Impact of Intracystic Hemorrhage on Therapeutic Outcomes in Macro/Mixed Cystic Lymphatic Malformation: A Retrospective Cohort Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. ICG Fluorescence Imaging System
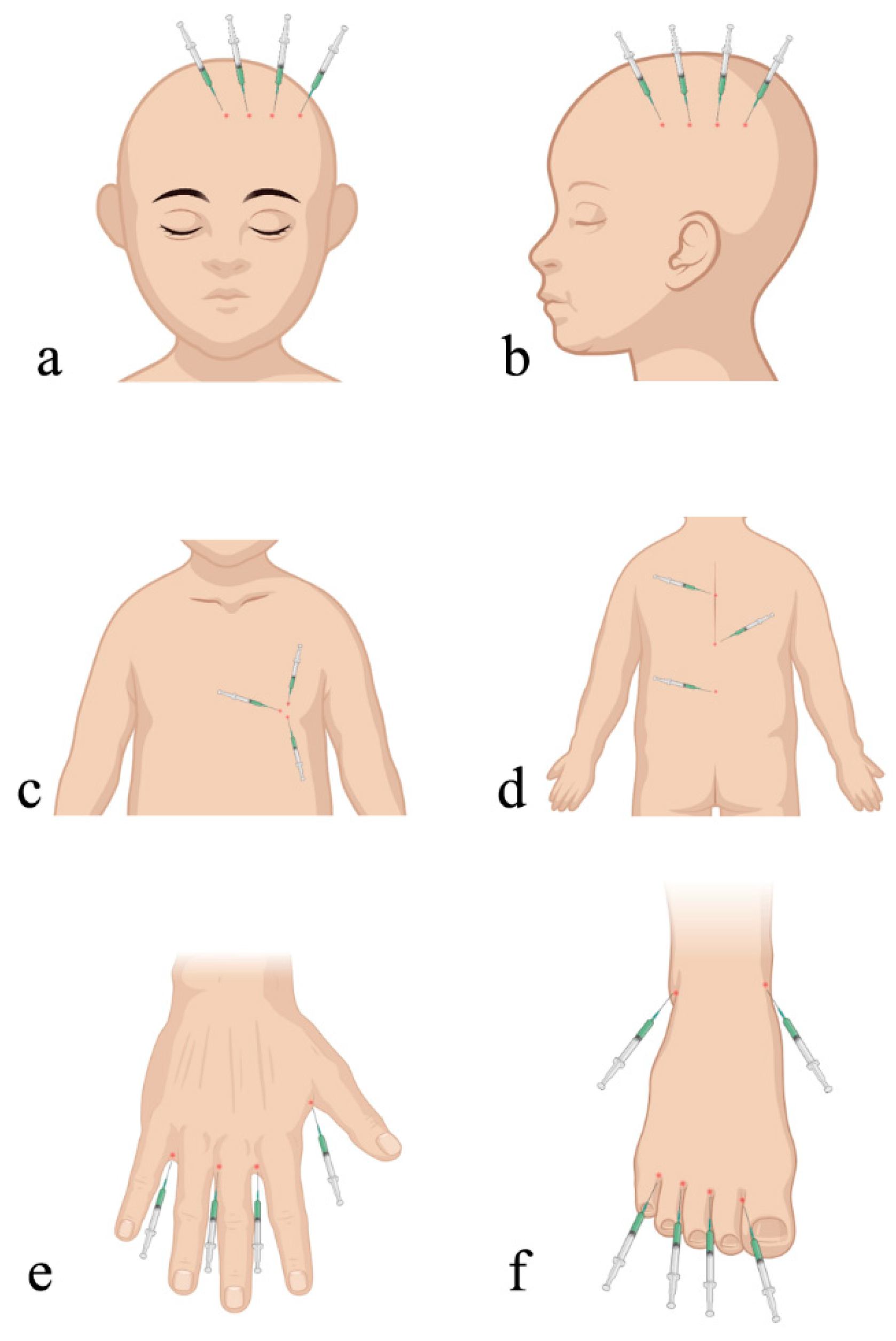
2.3. Surgical Technique
2.4. Postoperative Management
2.5. Evaluation of Curative Effects
2.6. Statistical Analysis
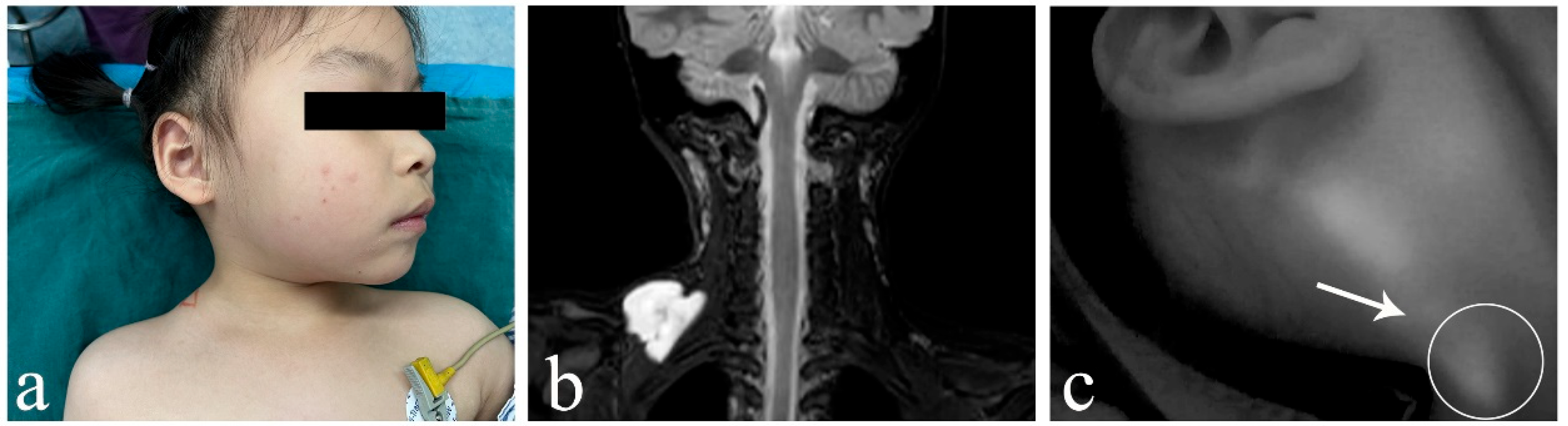
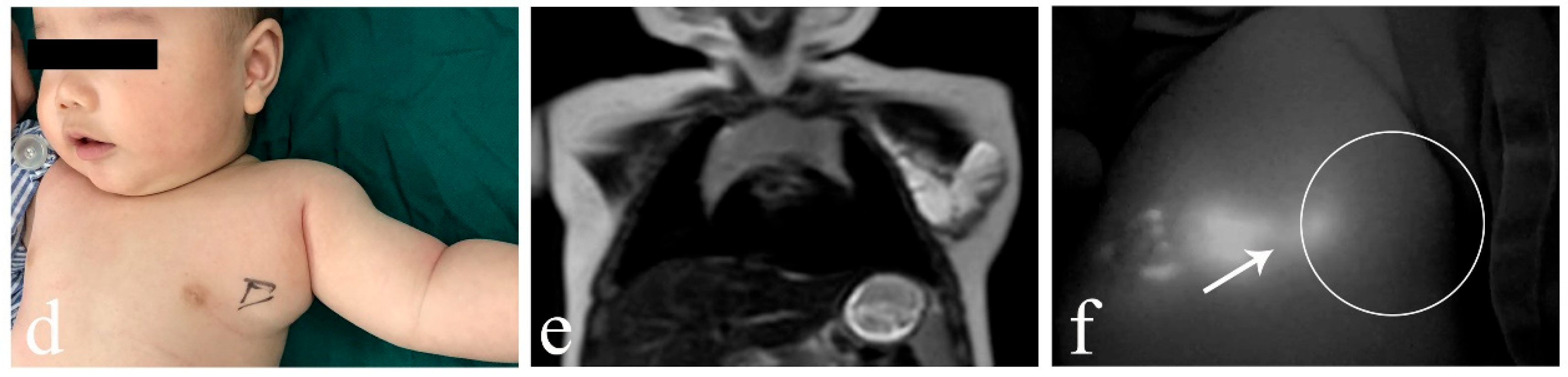
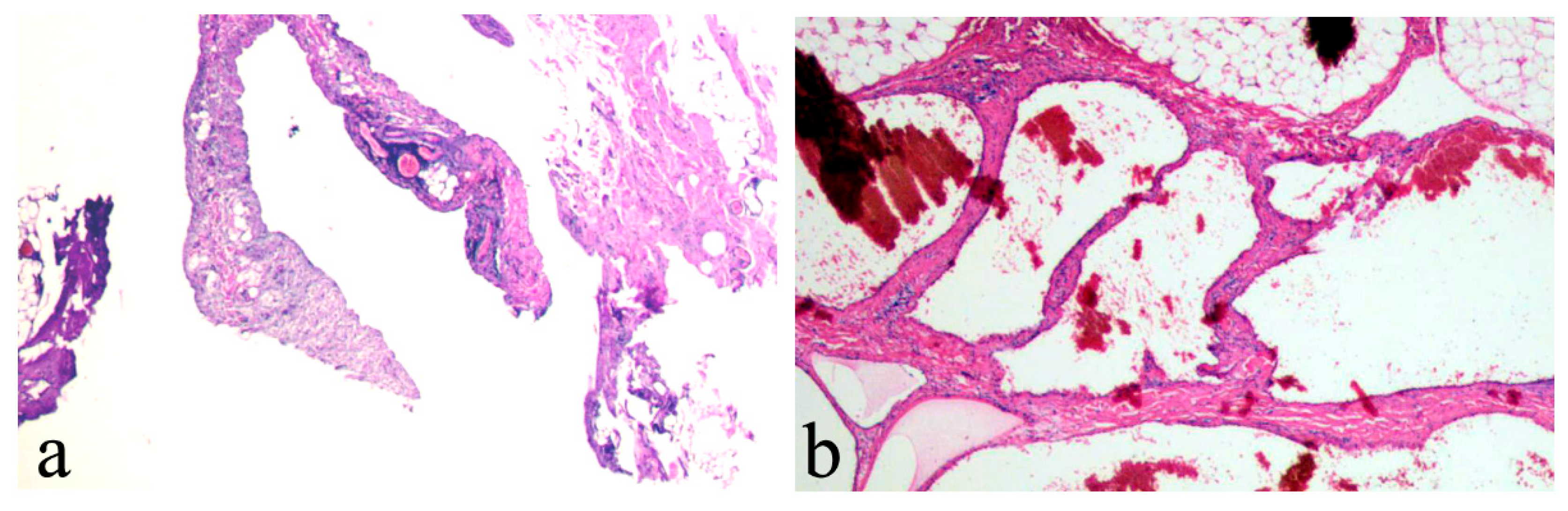
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, M.C.; Zimmerman, M.B.; Burke, D.K.; Bauman, N.M.; Sato, Y.; Smith, R.J.; Group, O.K.C.S. Efficacy and safety of OK-432 immunotherapy of lymphatic malformations. Laryngoscope 2009, 119, 107–115. [Google Scholar] [CrossRef]
- Defnet, A.M.; Bagrodia, N.; Hernandez, S.L.; Gwilliam, N.; Kandel, J.J. Pediatric lymphatic malformations: Evolving understanding and therapeutic options. Pediatr. Surg. Int. 2016, 32, 425–433. [Google Scholar] [CrossRef]
- Bjorklund, K.A.; Billmire, D.A. Mandibular Body Resection and Setback for Severe Malocclusion in Lymphatic Malformations. J. Craniofac. Surg. 2016, 27, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.A. New Frontiers in Our Understanding of Lymphatic Malformations of the Head and Neck: Natural History and Basic Research. Otolaryngol. Clin. N. Am. 2018, 51, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Kwak, R.; EPortnof, J.; Berke, D.M.; Lipari, B.; Waner, M. Analysis of skeletal mandibular abnormalities associated with cervicofacial lymphatic malformations. Laryngoscope 2011, 121, 91–101. [Google Scholar] [CrossRef]
- Faiz, K.; Finitsis, S.; Linton, J.; Shankar, J.J.S. Bleomycin for orbital and peri-orbital veno-lymphatic malformations—A systematic review. Interv. Neuroradiol. 2021, 27, 291–297. [Google Scholar] [CrossRef]
- De Maria, L.; De Sanctis, P.; Tollefson, M.; Mardini, S.; Garrity, J.A.; Morris, P.P.; Bendel, E.; Brinjikji, W. Sclerotherapy for low-flow vascular malformations of the orbital and periocular regions: Systematic review and meta-analysis. Surv. Ophthalmol. 2020, 65, 41–47. [Google Scholar] [CrossRef]
