Immature Platelet Fraction as a Sensitive Biomarker in Neonatal Sepsis: Diagnostic Performance Preceding Thrombocytopenia
Abstract
1. Introduction
2. Materials and Methods
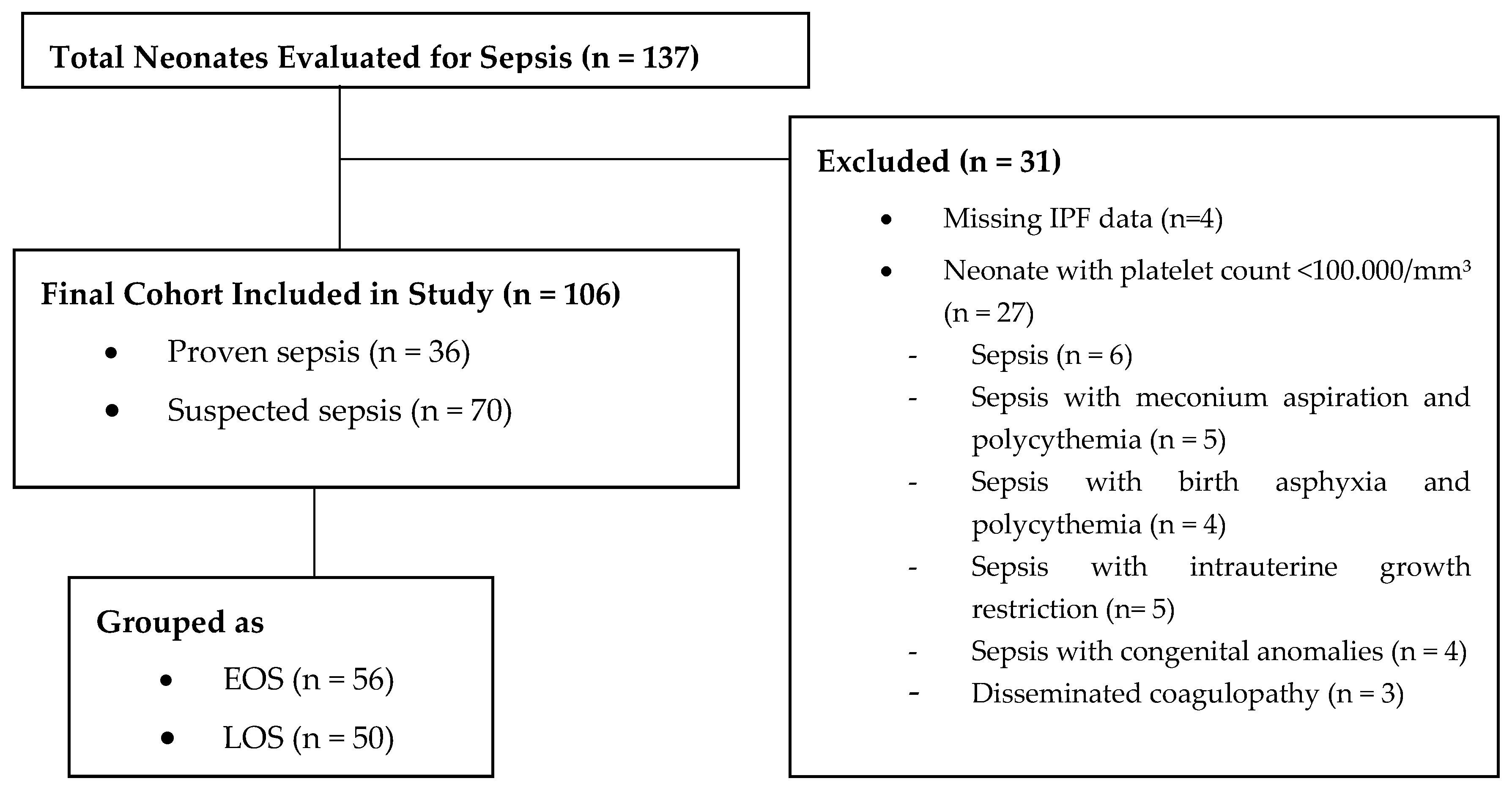
2.1. Study Design and Population
2.2. Blood Sampling and Analysis
2.3. Sepsis Criteria and Diagnosis
2.4. Measurement of IPF
2.5. Statistical Analyses
3. Results
3.1. Demographic and Initial Laboratory Characteristics
3.2. Temporal Changes in Inflammatory and Hematological Parameters
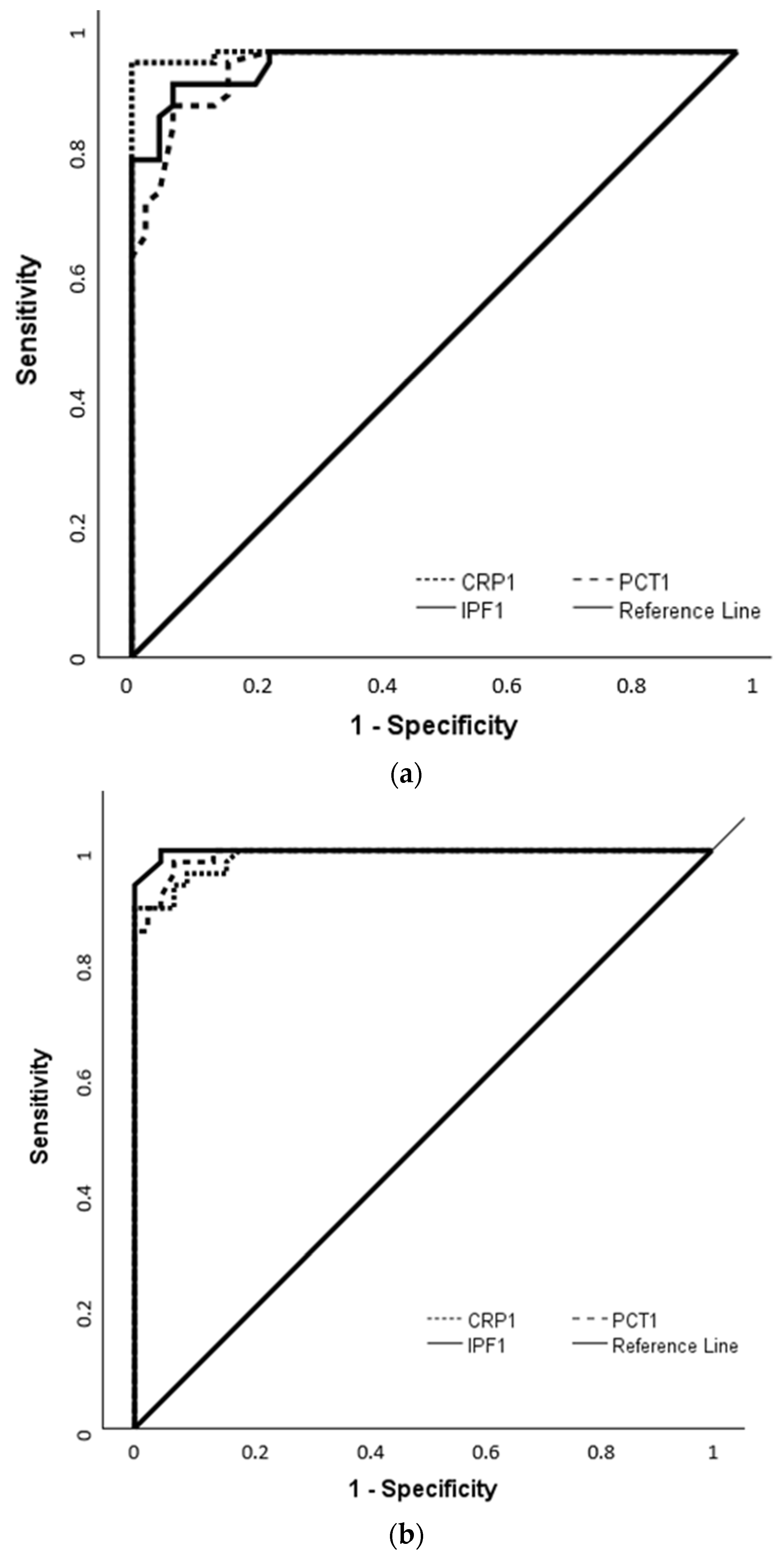
3.3. Diagnostic Performance of IPF in Comparison with CRP and PCT Based on ROC Analysis
3.4. Correlation Analysis of IPF with Hematological and Inflammatory Marker
3.5. Logistic Regression of Sepsis Predictors
3.6. Comparison of Culture-Positive and Culture-Negative Neonates
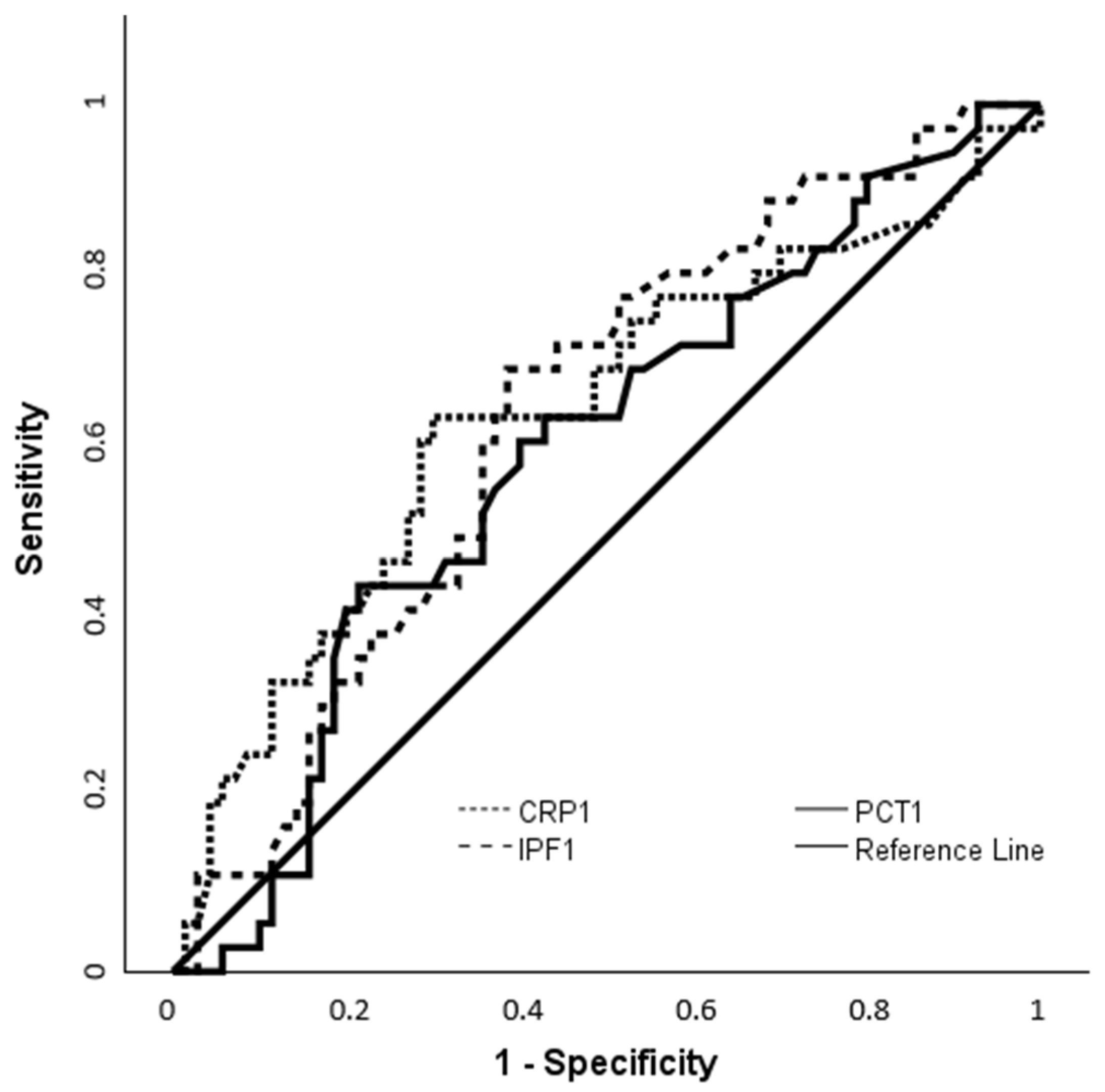
3.7. ROC-Based Assessment of IPF and CRP for Identifying Culture-Positive Cases
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Attia, H.M.H.; Parekh, R.; Dhandibhotla, S.; Sai, T.; Pradhan, A.; Alugula, S.; Cevallos-Cueva, M.; Hayes, B.K.; Athanti, S.; Abdin, Z.; et al. Insight into neonatal sepsis: An Overview. Cureus 2023, 15, e45530. [Google Scholar] [CrossRef] [PubMed]
