Non-Invasive Capnography Versus Pulse Oximetry for Early Detection of Respiratory Depression During Pediatric Procedural Sedation: A Prospective Observational Study
Abstract
1. Introduction
- Minimal sedation preserves normal responses to verbal commands, with minor cognitive impairment and no impact on ventilatory or cardiovascular function.
- Moderate sedation allows purposeful responses to verbal or tactile stimuli, with spontaneous ventilation and hemodynamic stability, without requiring airway intervention.
- Deep sedation is characterized by a response only to repeated or painful stimuli, potential ventilatory compromise requiring assistance, and usually preserved cardiovascular function.
- General anesthesia induces complete unconsciousness, with no response to painful stimuli, impaired spontaneous ventilation requiring support, and possible cardiovascular depression.
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sedation and Monitoring Protocol
2.3.1. Monitoring
- Pulse oximetry (SpO2) for oxygenation;
- Non-invasive, self-calibrating sidestream capnography (GE Healthcare E-miniC-00, GE Healthcare, White Marsh, MD, USA) via nasal cannulas (Natus®, Natus Medical Incorporated, Middleton, WI, USA) for EtCO2 measurement and respiratory rate monitoring;
- Standard vital signs, including heart rate and blood pressure.
2.3.2. Evaluation Scales
- Ramsay Sedation Scale to assess sedation level. Sedation levels were measured at minute 0, and every 5 min until the end of the procedure.
- FLACC Scale, Wong-Baker Faces Scale, and Visual Analog Scale (VAS) were used, depending on the patient’s age, to assess pain during the procedure.
2.4. Definition of Respiratory Events
- Apnea: Absence of breathing or capnographic waveform for more than 2 consecutive readings.
- Hypopneic Hypoventilation: EtCO2 < 30 mmHg or a decline greater than 10 mmHg from baseline with normal or decreased respiratory rate for more than 2 consecutive readings. It detects a decrease in the tidal volume while the patient is maintaining a normal respiratory rate, which leads to EtCO2 levels that are lower than normal, mainly because of a relative increase in dead space ventilation.
- Bradypneic Hypoventilation: Decreased respiratory rate with EtCO2 > 50 mmHg for more than 2 consecutive readings with an abnormally low respiratory rate per age.
- Desaturation: SpO2 < 95% measured by pulse oximetry for more than 2 consecutive readings.
- Respiratory rate (RR) [27] per age was defined as follows:
- ○
- 1–2 years: 20–40 breaths per minute (bpm);
- ○
- 2–8 years: 20–30 bpm;
- ○
- 8–12 years: 15–25 bpm;
- ○
- >12 years: 12–24 bpm.
- (1)
- Our monitoring system recorded vital parameters every 10 s, making 2 consecutive readings the shortest practical duration to confirm a sustained abnormality; and
- (2)
- Clinical relevance, as transient deviations shorter than this period are often self-resolving and may not warrant intervention.
2.5. Sample Size and Power Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Sedation and Pain Scores
- Ramsay sedation scale: Sedation depth was assessed using the Ramsay scale. At baseline (minute 0), the median score was 2 (range 1–3), indicating that patients were calm and cooperative. By minute 5, the median increased to 4 (range 3–5) and remained stable through minute 30, reflecting sustained moderate to deep sedation.
- Age-adjusted pain scales (FLACC, Wong-Baker Faces, or VAS): median pain score 0 during the first 15 min, slightly increasing to a median of 1 from minute 20 onward.
3.3. Respiratory Depression Events
3.3.1. Hypopneic Hypoventilation: 52 Episodes in 35 Patients
- Twelve events (23.0%) associated with desaturation.
3.3.2. Isolated Bradypneic Hypoventilation: 0 Episodes
- No events met the capnographic criteria for bradypneic hypoventilation.
3.3.3. Isolated Desaturation: 32 Episodes in 21 Patients
- Twenty-seven episodes with saturation 90–94%;
- Five episodes with saturation 85–89%;
- No episodes below 85%.
3.3.4. Apnea: 9 Episodes, All Detected Early by Capnography
- Median delay before detection by pulse oximetry: 10 s (range 0, +40 s);
- Lowest observed saturation: 60%, median 85% (interquartile range 79–91%).
- Three apneas began with a decrease in end-tidal CO2 (EtCO2), followed by a cessation of respiratory rate (RR = 0), and ended with oxygen desaturation.
- One apnea was characterized by an initial drop in EtCO2, followed by oxygen desaturation, and concluded with a cessation of respiratory rate (RR = 0), which had previously been abnormally low.
