Effects of Rope Therapy on Social Attention and Temperament Traits in Autistic Children
Abstract
1. Introduction
- Vestibular and Proprioceptive Regulation
- 2.
- Polyvagal Engagement
- 3.
- Cognitive–Motor Integration
- 4.
- Symbolic and Emotional Processing
2. Methodology
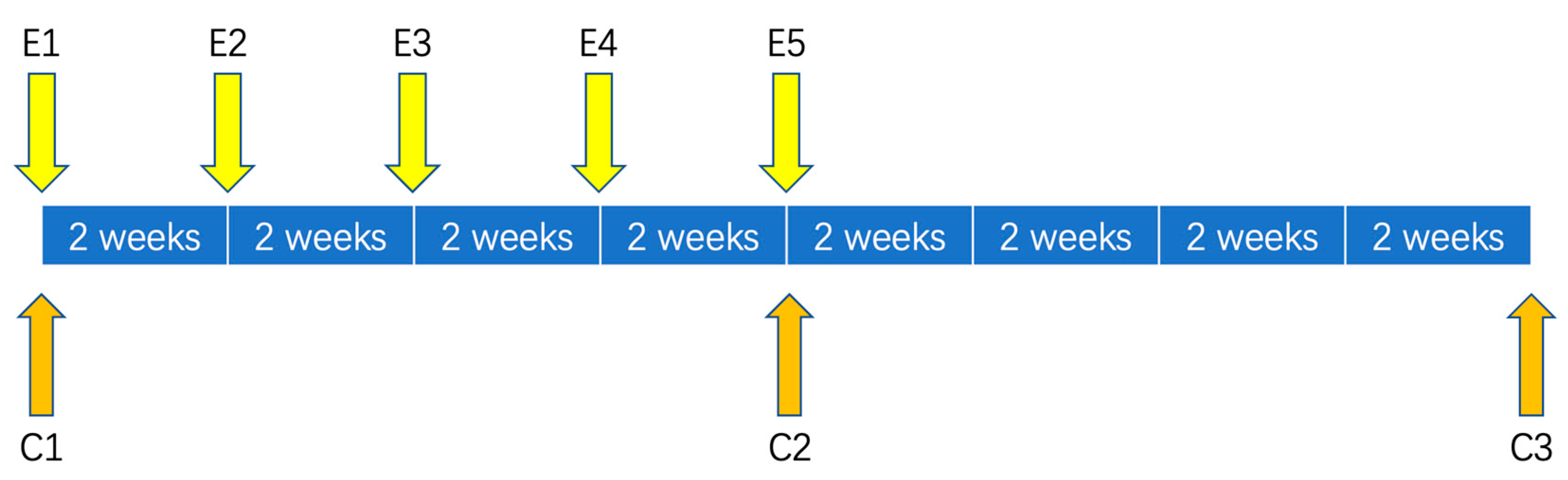
2.1. Study Design
2.2. Participants
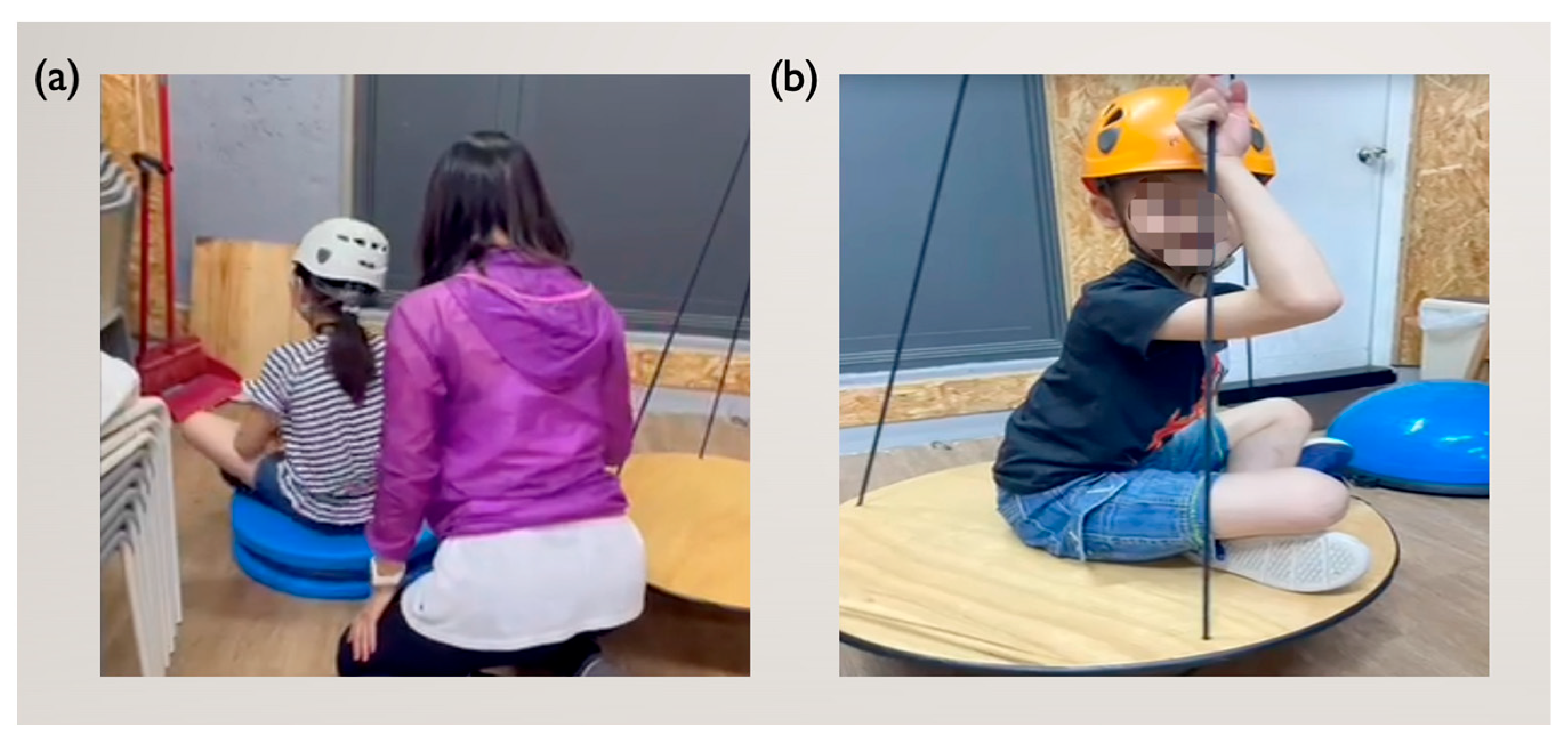
2.3. Intervention Design
2.4. Outcome Measures
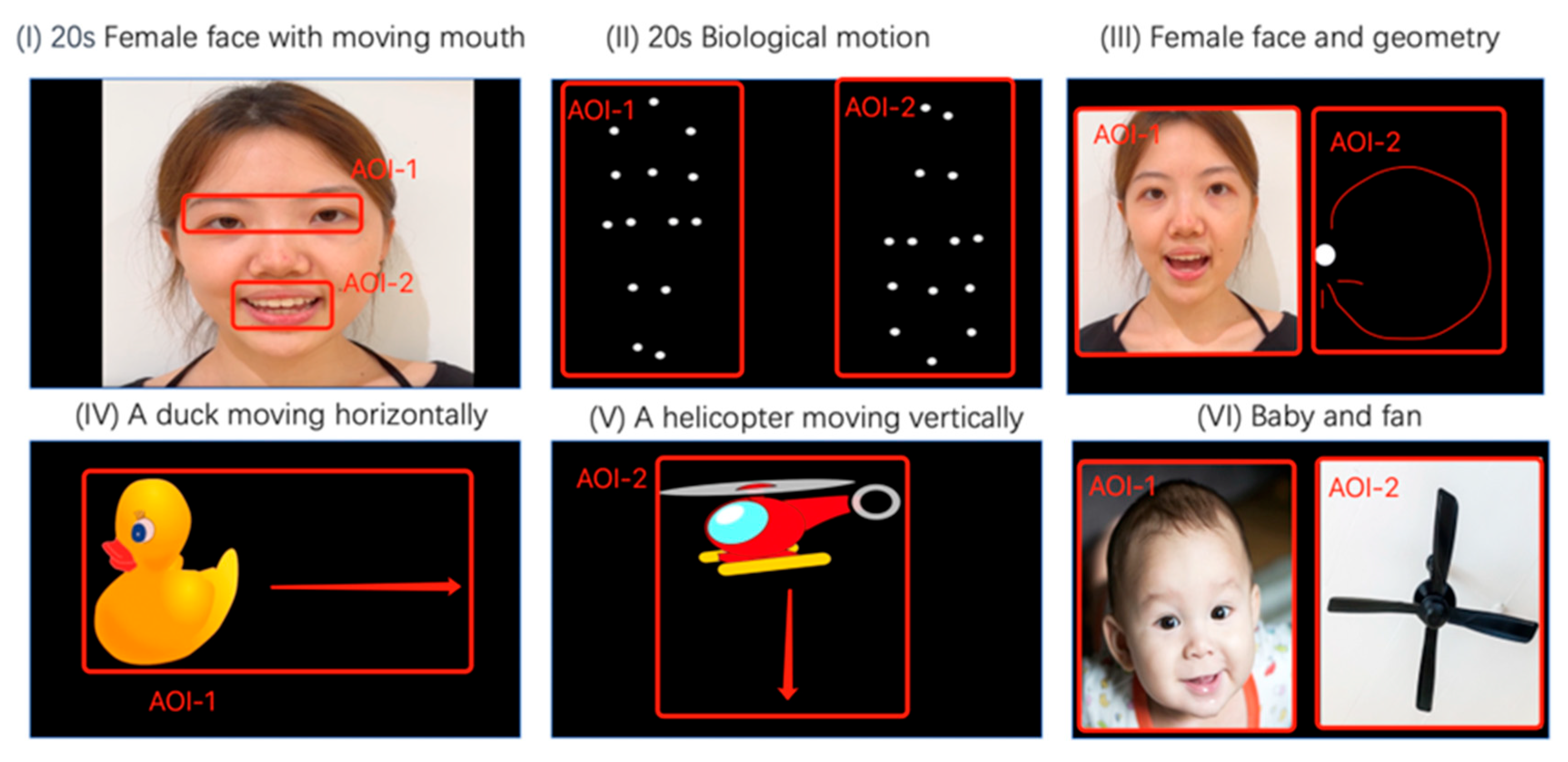
2.5. Stimulus Design
- Paradigm I (20 s): A woman’s face silently mouthing the alphabet was displayed. Attention to the eyes (AOI-1) and mouth (AOI-2) reflects social communication and language development [29].
- Paradigm II (20 s): A walking figure, both upright (AOI-1) and inverted (AOI-2), was presented in point-light display to assess attention to biological motion, which is typically diminished in autistic children, reflecting social communication and language development [29].
- Paradigm III (20 s): The same face from Paradigm I (AOI-1) was displayed alongside a moving dot (AOI-2) to test preference for social versus moving stimuli [29].