- Kariniotaki, C.; Thomou, C.; Gkentzi, D.; Panteris, E.; Dimitriou, G.; Hatzidaki, E. Neonatal sepsis: A comprehensive review. Antibiotics 2025, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.; Kornelisse, R.F.; Buonocore, G.; Maier, R.F.; Stocker, M. Culture-negative early-onset neonatal sepsis—At the crossroad between efficient sepsis care and antimicrobial stewardship. Front. Pediatr. 2018, 6, 285. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V. Effective biomarkers for diagnosis of neonatal sepsis. J. Pediatr. Infect Dis. Soc. 2014, 3, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Eichberger, J.; Resch, E.; Resch, B. Diagnosis of neonatal sepsis: The role of inflammatory markers. Front. Pediatr. 2022, 10, 840288. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.; Kunka, S.; Hart, D.; Oguni, S.; Machin, S.J. Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br. J. Haematol. 2004, 126, 93–99. [Google Scholar] [CrossRef] [PubMed]
- De Blasi, R.A.; Cardelli, P.; Costante, A.; Sandri, M.; Mercieri, M.; Arcioni, R. Immature platelet fraction in predicting sepsis in critically ill patients. Intensive Care Med. 2013, 39, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Enz Hubert, R.M.; Rodrigues, M.V.; Andregutto, B.D.; Santos, T.M.; de Fátima Pereira Gilberti, M.; de Castro, V.; Annichino-Bizzacchi, J.M.; Dragosavac, D.; Carvalho-Filho, M.A.; De Paula, E.V. Association of the immature platelet fraction with sepsis diagnosis and severity. Sci. Rep. 2015, 5, 8019. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Song, M.Y.; Yang, B.X.; Xia, R.X. Clinical significance of measuring reticulated platelets in infectious diseases. Medicine 2017, 96, e9424. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ha, S.O.; Cho, Y.-U.; Park, C.-J.; Jang, S.; Hong, S.-B. Immature platelet fraction in septic patients: Clinical relevance of immature platelet fraction is limited to the sensitive and accurate discrimination of septic patients from non-septic patients, not to the discrimination of sepsis severity. Ann. Lab. Med. 2016, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oncel, M.Y.; Ozdemir, R.; Yurttutan, S.; Canpolat, F.E.; Erdeve, O.; Oguz, S.S.; Uras, N.; Dilmen, U. Mean platelet volume in neonatal sepsis. J. Clin. Lab. Anal. 2012, 26, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Y.R. Platelet indices in neonatal sepsis: A review. World J. Clin. Infect. Dis. 2017, 7, 6–10. [Google Scholar] [CrossRef]
- Cremer, M.; Weimann, A.; Szekessy, D.; Hammer, H.; Bührer, C.; Dame, C. Low immature platelet fraction suggests decreased megakaryopoiesis in neonates with sepsis or necrotizing enterocolitis. J. Perinatol. 2013, 33, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Er, I.; Cetin, C.; Baydemir, C.; Günlemez, A. Can immature platelet fraction be an early predictor for congenital pneumonia? Turk. Pediatri. Ars. 2020, 55, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Garofoli, F.; Ciardelli, L.; Angelini, M.; Gentile, R.; Mazzucchelli, I.; Tinelli, C.; Bollani, L.; Tzialla, C. The role of immature platelet fraction (IPF%) in full-term and preterm infants: Italian data of a promising clinical biomarker in neonates. Int. J. Lab. Hematol. 2020, 42, e10–e13. [Google Scholar] [CrossRef] [PubMed]
- Septiane, I.; Kadi, F.A.; Yuniati, T.; Surtiretna, N.; Primadi, A. Mean platelet volume and immature platelet fraction as predictors of early onset neonatal sepsis risk in neonates of 28-36 weeks gestational age. Paediatr. Indones. 2022, 62, 265–273. [Google Scholar] [CrossRef]
- Indra, R.D.; Rikarni; Desiekawati; Rofinda, Z.D.; Yulia, D.; Yusri, E. Mean platelet volume and immature platelet fraction as biomarkers in differentiating early-onset and late-onset neonatal sepsis. Biosci. Med. 2025, 9, 7377–7387. [Google Scholar] [CrossRef]
- World Health Organization. Preterm Birth: Key Facts; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 9 June 2025).