- One apnea presented an initial increase in EtCO2, consistent with bradypneic hypoventilation, followed by oxygen desaturation and subsequent cessation of respiratory rate (RR = 0).
- The remaining four apneas showed a simultaneous drop in EtCO2, respiratory rate, and oxygen saturation.
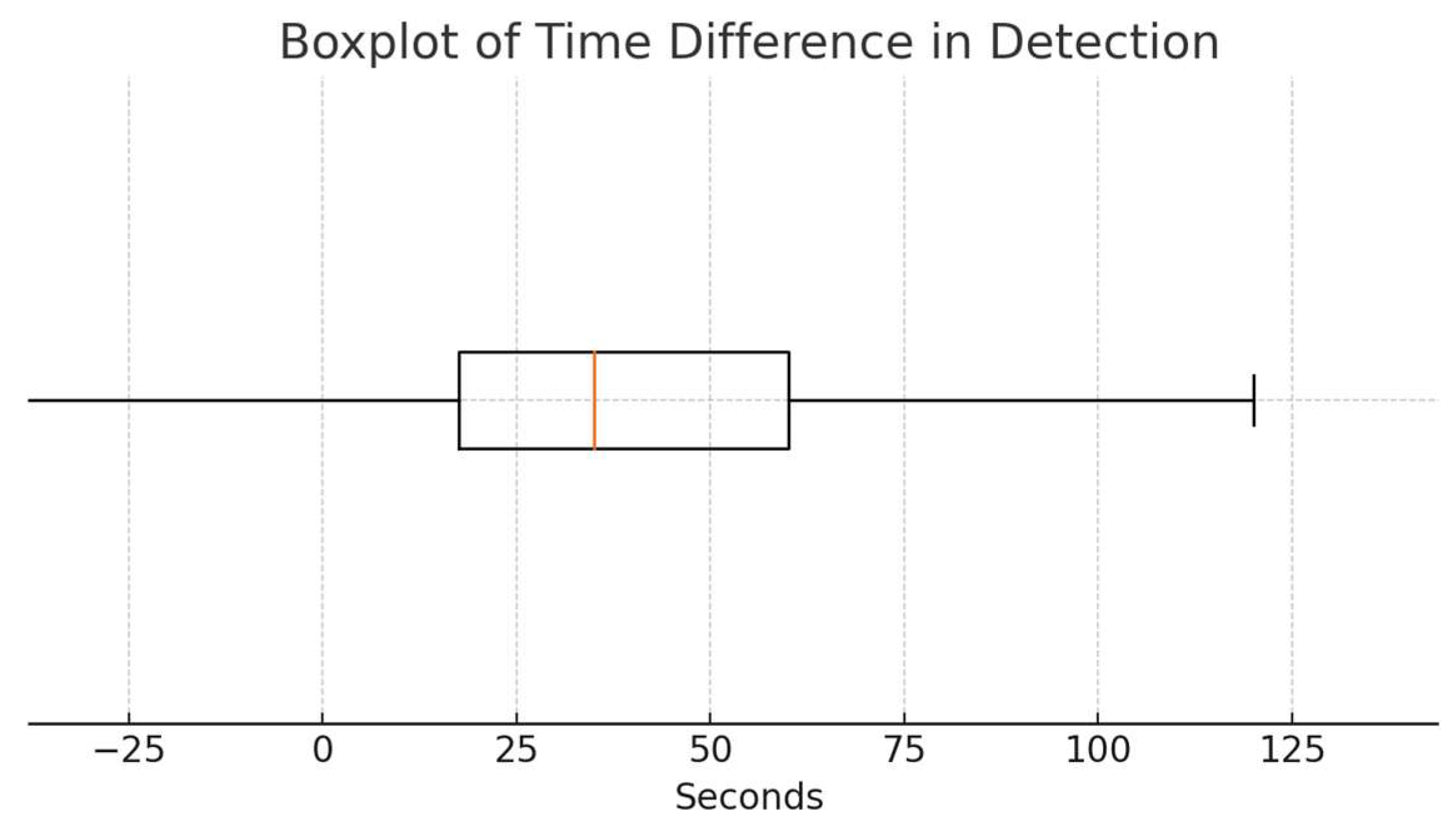
3.4. Time Difference Analysis
3.5. Clinical Interventions
3.6. ASA Classification
4. Discussion
4.1. Study Limitations
- Single-center design: Conducted in a tertiary pediatric hospital, limiting generalizability to other settings.