- Paradigm IV and V (20 s): A moving duck (AOI-1) and a helicopter (AOI-2) were displayed to assess sustained attention to animals versus objects [28].
- Paradigm VI (10 s): A baby’s face (AOI-1) was displayed next to an electronic fan (AOI-2) to evaluate preference for faces versus objects [28].
2.6. Taylor–Johnson Temperament Analysis
2.7. Data Processing
2.8. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Temperament Traits
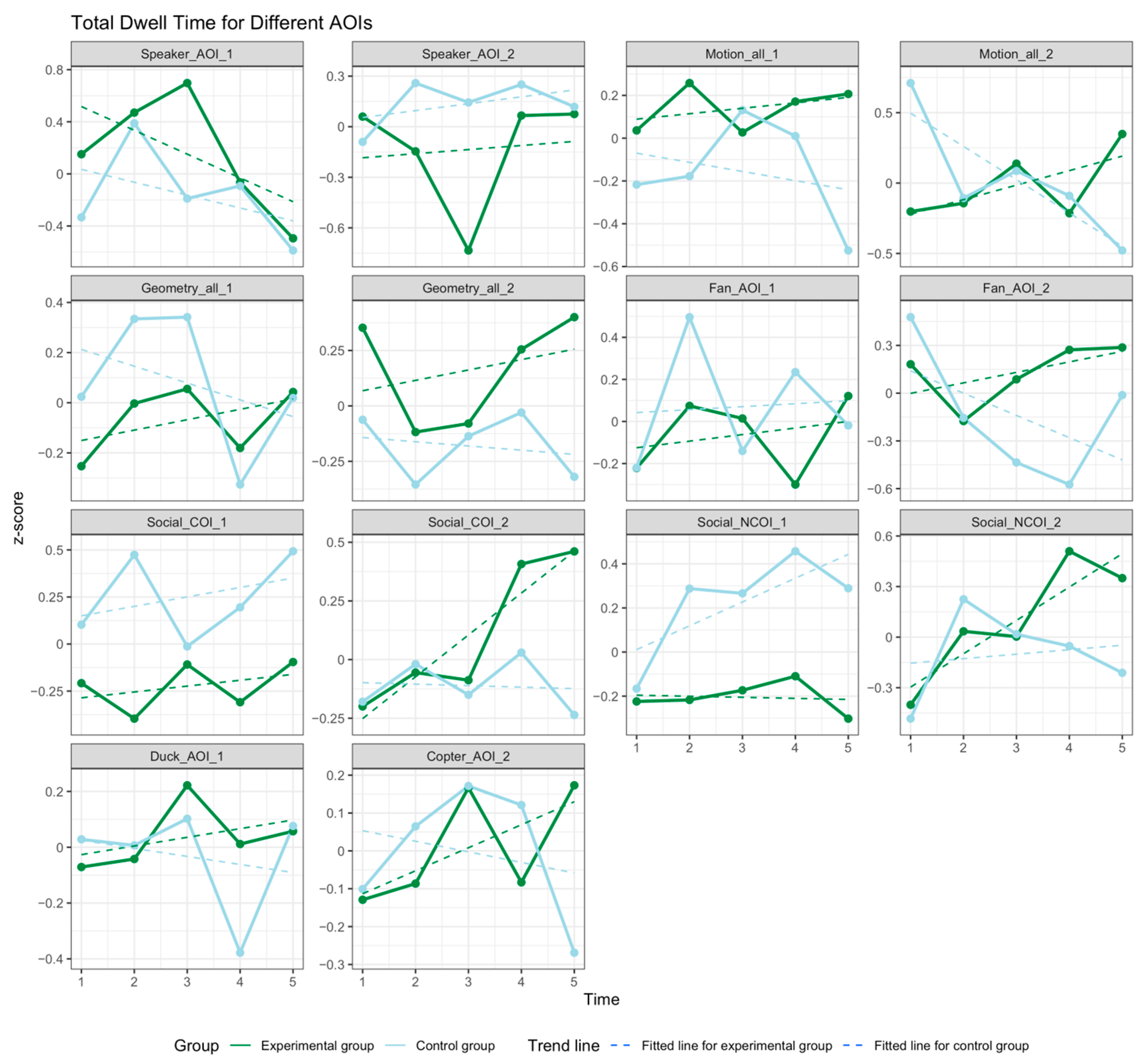
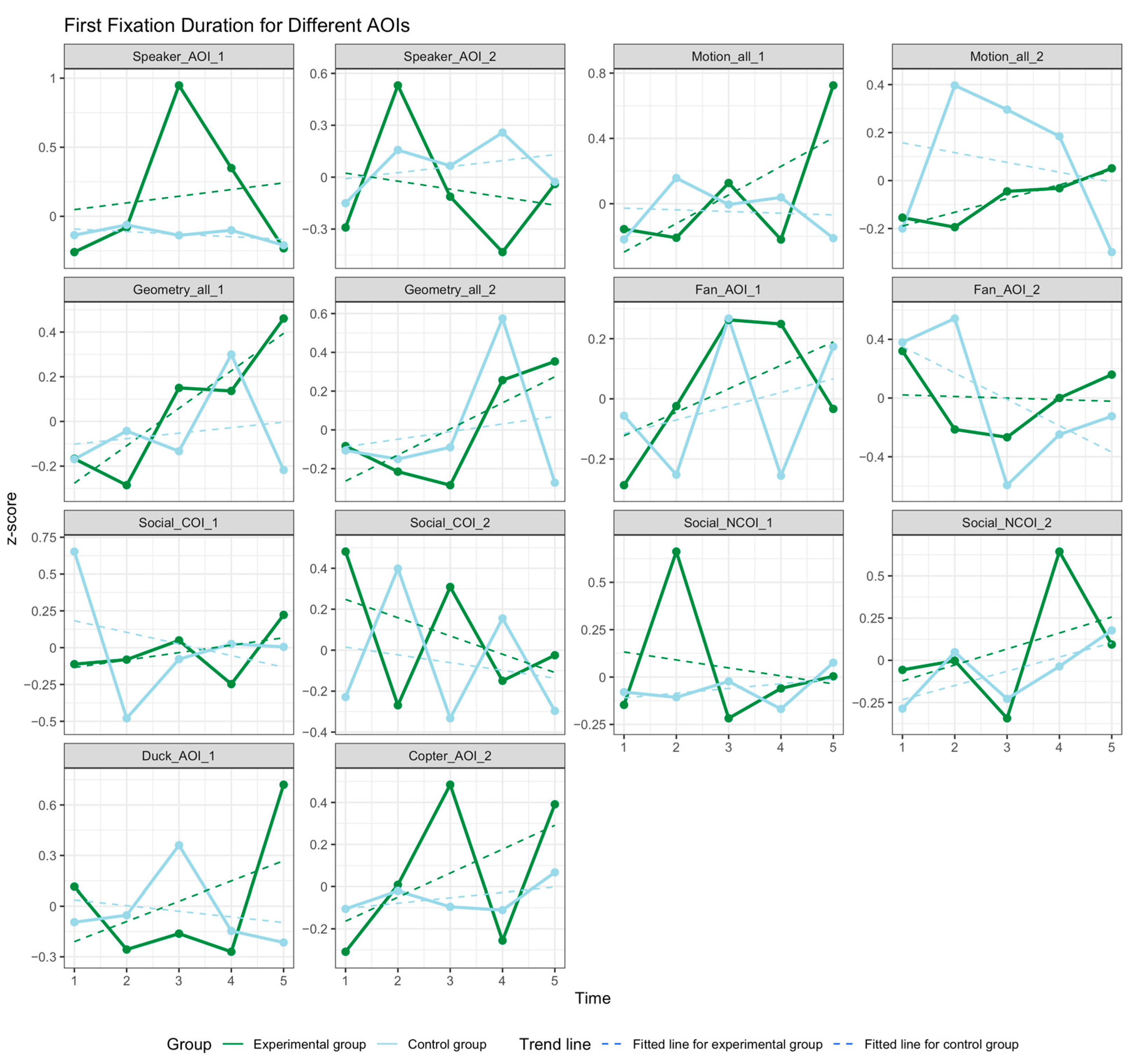
3.3. Social Attention
3.4. Temperament Trait Pattern Changes
4. Discussion
4.1. Study Limitations and Future Research
4.2. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| AOI | Effect | Df | Mean Square | F | p |
|---|---|---|---|---|---|
| Speaker_1 | Group | 1 | 30,575,193 | 3.259 | 0.074 * |
| Time | 4 | 5,554,721 | 0.592 | 0.669 | |
| Group: Time | 4 | 9,065,489 | 0.966 | 0.429 | |
| Error | 111 | 9,380,645 | |||
| Speaker_2 | Group | 1 | 47,061,800 | 2.265 | 0.135 |
| Time | 4 | 9,879,947 | 0.476 | 0.754 | |
| Group: Time | 4 | 19,595,296 | 0.943 | 0.442 | |
| Error | 111 | 20,777,333 | |||
| Duck_1 | Group | 1 | 1,239,590 | 0.137 | 0.712 |
| Time | 4 | 5,806,979 | 0.640 | 0.635 | |