- European Medicines Agency. Report on the Expert Meeting on Neonatal and Paediatric Sepsis; EMA: London, UK, 2010; EMA/CHMP/PEG/194074/2010; Available online: https://www.ema.europa.eu/en/documents/report/report-expert-meeting-neonatal-paediatric-sepsis_en.pdf (accessed on 9 June 2025).
- Roberts, I.; Stanworth, S.; Murray, N.A. Thrombocytopenia in the neonate. Blood Rev. 2008, 22, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.; Hart, D.; Kunka, S.; Oguni, S.; Machin, S.J. Immature platelet fraction measurement: A future guide to platelet transfusion requirement after haematopoietic stem cell transplantation. Transfus. Med. 2006, 16, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Schutt, R.C.; Hannawi, B.; DeLao, T.; Barker, C.M.; Kleiman, N.S. Association of immature platelets with adverse cardiovascular outcomes. J. Am. Coll. Cardiol. 2014, 64, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Adly, A.A.M.; Ragab, I.A.; Ismail, E.A.R.; Farahat, M.M. Evaluation of the immature platelet fraction in the diagnosis and prognosis of childhood immune thrombocytopenia. Platelets 2015, 26, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Goel, G.; Semwal, S.; Khare, A.; Joshi, D.; Amerneni, C.K.; Pakhare, A.; Kapoor, N. Immature Platelet Fraction: Its Clinical Utility in Thrombocytopenia Patients. J. Lab. Physicians 2021, 13, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Grace, R.F.; Lambert, M.P. An update on pediatric ITP: Differentiating primary ITP, IPD, and PID. Blood 2022, 140, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Yuko, S.; Takeda, T.; Hirota, A.; Hisaeda, Y.; Amakata, S.; Nakao, A.; Kawakami, T. Examination of the percentage of immature platelet fraction in term and preterm infants at birth. J. Clin. Neonatol. 2013, 2, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.J.; Kim, H.; Hur, M.; Choi, S.G.; Moon, H.-W.; Yun, Y.M.; Hong, S.N. Establishment of reference interval for immature platelet fraction. Int. J. Lab. Hematol. 2013, 35, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, A.; Garzia, M.; Leone, F.; Arcangeli, A.; Pagano, L.; Zini, G. Immature platelet fraction (IPF) in hospitalized patients with neutrophilia and suspected bacterial infection. J. Infect. 2009, 59, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H.; Kim, H.K.; Kim, J.-E.; Jung, J.S.; Han, K.-S.; Cho, H.-I. Prognostic value of immature platelet fraction and plasma thrombopoietin in disseminated intravascular coagulation. Blood Coagul. Fibrinolysis 2009, 20, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.; Son, B.H.; Seo, J.E.; Kim, I.R.; Park, C.K.; Kim, H.K. Improved Diagnostic and Prognostic Power of Combined Delta Neutrophil Index and Mean Platelet Volume in Pediatric Sepsis. Ann. Clin. Lab. Sci. 2018, 48, 223–230. [Google Scholar] [PubMed]