- Sample size and estimation approach: Although the sample (n = 101) was adequate for primary analyses, it limited subgroup comparisons (e.g., by age or comorbidities). Additionally, the sample size calculation was based on clinical trial assumptions rather than diagnostic accuracy frameworks.
- Selection bias: Given that this study was conducted in a major oncology center, the high proportion of oncologic patients (ASA III) may influence the frequency of respiratory events, although no statistical association was found.
- Monitoring and clinical decision-making: Although capnography and pulse oximetry were both used for monitoring, clinical decisions during procedures (e.g., adjustments in sedation, oxygen administration, or airway interventions) were based solely on pulse oximetry and standard clinical assessment.
- Our primary objective was to compare the timing of respiratory event detection between capnography and pulse oximetry, and thus, the study was not prospectively designed to capture detailed intervention data. As such, the number of cases requiring clinical interventions, including supplemental oxygen administration, airway repositioning, or bag-mask ventilation, was not systematically documented. However, correlating earlier detection with actual clinical interventions would provide critical insight into the practical significance of our findings.
- Equipment limitations: Use of nasal cannulas (rather than nasobuccal cannulas) may have underestimated EtCO2 values in cases of intermittent oral breathing. Sidestream capnography is limited by a smaller amount of gas analyzed, has a slower response time than microstream capnography, and there is a higher risk of gas mixing [14,21,32]. Although we recognize the importance of the capnography waveform, our monitoring system did not have the capability to store waveform tracings for later analysis. As a result, we collected data in real time during sedation, recording only numerical values. Consequently, it was not possible to perform a detailed morphological analysis of the four phases of the capnogram or to explore relationships between waveform characteristics, patient gender, or procedural complexity.
4.2. Clinical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSA | Procedural Sedation and Analgesia Multidisciplinary Digital Publishing Institute |
| EtCO2 | End-tidal Carbon Dioxide |
| SpO2 | Peripheral Oxygen Saturation (by pulse oximetry) |
| ASA | American Society of Anesthesiologists Physical Status Classification |
| IQR | Interquartile Range |
| VAS | Visual Analog Scale |
| FLACC | Face, Legs, Activity, Cry, Consolability (pain scale) |
| CI | Confidence Interval |
References
- Coté, C.J.; Wilson, S.; American Academy of Pediatrics; American Academy of Pediatric Dentistry. Guidelines for Monitoring and Management of Pediatric Patients Before, During, and After Sedation for Diagnostic and Therapeutic Procedures. Pediatrics 2019, 143, e20191000. [Google Scholar] [CrossRef] [PubMed]
- American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 2002, 96, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Mora Capín, A.; Míguez Navarro, C.; López López, R.; Marañón Pardillo, R. Utilidad de la capnografía en la monitorización durante procedimientos de sedoanalgesia. Influencia de la administración de oxígeno en los parámetros monitorizados [Usefulness of capnography for monitoring sedoanalgesia: Influence of oxygen on the parameters monitored]. An. Pediatría 2014, 80, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Lightdale, J.R.; Goldmann, D.A.; Feldman, H.A.; Newburg, A.R.; DiNardo, J.A.; Fox, V.L. Microstream capnography improves patient monitoring during moderate sedation: A randomized, controlled trial. Pediatrics 2006, 117, e1170–e1178. [Google Scholar] [CrossRef] [PubMed]
- The Joint Commission. Comprehensive Accreditation Manual for Hospitals (CAMH): The Official Handbook; The Joint Commission: Oakbrook Terrace, IL, USA, 2021. [Google Scholar]