- Ota, Y.; Lee, E.; Sella, E.; Agarwal, P. Vascular Malformations and Tumors: A Review of Classification and Imaging Features for Cardiothoracic Radiologists. Radiol. Cardiothorac. Imaging 2023, 5, e220328. [Google Scholar] [CrossRef]
- Efe, N.; Altas, E.; Mazlumoglu, M.R.; Aktan, B.; Ucuncu, H.; Eren, S.; Oner, F. Excellent Result with the Use of Single-Dose OK-432 in Cervical Macrocystic Lymphangioma. J. Craniofac. Surg. 2016, 27, 1802–1803. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Z.; Chan, Y.; Jiang, T. Airway Management of an Infant with Giant Neck Macro-Cystic Hygroma Utilizing a High-Flow Nasal Cannula. Cureus 2023, 15, e46865. [Google Scholar] [CrossRef]
- Alkwai, H.; Alkwai, H.; Al Namshan, M. Sudden Appearance of a Palpable Chest Wall Mass Secondary to Macrocystic Lymphatic Malformation: A Case Report. Children 2023, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Colbert, S.D.; Seager, L.; Haider, F.; Evans, B.T.; Anand, R.; Brennan, P.A. Lymphatic malformations of the head and neck-current concepts in management. Br. J. Oral. Maxillofac. Surg. 2013, 51, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.M.C.; Hammill, A.; Martini, J.; Treat, J. Sirolimus in the Treatment of Microcystic Lymphatic Malformations: A Systematic Review. Lymphat. Res. Biol. 2023, 21, 101–110. [Google Scholar] [CrossRef]
- Fernandes, S.; Yeung, P.; Heran, M.; Courtemanche, D.; Chadha, N.; Baird, R. Sclerosing agents in the management of lymphatic malformations in children: A systematic review. J. Pediatr. Surg. 2022, 57, 888–896. [Google Scholar] [CrossRef]
- Kalwani, N.M.; Rockson, S.G. Management of lymphatic vascular malformations: A systematic review of the literature. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 1077–1082. [Google Scholar] [CrossRef]
- Colletti, G.; Chiarini, L. Lymphatic malformations do not regress spontaneously. J. Pediatr. Surg. 2022, 57, 1711. [Google Scholar] [CrossRef]
- Bhatt, N.; Perakis, H.; Watts, T.L.; Borders, J.C. Traumatic hemorrhage and rapid expansion of a cervical lymphatic malformation. Ear Nose Throat J. 2011, 90, 20–22. [Google Scholar] [CrossRef]
