Neonatal Jaundice Requiring Phototherapy Risk Factors in a Newborn Nursery: Machine Learning Approach
Abstract
Highlights
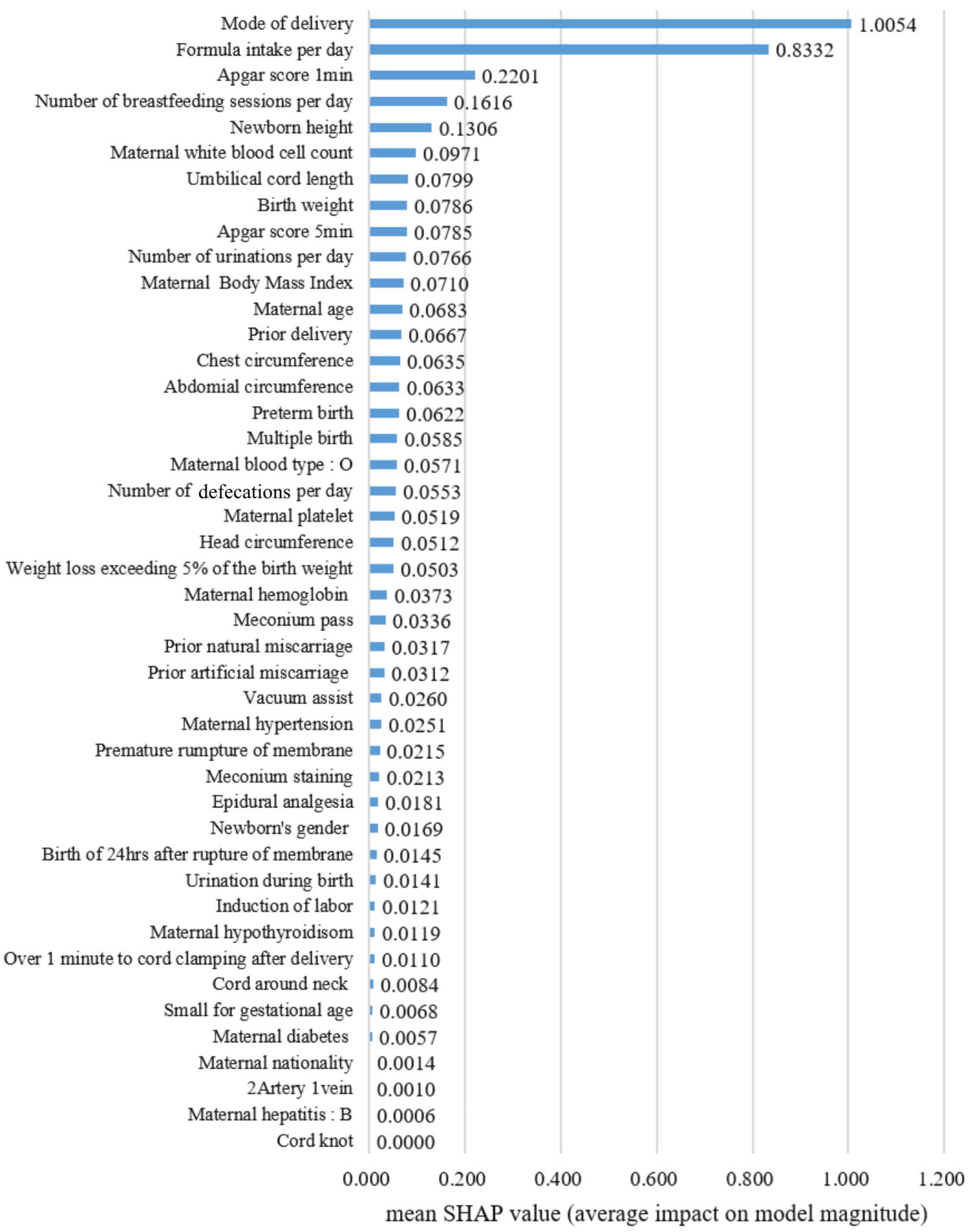
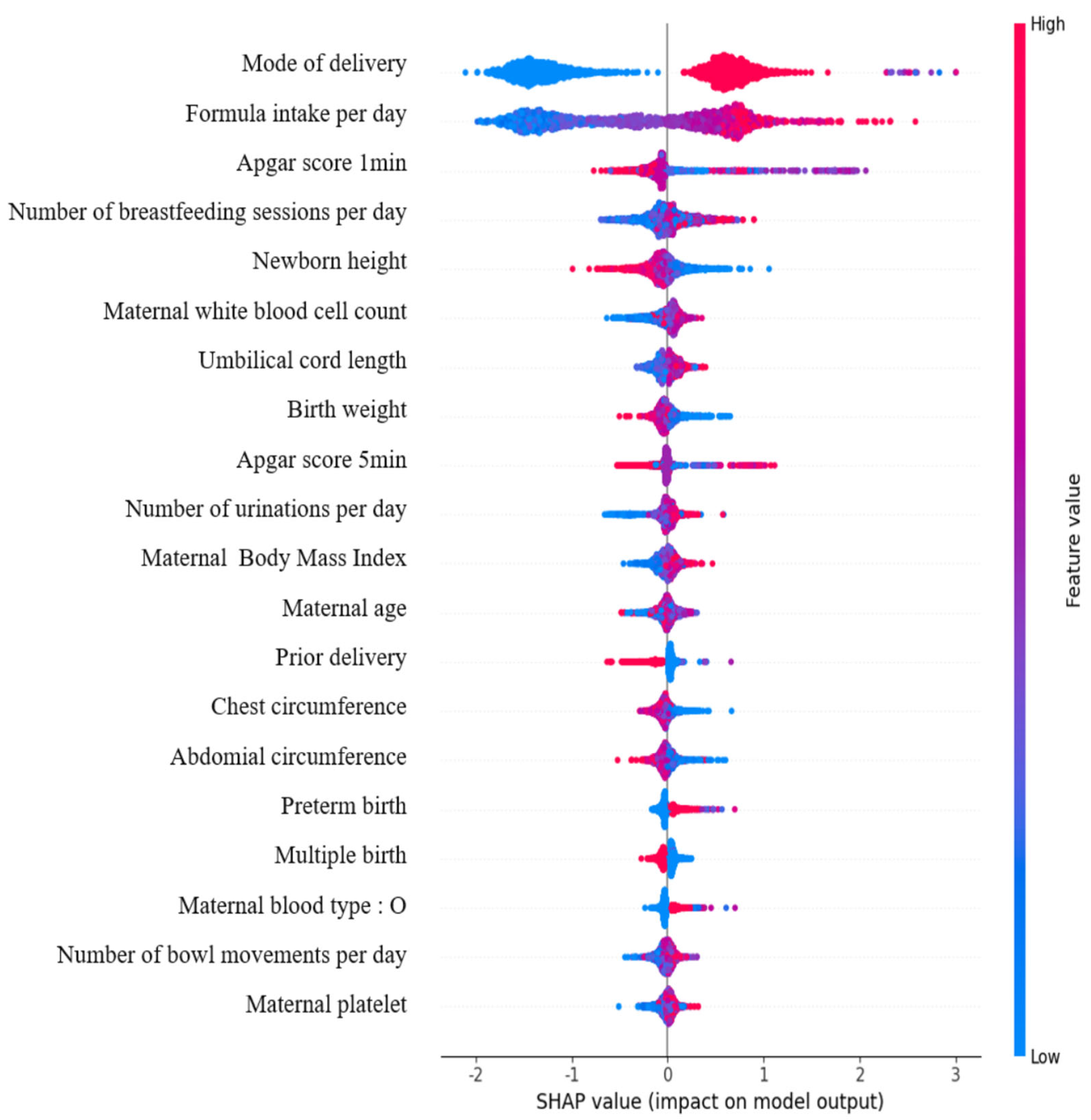
- Machine learning algorithms successfully identified the key perinatal factors, including mode of delivery, feeding patterns, maternal BMI, and neonatal birth weight, that are associated with the risk of neonatal jaundice requiring phototherapy.