- Langhan, M.L.; Chen, L.; Marshall, C.; Santucci, K.A. Detection of hypoventilation by capnography and its association with hypoxia in children undergoing sedation with ketamine. Pediatr. Emerg. Care 2011, 27, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.H.; Harrah, J.D.; Germann, C.A.; Dillon, D.C. Does end-tidal carbon dioxide monitoring detect respiratory events prior to current sedation monitoring practices? Acad. Emerg. Med. 2006, 13, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.D. End-tidal carbon dioxide monitoring during sedation with a combination of midazolam and ketamine for children undergoing painful, invasive procedures. Pediatr. Emerg. Care 1999, 15, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.R.; Heegaard, W.; Plummer, D. End-tidal carbon dioxide monitoring during procedural sedation. Acad. Emerg. Med. 2002, 9, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Vargo, J.J.; Zuccaro, G., Jr.; Dumot, J.A.; Conwell, D.L.; Morrow, J.B.; Shay, S.S. Automated graphic assessment of respiratory activity is superior to pulse oximetry and visual assessment for the detection of early respiratory depression during therapeutic upper endoscopy. Gastrointest. Endosc. 2002, 55, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Waugh, J.B.; Epps, C.A.; Khodneva, Y.A. Capnography enhances surveillance of respiratory events during procedural sedation: A meta-analysis. J. Clin. Anesth. 2011, 23, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Koyfman, A.; Vivirito, M.A. Capnography in the Emergency Department: A Review of Uses, Waveforms, and Limitations. J. Emerg. Med. 2017, 53, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Selby, S.T.; Abramo, T.; Hobart-Porter, N. An Update on End-Tidal CO2 Monitoring. Pediatr. Emerg. Care 2018, 34, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Langhan, M.L.; Chen, L. Current utilization of continuous end-tidal carbon dioxide monitoring in pediatric emergency departments. Pediatr. Emerg. Care 2008, 24, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Langhan, M.L.; Shabanova, V.; Li, F.Y.; Bernstein, S.L.; Shapiro, E.D. A randomized controlled trial of capnography during sedation in a pediatric emergency setting. Am. J. Emerg. Med. 2015, 33, 25–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aslan, N.; Yildizdas, D.; Horoz, O.O.; Arslan, D.; Coban, Y.; Sertdemir, Y. Effects of Sedation and/or Sedation/Analgesic Drugs Administered during Central Venous Catheterization on the Level of End-tidal Carbon Dioxide Measured by Nasal Cannula in Our PICU. Indian J. Crit. Care Med. 2020, 24, 705–708. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yldzdaş, D.; Yapcoǧlu, H.; Ylmaz, H.L. The value of capnography during sedation or sedation/analgesia in pediatric minor procedures. Pediatr. Emerg. Care 2004, 20, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Cacho, G.; Pérez-Calle, J.L.; Barbado, A.; Lledó, J.L.; Ojea, R.; Fernández-Rodríguez, C.M. Capnography is superior to pulse oximetry for the detection of respiratory depression during colonoscopy. Rev. Española Enfermedades Dig. 2010, 102, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Conway, A.; Douglas, C.; Sutherland, J. Capnography monitoring during procedural sedation and analgesia: A systematic review protocol. Syst. Rev. 2015, 4, 92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damam, S.; Meshram, R.J.; Taksande, A.; Lohiya, S.; Khurana, A.; Patel, A.; Khandelwal, R.; Nath, R.; Javvaji, C.K.; Kakkat, S. Navigating Pediatric Capnography: A Comprehensive Review of Scope and Limitations. Cureus J. Med. Sci. 2024, 16, e53289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, J.L.; Junkins, E.; Pribble, C.; Guenther, E. Capnography and depth of sedation during propofol sedation in children. Ann. Emerg. Med. 2007, 49, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Deitch, K.; Miner, J.; Chudnofsky, C.R.; Dominici, P.; Latta, D. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann. Emerg. Med. 2010, 55, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Cambra Lasaosa, F.J.; Pons Ódena, M. Pulsioximetría y capnografía. An. Pediatría 2003, 59, 252–285. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.J.; Kissoon, N.; Goodwin, S.R. End-tidal carbon dioxide monitoring in pediatric emergencies. Pediatr. Emerg. Care 2005, 21, 327–332. quiz 333–335. [Google Scholar] [CrossRef] [PubMed]
- Krauss, B.; Hess, D.R. Capnography for procedural sedation and analgesia in the emergency department. Ann. Emerg. Med. 2007, 50, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, C.; Cantais, A.; Gervaix, A.; Bressan, S.; Löllgen, R.; Krauss, B.; Pediatric Emergency Medicine Comfort and Analgesia Research in Europe (PemCARE) group of the Research in European Pediatric Emergency Medicine. Pediatric procedural sedation and analgesia in the emergency department: Surveying the current European practice. Eur. J. Pediatr. 2021, 180, 1799–1813, Erratum in: Eur. J. Pediatr. 2021, 180, 1815–1816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Advanced Life Support Group. Introduction. In Advanced Paediatric Life Support: A Practical Approach to Emergencies, 6th ed.; Advanced Life Support Group, Ed.; Wiley-Blackwell: Chichester, UK, 2016; pp. 3–11. [Google Scholar]