- Mishra, S.; Jaiswar, S.; Saad, S.; Tripathi, S.; Singh, N.; Deo, S.; Agarwal, M.; Mishra, N. Platelet indices as a predictive marker in neonatal sepsis and respiratory distress in preterm prelabor rupture of membranes. Int. J. Hematol. 2021, 113, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Delanghe, J.R.; Speeckaert, M.M. Translational research and biomarkers in neonatal sepsis. Clin. Chim. Acta 2015, 451, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Lotti, F.; Longini, M.; Rossetti, A.; Bindi, I.; Bazzini, F.; Belvisi, E.; Sarnacchiaro, P.; Scapellato, C.; Buonocore, G. C reactive protein in healthy term newborns during the first 48 hours of life. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F163–F166. [Google Scholar] [CrossRef] [PubMed]
- Bunduki, G.K.; Adu-Sarkodie, Y. The usefulness of C-reactive protein as a biomarker in predicting neonatal sepsis in a sub-Saharan African region. BMC Res. Notes 2020, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.; Sajan, R.; Reshma, G.G.; Gutjahr, G.; S, V.V.; Narmadha, M.P.; Bendapudi, P. Broadening Diagnostic Horizons: Specificity of Serial Negative CRPs in Predicting Blood Culture Negativity in Suspected Neonatal Sepsis. Cureus 2025, 17, e81660. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-G.; Tian, M.; Pan, S.-Y. Clinical utility of procalcitonin and its association with pathogenic microorganisms. Crit. Rev. Clin. Lab. Sci. 2022, 59, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.T.; Sun, L.C.; Lian, R.; Tao, Y.K.; Zhang, H.B.; Zhang, G. Diagnostic and predictive values of procalcitonin in bloodstream infections for nosocomial pneumonia. J. Crit. Care 2018, 44, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, A.; Ferrand, J.; Filhine-Tresarrieu, P.; Aissa, N.; Aimone-Gastin, I.; Namour, F.; Garcia, M.; Lozniewski, A.; Guéant, J.-L. Diagnostic Accuracy of Procalcitonin for Predicting Blood Culture Results in Patients With Suspected Bloodstream Infection: An Observational Study of 35,343 Consecutive Patients (A STROBE-Compliant Article). Medicine 2015, 94, e1774. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Shah, V.S. Intrapartum antibiotics for known maternal Group B streptococcal colonization. Cochrane Database Syst. Rev. 2013, 1, CD007467, Erratum in Cochrane Database Syst. Rev. 2014, 6, CD007467. [Google Scholar] [CrossRef]
| Characteristic | Control Group n = 44 1 | EOS Group n = 56 1 | LOS Group n = 50 1 | p |
|---|---|---|---|---|
| Gestational weeks | 35.5 (31–41) | 37.1 (25–41.5) | 30 (23.3–40.3) | <0.001 b,c,* |
| Preterm birth | 25 (56.8) | 27 (48.2) | 45 (90) | <0.001 b,c,* |
| Birth weight (kg) | 2.55 (1.32–4.08) | 2.73 (0.73–4.10) | 1.32 (0.54–3.9) | <0.001 b,c,* |
| Male | 32 (72.7) | 34 (60.7) | 27 (54) | 0.17 * |