- Specifically, Cesarean section delivery, increased breastfeeding and formula intake, and lower birth weight were found to significantly increase the likelihood of neonates needing phototherapy for jaundice.
- The development of predictive models leveraging electronic medical records offers a powerful tool for early risk stratification, enabling timely clinical interventions and the more effective management of neonatal jaundice.
- These findings emphasize the critical need for integrating comprehensive maternal and neonatal health data into real-time decision-making tools to help reduce complications and readmissions related to hyperbilirubinemia.
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansong-Assoku, B.; Adnan, M.; Daley, S.F.; Ankola, P.A. Neonatal jaundice. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532930/ (accessed on 17 July 2024).
- Jardine, L.A.; Woodgate, P. Neonatal jaundice. Am. Fam. Physician 2012, 85, 824–825. [Google Scholar]
- Mitra, S.; Rennie, J. Neonatal jaundice: Aetiology, diagnosis, and treatment. Br. J. Hosp. Med. 2017, 78, 699–704. [Google Scholar] [CrossRef]
- Khurshid, F.; Rao, S.P.; Sauve, C.; Gupta, S. Universal screening for hyperbilirubinemia in term healthy newborns at discharge: A systematic review and meta-analysis. J. Glob. Health 2022, 12, 12007. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Jaundice in Newborn Babies Under 28 Days: NICE Guidelines. CG98. 2010. Available online: https://www.nice.org.uk/guidance/cg98 (accessed on 20 July 2024).
- Rennie, J.; Burman-Roy, S.; Murphy, M.S. Neonatal jaundice: Summary of NICE guidance. BMJ 2010, 340, c2409. [Google Scholar] [CrossRef]
- Kemper, A.R.; Newman, T.B.; Slaughter, J.L.; Maisels, M.J.; Watchko, J.F.; Downs, S.M.; Grout, R.W.; Bundy, D.G.; Stark, A.R.; Bogen, D.L.; et al. Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics 2022, 150, e2022058859. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Dhillon, G.; Sasidharan, S.; Dhillon, H. Maternal and neonatal risk factors for neonatal jaundice and readmission—An Indian perspective. Acta Medica Int. 2021, 8, 44–49. [Google Scholar] [CrossRef]
- Lin, Q.; Zhu, D.; Chen, C.; Feng, Y.; Shen, F.; Wu, Z. Risk factors for neonatal hyperbilirubinemia: A systematic review and meta-analysis. Transl. Pediatr. 2022, 11, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Le Ray, I.; Sun, J.Y.; Wikman, A.; Reilly, M.; Johansson, S. Haemolytic and nonhaemolytic neonatal jaundice have different risk factor profiles. Acta Paediatr. 2016, 105, 1444–1450. [Google Scholar] [CrossRef]
- Tan, T.-J.; Chen, W.-J.; Lin, W.-C.; Yang, M.-C.; Tsai, C.-C.; Yang, Y.-N.; Yang, S.-N.; Liu, H.-K. Early-Term Neonates Demonstrate a Higher Likelihood of Requiring Phototherapy Compared to Those Born Full-Term. Children 2023, 10, 1819. [Google Scholar] [CrossRef]
- Rehna, T.; Thomas, S.A. Risk factors for early hyperbilirubinemia in neonates: A cross-sectional study. J. Curr. Res. Sci. Med. 2022, 8, 176–181. [Google Scholar] [CrossRef]
- Qattea, I.; Farghaly, M.A.A.; Elgendy, M.; Mohamed, M.A.; Aly, H. Neonatal hyperbilirubinemia and bilirubin neurotoxicity in hospitalized neonates: Analysis of the US database. Pediatr. Res. 2022, 91, 1662–1668. [Google Scholar] [CrossRef]