| Group: Time | 4 | 1,962,447 | 0.216 | 0.929 | |
| Error | 111 | 9,074,688 | |||
| Copter_2 | Group | 1 | 41,990 | 0.004 | 0.950 |
| Time | 4 | 2,560,020 | 0.237 | 0.917 | |
| Group: Time | 4 | 4,433,926 | 0.411 | 0.800 | |
| Error | 111 | 10,787,828 | |||
| Fan_1 | Group | 1 | 3,592,540 | 0.597 | 0.441 |
| Time | 4 | 14,145,420 | 2.351 | 0.058 * | |
| Group: Time | 4 | 3,743,333 | 0.622 | 0.648 | |
| Error | 111 | 6,016,053 | |||
| Fan_2 | Group | 1 | 10,799,313 | 2.159 | 0.145 |
| Time | 4 | 5,104,847 | 1.021 | 0.400 | |
| Group: Time | 4 | 6,332,881 | 1.266 | 0.288 | |
| Error | 111 | 5,001,615 | |||
| Motion_1 | Group | 1 | 53,437,934 | 2.708 | 0.103 |
| Time | 4 | 9,610,745 | 0.487 | 0.745 | |
| Group: Time | 4 | 10,482,561 | 0.531 | 0.713 | |
| Error | 111 | 19,736,405 | |||
| Motion_2 | Group | 1 | 827,789 | 0.069 | 0.793 |
| Time | 4 | 13,660,345 | 1.139 | 0.342 | |
| Group: Time | 4 | 30,112,539 | 2.511 | 0.046 ** | |
| Error | 111 | 11,992,811 | |||
| Geometry_1 | Group | 1 | 12,480,465 | 0.635 | 0.427 |
| Time | 4 | 3,711,877 | 0.189 | 0.944 | |
| Group: Time | 4 | 5,572,368 | 0.284 | 0.888 | |
| Error | 111 | 19,652,160 | |||
| Geometry_2 | Group | 1 | 68,993,915 | 3.564 | 0.062 * |