| Cesarean delivery | 34 (77.3) | 38 (67.9) | 40 (80) | 0.32 * |
| WBC-1 (×103/µL) | 12.07 (4.77–25.65) | 13.91 (4.06–37.14) | 11.82 (1.58–47.14) | 0.358 * |
| ANC-1 (×103/µL) | 5.41 (1.55–15.75) | 8.39 (0.65–35.31) | 4.30 (1.03–30.36) | 0.004 b,* |
| Platelet-1 (×103/µL) | 287.5 (130–644) | 218 (118–677) | 221 (110–509) | 0.016 a,* |
| MCV-1 (fL) | 100.6 ± 7.2 | 102.8 ± 6.7 | 96.9 ± 9.1 | <0.001 b,& |
| MPV-1 (fL) | 10.6 ± 1 | 11.3 ± 1.2 | 12.9 ± 1.2 | <0.001 a,b,c,& |
| Plateletcrit-1 (%) | 0.31 (0.09–0.73) | 0.24 (0.10–0.78) | 0.32 (0.02–1.15) | 0.008 a,b,* |
| PDW-1 (%) | 16.5 ± 0.4 | 16.8 ± 0.5 | 16.7 ± 0.4 | 0.009 a,& |
| IPF-1 (%) | 3.7 (0.9–6.5) | 9.2 (4.4–39.2) | 14.6 (6.1–32.3) | <0.001 a,b,c,* |
| CRP-1 (mg/L) | 6.5 (0.3–13.5) | 25.1 (11.2–100) | 35.8 (10–150) | <0.001 a,c,* |
| PCT-1 (ng/mL) | 0.75 (0.08–2.6) | 4.1 (1.2–100) | 5.4 (1.6–50) | <0.001 a,c, |
| Positive blood culture | 0 (0) | 1 (1.8) | 35 (70) | <0.001 b,c,* |
| Markers | EOS Group n = 56 1 | LOS Group n = 50 1 | p |
|---|---|---|---|
| WBC-1 (×103/µL) | 13.91 (4.06–37.14) | 11.82 (1.58–47. 14) | 0.414 * |
| WBC-2 (×103/µL) | 10.44 (1.67–27.19) | 10.28 (4.24–38.00) | |
| p ¥ | 0.010 | 0.233 | |
| ANC-1 (×103/µL) | 8.39 (0.65–35.31) | 4.30 (1.03–30.36) | 0.080 * |
| ANC-2 (×103/µL) | 4.53 (0.60–22.11) | 3.25 (1.27–16.17) | |
| p ¥ | <0.001 | 0.012 | |
| Platelet-1 (×103/µL) | 218 (118–677) | 221 (115–509) | 0.535 * |
| Platelet -2 (×103/µL) | 252.5 (211–320) | 288 (110–656) | |
| p ¥ | 0.088 | 0.032 | |
| MCV-1 (fL) | 102.8 ± 6.7 | 96.9 ± 9.1 | 0.016 & |
| MCV-2 (fL) | 101.7 ± 6.3 | 93.7 ± 8.2 | |
| p § | 0.038 | <0.001 | |
| MPV-1 (fL) | 11.3 ± 1.2 | 12.9 ± 1.2 | 0.006 & |
| MPV-2 (fL) | 11.6 ± 1.3 | 12.5 ± 1.2 | |
| p § | 0.040 | 0.069 | |
| Plateletcrit-1 (%) | 0.24 (0.10–0.78) | 0.32 (0.02–1.16) | 0.571 * |
| Plateletcrit-2 (%) | 0.31 (0.10–0.82) | 0.36 (0.12–0.93) | |
| p ¥ | 0.006 | 0.203 | |
| PDW-1 (%) | 16.7 (16–18.2) | 16.8 (15.7–17.4) | 0.959 * |
| PDW-2 (%) | 16.7 (15.9–18.1) | 16.6 (15.9–17.6) | |
| p ¥ | 0.677 | 0.781 | |
| IPF-1 (%) | 9.2 (4.4–39.2) | 14.6 (6.1–32.3) | 0.013 * |
| IPF-2 (%) | 6.4 (1.4–32.3) | 8.95 (3–23.7) | |
| p ¥ | <0.001 | <0.001 | |
| CRP-1 (mg/L) | 25.1 (11.2–100) | 35.8 (10–150) | 0.058 * |
| CRP-2 (mg/L) | 6.02 (1.3–45.7) | 8 (1.3–58) | |
| p ¥ | <0.001 | <0.001 | |
| PCT-1 (ng/mL) | 4.1 (1.2–100) | 5.4 (1.6–50) | 0.173 * |
| PCT-2 (ng/mL) | 1.1 (0.1–16) | 1.4 (0.1–14) | |
| p ¥ | <0.001 | <0.001 |
| Group | Cut-Off | AUC (95% CI) | p-Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| EOS group | |||||
| IPF-1 | 5.5 | 0.982 (0.963–1.000) | <0.001 | 94.6 | 93.2 |
| CRP-1 | 14 | 0.998 (0.992–1.003) | <0.001 | 98.2 | 100 |
| PCT-1 | 1.9 | 0.975 (0.951–0.999) | <0.001 | 91.1 | 93.2 |
| LOS group | |||||