- Garosi, E.; Mohammadi, F.; Ranjkesh, F. The relationship between neonatal jaundice and maternal and neonatal factors. Iran. J. Neonatol. 2016, 7, 37–40. [Google Scholar]
- De Luca, D.; Carnielli, V.P.; Paolillo, P. Neonatal hyperbilirubinemia and early discharge from the maternity ward. Eur. J. Pediatr. 2009, 168, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Maisels, M.J. Neonatal hyperbilirubinemia and kernicterus—Not gone but sometimes forgotten. Early Hum. Dev. 2009, 85, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Merino-Andrés, J.; Pérez-Nombela, S.; Álvarez-Bueno, C.; Hidalgo-Robles, Á.; Ruiz-Becerro, I.; Fernández-Rego, F.J. Neonatal hyperbilirubinemia and repercussions on neurodevelopment: A systematic review. Child Care Health Dev. 2024, 50, e13183. [Google Scholar] [CrossRef] [PubMed]
- Blumovich, A.; Mangel, L.; Yochpaz, S.; Mandel, D.; Marom, R. Risk factors for readmission for phototherapy due to jaundice in healthy newborns: A retrospective, observational study. BMC Pediatr. 2020, 20, 248. [Google Scholar] [CrossRef]
- Lain, S.J.; Roberts, C.L.; Bowen, J.R.; Nassar, N. Early discharge of infants and risk of readmission for jaundice. Pediatrics 2015, 135, 314–321. [Google Scholar] [CrossRef]
- Mercer, R.T. Nursing support of the process of becoming a mother. J. Obstet. Gynecol. Neonatal Nurs. 2006, 35, 649–651. [Google Scholar] [CrossRef]
- Henry, J.F.; Sherwin, B.B. Hormones and cognitive functioning during late pregnancy and postpartum: A longitudinal study. Behav. Neurosci. 2012, 126, 73–85. [Google Scholar] [CrossRef]
- Dietz, C.; Swinkels, S.H.N.; van Daalen, E.; van Engeland, H.; Buitelaar, J.K. Parental Compliance After Screening Social Development in Toddlers. Arch. Pediatr. Adolesc. Med. 2007, 161, 363–368. [Google Scholar] [CrossRef][Green Version]
- Kinshella, M.-L.W.; Salimu, S.; Chiwaya, B.; Chikoti, F.; Chirambo, L.; Mwaungulu, E.; Banda, M.; Hiwa, T.; Vidler, M.; Molyneux, E.M.; et al. Challenges and recommendations to improve implementation of phototherapy among neonates in Malawian hospitals. BMC Pediatr. 2022, 22, 367. [Google Scholar] [CrossRef]
- Hegde, D.; Rath, C.; Amarasekara, S.; Saraswati, C.; Patole, S.; Rao, S. Performance of smartphone application to accurately quantify hyperbilirubinemia in neonates: A systematic review with meta-analysis. Eur. J. Pediatr. 2023, 182, 3957–3971. [Google Scholar] [CrossRef] [PubMed]
- Soldi, A.; Tonetto, P.; Varalda, A.; Bertino, E. Neonatal jaundice and human milk. J. Matern.-Fetal Neonatal Med. 2011, 24 (Suppl. S1), 85–87. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Guo, Y.; Huang, M.; He, J.; Qiu, X. Breast milk constituents and the development of breast milk jaundice in neonates: A systematic review. Nutrients 2023, 15, 2261. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.W.; Fan, W.Q. Earlier nutrient fortification of breast milk fed low birth weight infants improves jaundice related outcomes. Nutrients 2020, 12, 2116. [Google Scholar] [CrossRef]
- WHO. Baby-Friendly Hospital Initiative: Revised, Updated and Expanded for Integrated Care (Section 3). Wellstart International. 2009. Available online: https://www.who.int/publications/i/item/9789241594950 (accessed on 8 June 2024).