- Elnahry, A.G.; Habib, M.M. Needle aspiration for orbital venolymphatic malformation presenting with intralesional hemorrhage in adulthood. Orbit 2020, 39, 75–76. [Google Scholar] [CrossRef]
- Haricharan, R.N.; Nawaz, M.; Bettolli, M.; Ferretti, E. Antenatal hemorrhage of a cervical lymphatic malformation presenting as a draining neck mass: An unusual presentation. J. Neonatal Perinat. Med. 2014, 7, 81–84. [Google Scholar] [CrossRef]
- Chen, W.; Luan, J.; Xu, H.; Chen, J.; Xu, R.; Sun, G.; Li, X. Ultrasonography findings of pediatric head and neck lymphatic malformations: A 10-year experience of 140 surgical cases. J. Clin. Ultrasound 2024, 52, 1288–1295. [Google Scholar] [CrossRef]
- Makinen, T.; Boon, L.M.; Vikkula, M.; Alitalo, K. Lymphatic Malformations: Genetics, Mechanisms and Therapeutic Strategies. Circ. Res. 2021, 129, 136–154. [Google Scholar] [CrossRef]
- Zhao, J.; Thompson, E.; Weiss, C.R.; Walsh, J. Treatments and Outcomes of Pediatric Head and Neck Lymphatic Malformations: A 20-Year Single Institution Experience. Otolaryngol. Head Neck Surg. 2025, 172, 1026–1035. [Google Scholar] [CrossRef]
- Wiegand, S.; Wichmann, G.; Dietz, A.; Werner, J.A. Association between malformation type, location and functional deficits in lymphatic malformations of the head and neck in children. Eur. Arch. Otorhinolaryngol. 2023, 280, 2535–2540. [Google Scholar] [CrossRef]
- Han, T.; Chen, H.; Cui, J.; Shen, W. Management of Macrocystic Lymphatic Malformation in the Cervicofacial Region: Ultrasound-Guided Iodine Tincture Cauterization Combined with Intralesional Negative Pressure. Ann. Plast. Surg. 2022, 88, e9–e12. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jin, Y.; Lin, X.; Chen, H.; Ma, G.; Hu, X.; Qiu, Y.; Yu, W.; Chang, L.; Wang, T. Management of periorbital microcystic lymphatic malformation with blepharoptosis: Surgical treatment combined with intralesional bleomycin injection. J. Pediatr. Surg. 2015, 50, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Alqutub, A.; Baamir, N.J.; Mofti, Z.; Zawawi, F.; Al-Khatib, T. Sclerotherapy vs. surgical excision for lymphatic malformations of the head and neck: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2024, 281, 5571–5617. