1.18 | 0.96 | 1.47 | 1.30 | 1.09 | 1.75 | 1.15 | 1.89 | undecided for | undecided for | |
| Overall | 34 | 1.58 | 1.12 | 2.20 | 2.25 | 1.79 | 3.05 | 20.51 | >106 | strong for | extreme for | |
| Funisitis | Fun+ vs. Fun−/CA− | 6 | 0.97 | 0.68 | 1.39 | 1.26 | 1.07 | 1.84 | 0.55 | 0.56 | undecided against | undecided against |
| Fun+ vs. Fun−/CA+ | 4 | 0.88 | 0.55 | 1.35 | 1.34 | 1.08 | 2.44 | 0.76 | 0.75 | undecided against | undecided against | |
| Fun+ vs. Fun− | 5 | 0.93 | 0.65 | 1.33 | 1.26 | 1.08 | 1.85 | 0.60 | 0.53 | undecided against | undecided against | |
| Hypertensive disorders of pregnancy | Any | 14 | 0.66 | 0.54 | 0.80 | 1.20 | 1.07 | 1.55 | 196.19 | 0.32 | extreme for | moderate against |
| Preeclampsia | 15 | 0.75 | 0.56 | 1.00 | 1.32 | 1.08 | 1.92 | 3.25 | 0.92 | moderate for | undecided against | |
| Preeclampsia/HELLP | 2 | 0.65 | 0.40 | 1.22 | 1.65 | 1.12 | 4.19 | 2.82 | 3.89 | undecided for | moderate for | |
| Overall | 31 | 0.63 | 0.54 | 0.75 | 1.21 | 1.08 | 1.47 | 2936.82 | 0.88 | extreme for | undecided against | |
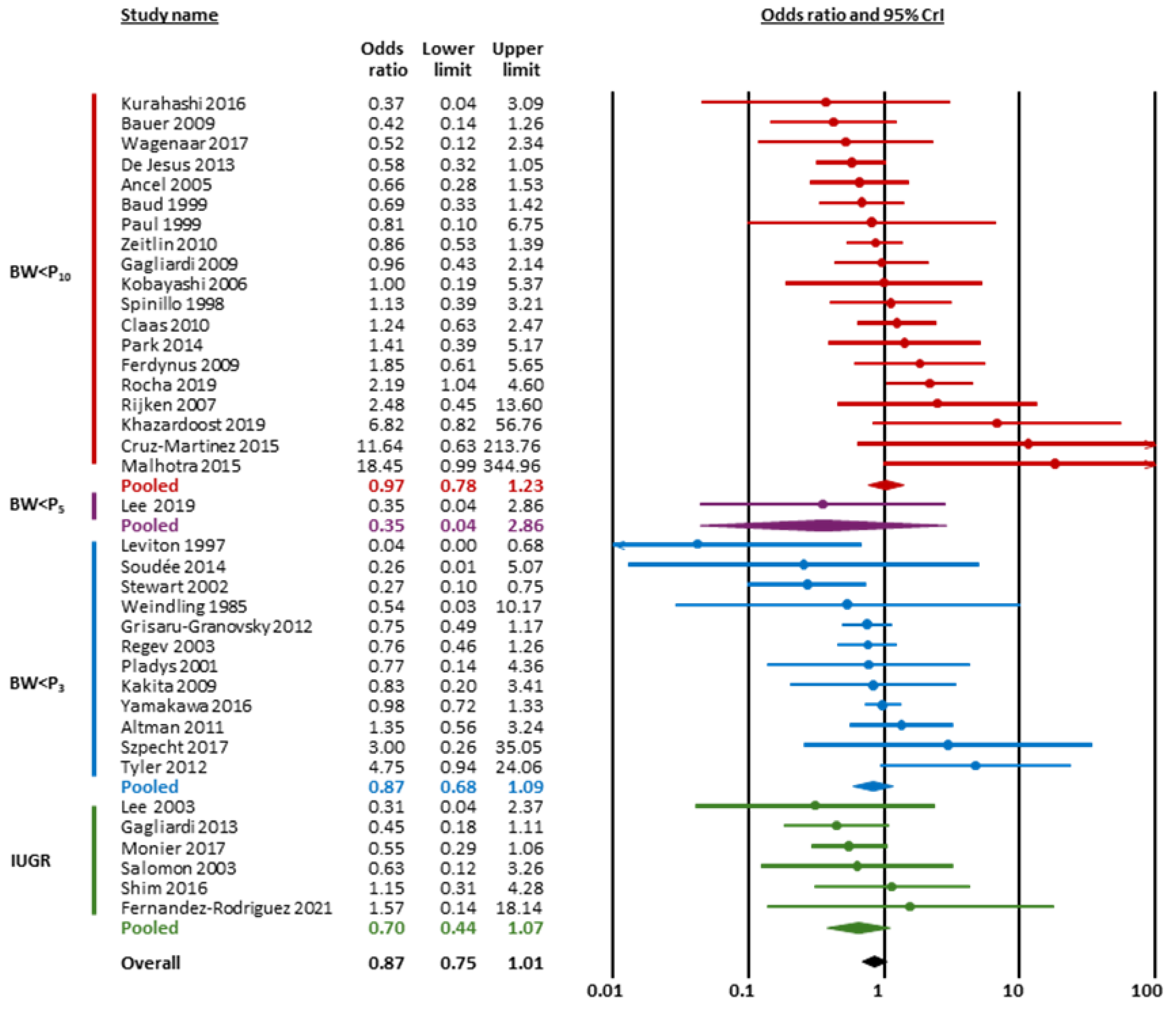
| SGA/IUGR | BW < P10 | 19 | 0.97 | 0.78 | 1.23 | 1.27 | 1.08 | 1.69 | 0.37 | 0.82 | undecided against | undecided against |
| BW < P3 | 11 | 0.87 | 0.68 | 1.09 | 1.28 | 1.08 | 1.91 | 0.84 | 0.63 | undecided against | undecided against | |
| IUGR | 6 | 0.70 | 0.44 | 1.07 | 1.34 | 1.08 | 2.28 | 2.41 | 0.85 | undecided for | undecided against | |
| Overall | 37 | 0.87 | 0.75 | 1.01 | 1.24 | 1.08 | 1.56 | 1.41 | 0.68 | undecided for | undecided against | |
| Condition | k | GA Difference (Hedges’ g) | 95% CrI | Tau | 95% CrI | BF10 | BFrf | Evidence for Effect | Evidence for Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | ||||||||