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004, 114, 297–316. [Google Scholar] [CrossRef]
- Flaherman, V.J.; Maisels, M.J. ABM Clinical Protocol #22: Guidelines for management of jaundice in the breastfeeding infant 35 weeks or more of gestation—Revised 2017. Breastfeed. Med. 2017, 12, 250–257. [Google Scholar] [CrossRef]
- Wilde, V.K. Breastfeeding insufficiencies: Common and preventable harm to neonates. Curēus 2021, 13, e18478. [Google Scholar] [CrossRef]
- Tavakolizadeh, R.; Izadi, A.; Seirafi, G.; Khedmat, L.; Mojtahedi, S.Y. Maternal risk factors for neonatal jaundice: A hospital-based cross-sectional study in Tehran. Eur. J. Transl. Myol. 2018, 28, 7618. [Google Scholar] [CrossRef]
- Steffens, B.; Koch, G.; Engel, C.; Franz, A.R.; Pfister, M.; Wellmann, S. Assessing accuracy of BiliPredics algorithm in predicting individual bilirubin progression in neonates—Results from a prospective multi-center study. Front. Digit. Health 2025, 7, 1497165. [Google Scholar] [CrossRef]
- Norman, M.; Aberg, K.; Holmsten, K.; Weibel, V.; Ekeus, C. Predicting nonhemolytic neonatal hyperbilirubinemia. Pediatrics 2015, 136, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, B.O.; Osibanjo, F.B.; Slusher, T.M. Risk factors for severe neonatal hyperbilirubinemia in low and middle-income countries: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0117229. [Google Scholar] [CrossRef] [PubMed]
- Guedalia, J.; Farkash, R.; Wasserteil, N.; Kasirer, Y.; Rottenstreich, M.; Unger, R.; Grisaru Granovsky, S. Primary risk stratification for neonatal jaundice among term neonates using machine learning algorithm. Early Hum. Dev. 2022, 165, 105538. [Google Scholar] [CrossRef] [PubMed]
- Daunhawer, I.; Kasser, S.; Koch, G.; Sieber, L.; Cakal, H.; Tütsch, J.; Pfister, M.; Wellmann, S.; Vogt, J.E. Enhanced early prediction of clinically relevant neonatal hyperbilirubinemia with machine learning. Pediatr. Res. 2019, 86, 122–127. [Google Scholar] [CrossRef]
| Characteristics | Non-Phototherapy Group (N = 6543) | Phototherapy Group (N = 1699) | x2 | p | |||