| IPF-1 | 6 | 0.998 (0.994–1.002) | <0.001 | 100 | 95.5 |
| CRP-1 | 13.75 | 0.989 (0.975–1.002) | <0.001 | 90 | 100 |
| PCT-1 | 1.9 | 0.992 (0.981–1.003) | <0.001 | 98 | 93.2 |
| Variables | OR | 95% CI | p-Value |
|---|---|---|---|
| Preterm birth | 2.118 | 1.033–4.342 | 0.041 |
| Male Gender | 0.508 | 0.236–1.095 | 0.084 |
| Cesarean delivery | 0.819 | 0.358–0.819 | 0.637 |
| WBC-1 | 1.000 | 1.000–1.000 | 0.226 |
| ANC-1 | 1.000 | 1.000–1.000 | 0.247 |
| Platelet-1 | 1.000 | 1.000–1.000 | 0.035 |
| MCV-1 | 0.991 | 0.948–1.036 | 0.690 |
| MPV-1 | 2.622 | 1.769–3.885 | <0.001 |
| Plateletcrit-1 | 0.666 | 0.075–5.930 | 0.715 |
| PDW-1 | 3.576 | 1.467–8.717 | 0.005 |
| IPF-1 | 6.675 | 2.925–15.234 | <0.001 |
| CRP-1 | 2.425 | 1.575–3.732 | <0.001 |
| PCT-1 | 25.088 | 6.970–90.307 | <0.001 |
| Parameters | Culture-Negative Neonates n = 70 1 | Culture-Positive Neonates n = 36 1 | p-Value |
|---|---|---|---|
| Gestational weeks | 35.1 ± 4.9 | 29.8 ± 3.8 | <0.001 & |
| Birth weight (kg) | 2453 ± 1025.3 | 1408.3 ± 589.3 | <0.001 & |
| WBC-1 (×103/µL) | 13.76 (4.06–37.14) | 11.42 (1.58–22.96) | 0.025 * |
| ANC-1 (×103/µL) | 7.65 (0.65–35.31) | 3.84 (1.03–17.98) | <0.001 * |
| Platelet-1 (×103/µL) | 220 (115- 577) | 216.5 (110–488) | 0.247 * |
| MCV-1 (fL) | 101.6 (77–124.8) | 97.8 (81.5–122) | 0.024 * |
| MPV-1 (fL) | 11.5 ± 1.3 | 13 ± 1.3 | <0.001 & |
| Plateletcrit-1 (%) | 0.3 (0–1.2) | 0.3 (0–0.5) | 0.610 * |
| PDW-1 (%) | 16.7 ± 0.4 | 16.7 ± 0.4 | 0.725 & |
| IPF-1 (%) | 9.8 (4.4–39.2) | 13.1 (6.1–32.3) | 0.017 * |
| CRP-1 (mg/L) | 25 (10.2–150) | 38.3 (10–148) | 0.015 * |
| PCT-1 (ng/mL) | 4.1 (1.2–100) | 5.4 (1.6–50) | 0.100 * |
| Markers | Cut-Off | AUC (95% CI) | p-Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| IPF-1 | 10.85 | 0.642 (0.534–0.749) | 0.010 | 69.4 | 61.4 |
| CRP-1 | 29.9 | 0.645 (0.528–0.761) | 0.015 | 63.9 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Er, I.; Arpa, M. Immature Platelet Fraction as a Sensitive Biomarker in Neonatal Sepsis: Diagnostic Performance Preceding Thrombocytopenia. Children 2025, 12, 931. https://doi.org/10.3390/children12070931
Er I, Arpa M. Immature Platelet Fraction as a Sensitive Biomarker in Neonatal Sepsis: Diagnostic Performance Preceding Thrombocytopenia. Children. 2025; 12(7):931. https://doi.org/10.3390/children12070931
Chicago/Turabian StyleEr, Ilkay, and Medeni Arpa. 2025. "Immature Platelet Fraction as a Sensitive Biomarker in Neonatal Sepsis: Diagnostic Performance Preceding Thrombocytopenia" Children 12, no. 7: 931. https://doi.org/10.3390/children12070931
APA StyleEr, I., & Arpa, M. (2025). Immature Platelet Fraction as a Sensitive Biomarker in Neonatal Sepsis: Diagnostic Performance Preceding Thrombocytopenia. Children, 12(7), 931. https://doi.org/10.3390/children12070931