- Lee, J.H.; Ko, H.; Park, J.B.; Ji, S.H.; Kim, J.T. Oxygen supplementation in pediatric sedation-prospective, multicenter, randomized controlled trial. Anesthesiology 2025, 143, 132. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.; Struys, M.M.R.F.; Pollock, R.F.; Mestek, M.; Lightdale, J.R. Patient safety during procedural sedation using capnography monitoring: A systematic review and meta-analysis. BMJ Open. 2017, 7, e013402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nitzan, M.; Romem, A.; Koppel, R. Pulse oximetry: Fundamentals and technology update. Med. Devices (Auckl. NZ) 2014, 7, 231–239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bass, J.L.; Corwin, M.; Gozal, D.; Moore, C.; Nishida, H.; Parker, S.; Schonwald, A.; Wilker, R.E.; Stehle, S.; Kinane, T.B. The effect of chronic or intermittent hypoxia on cognition in childhood: A review of the evidence. Pediatrics 2004, 114, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Tolnai, J.; Rárosi, F.; Tóth, I.; Babik, B.; Novák, Z.; Peták, F.; Fodor, G.H. Relationships between capnogram parameters by mainstream and sidestream techniques at different breathing frequencies. Sci. Rep. 2024, 14, 25443. [Google Scholar] [CrossRef] [PubMed]
| Exclusion Criteria |
|---|
| Emergency situations Patients with abnormal baseline EtCO2 or oxygen saturation (O2 Sat.) 1 |
| Intubation |
| Patients with liver or kidney disease |
| Patients requiring home oxygen therapy |
| Intolerance to nasal cannulas |
| Injuries to the choanae or nasal mucosa |
| Difficult intubation predictors (craniofacial malformations, short neck, or Mallampati score 3–4) |
| History of severe allergic reaction to sedative drugs |
| Demographic Information | |
|---|---|
| Age | Median 10.57 years (Interquartile range: 1.07 to 17.91 years) |
| ASA | Median 3 (Interquartile range: 1 to 3) |
| Fasting time | Median 11.30 h (Interquartile range: 3 to 28 h) |
| Sex | 47 boys |
| 54 girls | |
| Procedure | Count |
|---|---|
| Lumbar puncture | 33 |
| Bone marrow aspiration | 32 |
| Fracture reduction | 9 |
| Bone marrow aspiration and lumbar puncture | 6 |
| Central venous catheter placement | 4 |
| Digestive endoscopy | 4 |
| Peripherally inserted central catheter (PICC) placement | 3 |
| Abscess drainage | 2 |
| Skin biopsy | 1 |
| Molluscum contagiosum cryotherapy | 1 |
| Pneumothorax drainage | 1 |
| Fecaloma extraction | 1 |
| Magnetic resonance imaging (MRI) | 1 |
| Urinary catheterization | 1 |
| Respiratory Depression Events | Capnography | Pulse Oximetry |
|---|---|---|
| Hypopneic Hypoventilation | 52 | 12 * |
| Bradypneic Hypoventilation | 0 | 0 |
| Apnea | 9 | 9 |
| Isolated Desaturation | - | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Català Altarriba, L.; Yeh Hsi, S.; Ravit, A.M.; Brió Sanagustín, S.; González-Rioja, X. Non-Invasive Capnography Versus Pulse Oximetry for Early Detection of Respiratory Depression During Pediatric Procedural Sedation: A Prospective Observational Study. Children 2025, 12, 938. https://doi.org/10.3390/children12070938
Català Altarriba L, Yeh Hsi S, Ravit AM, Brió Sanagustín S, González-Rioja X. Non-Invasive Capnography Versus Pulse Oximetry for Early Detection of Respiratory Depression During Pediatric Procedural Sedation: A Prospective Observational Study. Children. 2025; 12(7):938. https://doi.org/10.3390/children12070938
Chicago/Turabian StyleCatalà Altarriba, Laura, Sean Yeh Hsi, Aude Marie Ravit, Sònia Brió Sanagustín, and Xoan González-Rioja. 2025. "Non-Invasive Capnography Versus Pulse Oximetry for Early Detection of Respiratory Depression During Pediatric Procedural Sedation: A Prospective Observational Study" Children 12, no. 7: 938. https://doi.org/10.3390/children12070938
APA StyleCatalà Altarriba, L., Yeh Hsi, S., Ravit, A. M., Brió Sanagustín, S., & González-Rioja, X. (2025). Non-Invasive Capnography Versus Pulse Oximetry for Early Detection of Respiratory Depression During Pediatric Procedural Sedation: A Prospective Observational Study. Children, 12(7), 938. https://doi.org/10.3390/children12070938