| Time | 4 | 2,062,186 | 0.107 | 0.980 | |
| Group: Time | 4 | 6,714,346 | 0.347 | 0.846 | |
| Error | 111 | 19,356,268 | |||
| Social_COI_1 | Group | 1 | 411,490,489 | 7.091 | 0.009 *** |
| Time | 4 | 49,535,009 | 0.854 | 0.494 | |
| Group: Time | 4 | 30,530,272 | 0.526 | 0.717 | |
| Error | 111 | 58,027,302 | |||
| Social_COI_2 | Group | 1 | 96,141,444 | 1.480 | 0.226 |
| Time | 4 | 43,640,569 | 0.672 | 0.613 | |
| Group: Time | 4 | 42,639,732 | 0.656 | 0.624 | |
| Error | 111 | 64,970,748 | |||
| Social_NCOI_1 | Group | 1 | 345,392,987 | 5.674 | 0.019 ** |
| Time | 4 | 53,553,197 | 0.880 | 0.479 | |
| Group: Time | 4 | 17,251,416 | 0.283 | 0.888 | |
| Error | 111 | 60,876,216 | |||
| Social_NCOI_2 | Group | 1 | 79,075,380 | 1.324 | 0.252 |
| Time | 4 | 22,361,182 | 0.374 | 0.827 | |
| Group: Time | 4 | 46,164,060 | 0.773 | 0.545 | |
| Error | 111 | 59,746,994 |
| AOI | Effect | Df | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Speaker_1 | Group | 1 | 3,347,642 | 2.09 | 0.151 |
| Time | 4 | 2,377,236 | 1.484 | 0.212 | |
| Group: Time | 4 | 2,016,773 | 1.259 | 0.291 | |
| Residuals | 105 | 1,601,620 | |||
| Speaker_2 | Group | 1 | 374,223 | 0.434 | 0.511 |