| Chorioamnionitis | 14 | −0.52 | −0.75 | −0.27 | 0.44 | 0.30 | 0.66 | 226.52 | >106 | extreme for | extreme for |
| Hypertensive disorders of pregnancy | 7 | 0.37 | 0.10 | 0.62 | 0.35 | 0.19 | 0.67 | 9.78 | >106 | moderate for | extreme for |
| SGA/IUGR | 12 | 0.21 | −0.12 | 0.54 | 0.62 | 0.39 | 0.97 | 0.93 | >106 | undecided against | extreme for |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lissone, N.P.A.; Hundscheid, T.M.; Galán-Henríquez, G.M.; González-Luis, G.E.; Bartoš, F.; Villamor, E. Association Between Endotype of Prematurity and Cystic Periventricular Leukomalacia: A Bayesian Model-Averaged Meta-Analysis. Children 2025, 12, 1065. https://doi.org/10.3390/children12081065
Lissone NPA, Hundscheid TM, Galán-Henríquez GM, González-Luis GE, Bartoš F, Villamor E. Association Between Endotype of Prematurity and Cystic Periventricular Leukomalacia: A Bayesian Model-Averaged Meta-Analysis. Children. 2025; 12(8):1065. https://doi.org/10.3390/children12081065
Chicago/Turabian StyleLissone, Neirude P. A., Tamara M. Hundscheid, Gloria M. Galán-Henríquez, Gema E. González-Luis, František Bartoš, and Eduardo Villamor. 2025. "Association Between Endotype of Prematurity and Cystic Periventricular Leukomalacia: A Bayesian Model-Averaged Meta-Analysis" Children 12, no. 8: 1065. https://doi.org/10.3390/children12081065
APA StyleLissone, N. P. A., Hundscheid, T. M., Galán-Henríquez, G. M., González-Luis, G. E., Bartoš, F., & Villamor, E. (2025). Association Between Endotype of Prematurity and Cystic Periventricular Leukomalacia: A Bayesian Model-Averaged Meta-Analysis. Children, 12(8), 1065. https://doi.org/10.3390/children12081065