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, H.; Chen, F.; Xu, M.; Xu, R.; Wang, Q.; Li, X. Management of the head and neck lymphatic malformations in children: A 7-year experience of 91 surgical cases. Am. J. Otolaryngol. 2023, 44, 103897. [Google Scholar] [CrossRef]
- Sun, J.; Wang, C.; Li, J.; Song, D.; Guo, L. The efficacy of bleomycin sclerotherapy in the treatment of lymphatic malformations: A review and meta-analysis. Braz. J. Otorhinolaryngol. 2023, 89, 101285. [Google Scholar] [CrossRef]
- Marchand, A.; Caille, A.; Gissot, V.; Giraudeau, B.; Lengelle, C.; Bourgoin, H.; Largeau, B.; Leducq, S.; Maruani, A. Topical sirolimus solution for lingual microcystic lymphatic malformations in children and adults (TOPGUN): Study protocol for a multicenter, randomized, assessor-blinded, controlled, stepped-wedge clinical trial. Trials 2022, 23, 557. [Google Scholar] [CrossRef]
- Sporns, P.B.; Psychogios, M.; Blackham, K.; Zech, C.; Wildgruber, M.; Takes, M. Ultrasonography-guided radiofrequency ablation of vascular malformations-The moving shot technique. Front. Med. 2023, 10, 1345904. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Sun, N.; Liu, Q.; Li, Y.; Peng, Y.; Cheng, X.; Zhang, J.; Liu, Y.; Feng, G.; et al. Differences in Efficacy and Safety of Sirolimus and Sildenafil in Pediatric Lymphatic Malformations. Laryngoscope 2023, 133, 3192–3199. [Google Scholar] [CrossRef]
- Price, C.; Last, S.P.; Sale, P.; Abeysuriya, D.; Herbert, H. Innovative multimodal treatment of an expanding paediatric cervical lymphatic malformation-overcoming refractory disease. BMJ Case Rep. 2025, 18, e26734. [Google Scholar] [CrossRef]
- Peiser, G.; Chand, R.; Amaral, J.G.; Carcao, M.; Willis, L.; Downey, A.C.; Gasparetto, A. Complications of pediatric macrocystic lymphatic malformations of the head and neck: A survival analysis of treated and untreated patients. Emerg. Radiol. 2024, 31, 669–675. [Google Scholar] [CrossRef]