| Time | 4 | 1,237,435 | 1.437 | 0.227 | |
| Group: Time | 4 | 750,427 | 0.871 | 0.484 | |
| Residuals | 105 | 861,291 | |||
| Duck_1 | Group | 1 | 119,833 | 0.109 | 0.742 |
| Time | 4 | 1,395,261 | 1.268 | 0.287 | |
| Group: Time | 4 | 1,716,430 | 1.56 | 0.191 | |
| Residuals | 105 | 1,100,450 | |||
| Copter_2 | Group | 1 | 220,376 | 0.505 | 0.4788 |
| Time | 4 | 1,485,896 | 3.406 | 0.0116 ** | |
| Group: Time | 4 | 372,462 | 0.854 | 0.4943 | |
| Residuals | 105 | 436,198 | |||
| Fan_1 | Group | 1 | 69,065 | 0.122 | 0.728 |
| Time | 4 | 187,239 | 0.33 | 0.857 | |
| Group: Time | 4 | 321,836 | 0.567 | 0.687 | |
| Residuals | 105 | 567,149 | |||
| Fan_2 | Group | 1 | 1090 | 0.011 | 0.918 |
| Time | 4 | 120,350 | 1.165 | 0.331 | |
| Group: Time | 4 | 138,500 | 1.34 | 0.26 | |
| Residuals | 105 | 103,347 | |||
| Motion_1 | Group | 1 | 1,147,740 | 0.27 | 0.604 |
| Time | 4 | 1,853,803 | 0.436 | 0.782 | |
| Group: Time | 4 | 6,048,872 | 1.423 | 0.231 | |
| Residuals | 105 | 4,250,001 | |||
| Motion_2 | Group | 1 | 1,245,692 | 0.577 | 0.449 |
| Time | 4 | 483,540 | 0.224 | 0.924 | |
| Group: Time | 4 | 1,419,753 | 0.658 | 0.623 | |
| Residuals | 105 | 2,157,473 | |||