| Gender | M | 3181 | 79.2% | 837 | 20.8% | 0.226 | 0.643 |
| F | 3362 | 79.6% | 862 | 20.4% | |||
| Multiple pregnancies | No | 3547 | 78.5% | 971 | 21.5% | 4.709 | 0.031 |
| Yes | 2996 | 80.5% | 728 | 19.5% | |||
| Weight loss exceeding 5% of birth weight | No | 5255 | 81.3% | 1205 | 18.7% | 70.188 | <0.001 |
| Yes | 1288 | 72.3% | 494 | 27.7% | |||
| Maternal country | Korea | 6363 | 79.5% | 1641 | 20.5% | 2.113 | 0.144 |
| Other | 180 | 75.6% | 58 | 24.4% | |||
| Maternal ABO blood group | Non-O | 4819 | 80.1% | 1199 | 19.9% | 6.495 | 0.012 |
| O | 1724 | 77.5% | 500 | 22.5% | |||
| Maternal HBsAg positive | No | 6437 | 79.3% | 1677 | 20.7% | 0.933 | 0.379 |
| Yes | 106 | 82.8% | 22 | 17.2% | |||
| Gestational DM | No | 5978 | 79.6% | 1529 | 20.4% | 3.120 | 0.085 |
| Yes | 565 | 76.9% | 170 | 23.1% | |||
| Gestational hypertensive disorders | No | 5932 | 80.2% | 1465 | 19.8% | 28.827 | 0.000 |
| Yes | 611 | 72.3% | 234 | 27.7% | |||
| Maternal thyroid disease | No | 6113 | 79.6% | 1566 | 20.4% | 3.344 | 0.075 |
| Yes | 430 | 76.4% | 133 | 23.6% | |||
| Premature rupture of membrane | No | 5596 | 78.6% | 1521 | 21.4% | 18.279 | <0.001 |
| Yes | 947 | 84.2% | 178 | 15.8% | |||
| Parity | 1 | 4782 | 78.5% | 1310 | 21.5% | 11.296 | 0.001 |
| 2+ | 1761 | 81.9% | 389 | 18.1% | |||
| Prior artificial miscarriage | 0 | 6111 | 79.8% | 1550 | 20.2% | 9.670 | 0.002 |
| 1+ | 432 | 74.4% | 149 | 25.6% | |||
| Prior natural miscarriage | 0 | 4921 | 79.4% | 1275 | 20.6% | 0.020 | 0.900 |
| 1+ | 1622 | 79.3% | 424 | 20.7% | |||
| Induction of labor | No | 3165 | 71.2% | 1281 | 28.8% | 396.5 | <0.001 |
| Yes | 3378 | 89.0% | 418 | 11.0% | |||
| Epidural analgesia | No | 5564 | 77.8% | 1592 | 22.2% | 88.514 | <0.001 |
| Yes | 979 | 90.1% | 107 | 9.9% | |||
| Delayed cord clamping | No | 6380 | 79.2% | 1676 | 20.8% | 7.911 | 0.004 |
| Yes | 163 | 87.6% | 23 | 12.4% | |||
| Type of delivery | Normal | 4125 | 93.0% | 309 | 7.0% | 1091.884 | <0.001 |
| Cesarean section | 2418 | 63.5% | 1390 | 36.5% | |||