- Papaccio, M.; Bernardi, M.; Tonegatti, L.G.; Alberti, D.; Sartori, E.; Signorelli, M. A case series of fetal lymphatic malformations and a review of the literature. J. Neonatal Perinat. Med. 2023, 16, 747–754. [Google Scholar] [CrossRef]
- Cronan, J.; Gill, A.E.; Shah, J.H.; Hawkins, C.M. The Role of Interventional Radiologists in the Treatment of Congenital Lymphatic Malformations. Semin. Interv. Radiol. 2020, 37, 285–294. [Google Scholar] [CrossRef]
- Chen, W.; Xu, H.; Zhang, L.; Xu, R.; Li, X.; Sun, G. Imaging manifestations of head and neck lymphatic malformations: A single-center experience of 170 surgical cases. Head Neck 2024, 46, 1475–1485. [Google Scholar] [CrossRef]
- Levy, A.D.; Cantisani, V.; Miettinen, M. Abdominal lymphangiomas: Imaging features with pathologic correlation. AJR Am. J. Roentgenol. 2004, 182, 1485–1491. [Google Scholar] [CrossRef]
- Yang, C.; Qiu, T.; Yang, M.; Zhou, J.; Gong, X.; Yang, K.; Zhang, Z.; Lan, Y.; Zhang, X.; Zhou, Z.; et al. Clinical characteristics and risk factors for acute abdomen in patients with abdominal lymphatic malformations. J. Vasc. Surg. Venous Lymphat. Disord. 2025, 13, 101969. [Google Scholar] [CrossRef] [PubMed]
- Fuad, M.; Goh, B.S.; Lokman, F.L.; Mohamad Yunus, M.R. Blue Parotid Unveiled: A Rare Case of Traumatic Hemorrhagic Parotid Lymphangioma in an Eight-Year-Old Boy. Cureus 2023, 15, e46415. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Watanabe, S.; Iida, T.; Watanabe, A. Flow Pattern Classification in Lymphatic Malformations by Indocyanine Green Lymphography. Plast. Reconstr. Surg. 2019, 143, 558e–564e. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | ICH (n = 36) | Without ICH (n = 47) | X2 | p Value |
|---|---|---|---|---|
| Gender | 0.538 | 0.495 | ||
| Male | 25 | 29 | ||
| Female | 11 | 18 | ||
| Age (years) | 2.418 | 0.157 | ||
| 0~3 | 21 | 35 | ||
| >3 | 15 | 12 | ||
| Histological typing | 0.072 | 0.820 | ||
| Macrocystic | 24 | 30 | ||
| Mixed cystic | 12 | 17 | ||
| Lesion location | 0.057 | 0.972 | ||
| Cervicofacial region | 20 | 25 | ||
| Extremities | 7 | 10 | ||
| Trunk | 9 | 12 | ||
| Maximum diameter (cm) | 1.030 | 0.371 | ||
| ≤5 | 17 | 17 | ||
| >5 | 19 | 30 |
| Characteristics | n | Excellent Outcome (n, %) | Subsequent Treatment (n, %) |