| Geometry_1 | Group | 1 | 553,315 | 0.27 | 0.604 |
| Time | 4 | 2,208,905 | 1.078 | 0.371 | |
| Group: Time | 4 | 1,379,447 | 0.673 | 0.612 | |
| Residuals | 105 | 2,049,865 | |||
| Geometry_2 | Group | 1 | 17,489 | 0.007 | 0.932 |
| Time | 4 | 207,089 | 0.086 | 0.987 | |
| Group: Time | 4 | 1,979,743 | 0.823 | 0.514 | |
| Residuals | 105 | 2,406,820 | |||
| Social_COI_1 | Group | 1 | 16,057 | 0.133 | 0.716 |
| Time | 4 | 65,201 | 0.541 | 0.706 | |
| Group: Time | 4 | 154,331 | 1.281 | 0.282 | |
| Residuals | 105 | 120,511 | |||
| Social_COI_2 | Group | 1 | 25,614 | 0.384 | 0.5369 |
| Time | 4 | 26,254 | 0.393 | 0.8129 | |
| Group: Time | 4 | 150,268 | 2.252 | 0.0684 * | |
| Residuals | 105 | 66,724 | |||
| Social_NCOI_1 | Group | 1 | 1,012,909 | 0.4 | 0.529 |
| Time | 4 | 3,356,164 | 1.324 | 0.266 | |
| Group: Time | 4 | 2,153,334 | 0.85 | 0.497 | |
| Residuals | 105 | 2,534,193 | |||
| Social_NCOI_2 | Group | 1 | 75,918 | 0.558 | 0.457 |
| Time | 4 | 84,495 | 0.622 | 0.648 | |
| Group: Time | 4 | 85,799 | 0.631 | 0.641 | |
| Residuals | 105 | 135,947 |
| Scale | Source | Df | F | p-Value | Partial Eta Squared |
|---|---|---|---|---|---|