| Vacuum assist | No | 5157 | 76.7% | 1566 | 23.3% | 160.01 | <0.001 |
| Yes | 1386 | 91.2% | 133 | 8.8% | |||
| Small for gestational age | No | 4788 | 80.7% | 1148 | 19.3% | 21.053 | <0.001 |
| Yes | 1755 | 76.1% | 551 | 23.9% | |||
| Preterm birth | No | 4565 | 80.9% | 1076 | 19.1% | 25.881 | <0.001 |
| Yes | 1978 | 76.0% | 623 | 24.0% | |||
| Meconium pass | No | 5069 | 77.3% | 1485 | 22.7% | 81.699 | <0.001 |
| Yes | 1474 | 87.3% | 214 | 12.7% | |||
| Meconium staining | No | 6128 | 79.0% | 1633 | 21.0% | 14.829 | <0.001 |
| Yes | 415 | 86.3% | 66 | 13.7% | |||
| Cord around neck | No | 5262 | 78.7% | 1427 | 21.3% | 11.233 | 0.001 |
| Yes | 1281 | 82.5% | 272 | 17.5% | |||
| Cord knot | No | 6503 | 79.5% | 1682 | 20.5% | 2.976 | 0.064 |
| Yes | 40 | 70.2% | 17 | 29.8% | |||
| Umbilical cord vessels | 2 arteries 1 vein | 6497 | 79.4% | 1688 | 20.6% | 0.061 | 0.480 |
| 1 artery 1 vein | 46 | 80.7% | 11 | 19.3% | |||
| Urination during birth | No | 5237 | 79.8% | 1324 | 20.2% | 3.704 | 0.058 |
| Yes | 1306 | 77.7% | 375 | 22.3% | |||
| Prolonged rupture of membrane 1 | No | 6316 | 79.3% | 1645 | 20.7% | 0.347 | 0.600 |
| Yes | 227 | 80.8% | 54 | 19.2% | |||
| Characteristics | Non-Phototherapy Group (N = 6543) | Phototherapy Group (N = 1699) | F | p | |||
| Mean | SD | Mean | SD | ||||
| Birth weight | 2.85 | ±0.50 | 2.79 | ±0.54 | 3.915 | <0.001 | |
| Birth height | 48.01 | ±2.31 | 47.47 | ±2.39 | 8.537 | <0.001 | |
| Head circumference | 33.85 | ±5.39 | 33.70 | ±1.77 | 1.098 | 0.272 | |
| Chest circumference | 30.57 | ±2.12 | 30.43 | ±2.32 | 2.281 | 0.023 | |
| Abdominal circumference | 28.32 | ±4.08 | 28.16 | ±2.39 | 1.513 | 0.130 | |
| Number of defecations (per day) | 5.41 | ±3.27 | 6.18 | ±4.97 | −7.665 | <0.001 | |
| Number of urinations (per day) | 5.90 | ±1.87 | 7.11 | ±1.53 | −27.541 | <0.001 | |
| Number of breastfeeding sessions (per day) | 2.57 | ±3.59 | 2.21 | ±3.09 | 3.766 | <0.001 | |
| Formula intake (per day) | 166.85 | ±55.79 | 218.46 | ±54.3 | −34.153 | <0.001 | |