|---|---|---|---|
| Gender | |||
| Male | 54 | 31 (57.4) | 9 (16.7) |
| Female | 29 | 17 (58.6) | 6 (20.7) |
| Age (years) | |||
| 0~3 | 56 | * 27 (48.2) | 12 (21.4) |
| >3 | 27 | 21 (77.8) | 3 (11.1) |
| Histological typing | |||
| Macrocystic | 54 | 34 (63.0) | 8 (14.8) |
| Mixed cystic | 29 | 14 (48.3) | 7 (24.1) |
| Lesion location | |||
| Cervicofacial region | 45 | * 20 (44.4) | 8 (17.8) |
| Extremities | 17 | 13 (76.5) | 2 (11.8) |
| Trunk | 21 | 15 (71.4) | 5 (23.8) |
| Maximum diameter (cm) | |||
| ≤5 | 34 | 23 (67.6) | 4 (11.8) |
| >5 | 49 | 25 (51.0) | 11 (22.4) |
| Intracystic condition | |||
| ICH | 36 | * 26 (72.2) | * 2 (5.6) |
| Without ICH | 47 | 22 (46.8) | 13 (27.7) |
| Characteristics | ICH (n = 36) | Without ICH (n = 47) | p Value |
|---|---|---|---|
| Duration of negative drainage (days) | 5.42 ± 1.36 | 6.98 ± 2.17 | <0.001 |
| Local infection (n) | 2 (5.6) | 4 (8.5) | 0.693 |
| Scar hyperplasia (n) | 3 (8.3) | 2 (4.3) | 0.648 |
| Follow-up (months) | 9.00 ± 2.69 | 9.49 ± 3.05 | 0.449 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, T.; Ye, D.; Cui, J.; Huang, S.; Shen, W. Impact of Intracystic Hemorrhage on Therapeutic Outcomes in Macro/Mixed Cystic Lymphatic Malformation: A Retrospective Cohort Study. Children 2025, 12, 935. https://doi.org/10.3390/children12070935
Han T, Ye D, Cui J, Huang S, Shen W. Impact of Intracystic Hemorrhage on Therapeutic Outcomes in Macro/Mixed Cystic Lymphatic Malformation: A Retrospective Cohort Study. Children. 2025; 12(7):935. https://doi.org/10.3390/children12070935
Chicago/Turabian StyleHan, Tao, Daolin Ye, Jie Cui, Songming Huang, and Weimin Shen. 2025. "Impact of Intracystic Hemorrhage on Therapeutic Outcomes in Macro/Mixed Cystic Lymphatic Malformation: A Retrospective Cohort Study" Children 12, no. 7: 935. https://doi.org/10.3390/children12070935
APA StyleHan, T., Ye, D., Cui, J., Huang, S., & Shen, W. (2025). Impact of Intracystic Hemorrhage on Therapeutic Outcomes in Macro/Mixed Cystic Lymphatic Malformation: A Retrospective Cohort Study. Children, 12(7), 935. https://doi.org/10.3390/children12070935