| Weight loss rate of the birth weight | 3.53 | ±1.87 | 4.00 | ±2.00 | −9.153 | <0.001 | |
| Maternal age | 40.24 | ±4.27 | 40.51 | ±4.24 | −2.302 | 0.021 | |
| Maternal body mass index | 27.33 | ±6.63 | 28.00 | ±5.87 | 3.361 | <0.001 | |
| Maternal white blood cell count | 8.51 | ±12.23 | 8.47 | ±2.19 | 0.136 | 0.892 | |
| Maternal hemoglobin | 11.94 | ±2.04 | 12.11 | ±4.75 | −2.240 | 0.025 | |
| Maternal platelet count | 207.97 | ±65.44 | 212.90 | ±61.27 | −2.807 | 0.005 | |
| Apgar score 1 min | 7.88 | ±0.86 | 7.77 | ±0.99 | 4.339 | <0.001 | |
| Apgar score 5 min | 9.03 | ±0.56 | 9.00 | ±0.59 | 2.061 | 0.039 | |
| Umbilical cord length | 50.20 | ±33.01 | 49.11 | ±26.17 | 1.251 | 0.211 | |
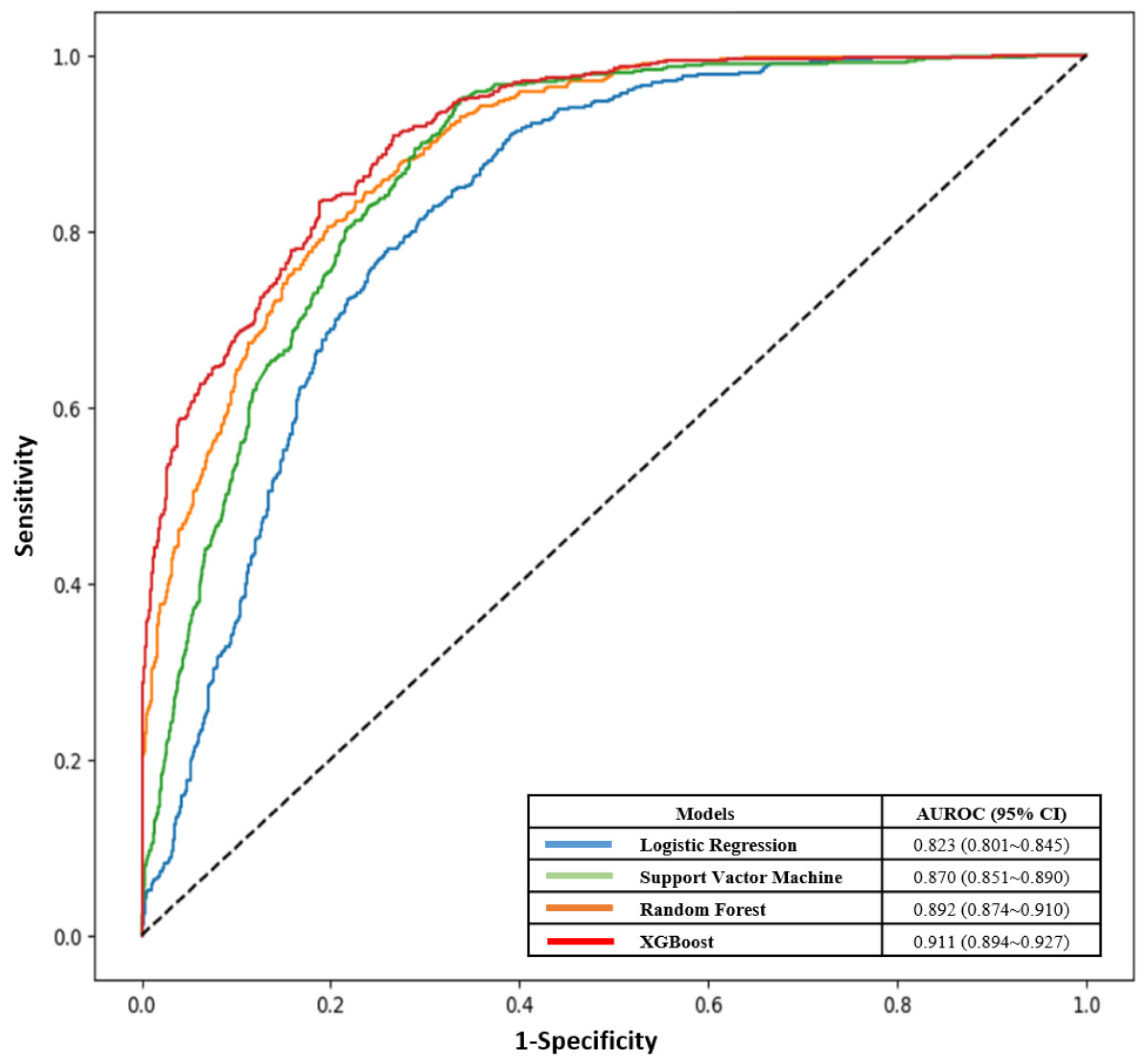
| Models | Accuracy | Precision | Recall | F1-Score | AUROC (95% CI) |
|---|---|---|---|---|---|
| Logistic Regression | 0.754 | 0.632 | 0.566 | 0.597 | 0.823 (0.801~0.845) |
| Support Vactor Machine | 0.79 | 0.665 | 0.699 | 0.682 | 0.870 (0.851~0.890) |
| Random Forest | 0.815 | 0.710 | 0.716 | 0.713 | 0.892 (0.874~0.910) |
| XGBoost | 0.827 | 0.739 | 0.713 | 0.726 | 0.911 (0.894~0.927) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Park, S.; Lee, H. Neonatal Jaundice Requiring Phototherapy Risk Factors in a Newborn Nursery: Machine Learning Approach. Children 2025, 12, 1020. https://doi.org/10.3390/children12081020
Choi Y, Park S, Lee H. Neonatal Jaundice Requiring Phototherapy Risk Factors in a Newborn Nursery: Machine Learning Approach. Children. 2025; 12(8):1020. https://doi.org/10.3390/children12081020
Chicago/Turabian StyleChoi, Yunjin, Sunyoung Park, and Hyungbok Lee. 2025. "Neonatal Jaundice Requiring Phototherapy Risk Factors in a Newborn Nursery: Machine Learning Approach" Children 12, no. 8: 1020. https://doi.org/10.3390/children12081020
APA StyleChoi, Y., Park, S., & Lee, H. (2025). Neonatal Jaundice Requiring Phototherapy Risk Factors in a Newborn Nursery: Machine Learning Approach. Children, 12(8), 1020. https://doi.org/10.3390/children12081020