| 1. Overall Adjustment | Group | 1, 22 | 0.006 | 0.964 | 0.000 |
| Time | 2, 44 | 2.528 | 0.091 | 0.103 | |
| Time × Group | 2, 44 | 2.196 | 0.126 | 0.091 | |
| 2. Self-Esteem | Group | 1, 22 | 0.248 | 0.623 | 0.011 |
| Time | 2, 44 | 0.935 | 0.384 | 0.043 | |
| Time × Group | 2, 44 | 0.935 | 0.384 | 0.043 | |
| 3. Outgoing/Gregarious | Group | 1, 22 | 0.401 | 0.533 | 0.018 |
| Time | 2, 44 | 0.573 | 0.629 | 0.028 | |
| Time × Group | 2, 44 | 0.240 | 0.770 | 0.012 | |
| 4. Interpersonal Effectiveness | Group | 1, 22 | 0.259 | 0.616 | 0.012 |
| Time | 2, 44 | 2.777 | 0.029 | 0.149 | |
| Time × Group | 2, 44 | 1.527 | 0.233 | 0.088 | |
| 5. Alienating | Group | 1, 22 | 0.397 | 0.535 | 0.018 |
| Time | 2, 44 | 1.946 | 0.017 | 0.168 | |
| Time × Group | 2, 44 | 1.072 | 0.351 | 0.094 | |
| 6. Industrious/Persevering | Group | 1, 22 | 0.001 | 0.974 | 0.000 |
| Time | 2, 44 | 1.765 | 0.166 | 0.078 | |
| Time × Group | 2, 44 | 0.765 | 0.451 | 0.036 | |
| 7. Persuasive/Influential | Group | 1, 22 | 0.187 | 0.669 | 0.008 |
| Time | 2, 44 | 0.146 | 0.864 | 0.007 | |
| Time × Group | 2, 44 | 0.229 | 0.796 | 0.010 |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Chevallier, C.; Kohls, G.; Troiani, V.; Brodkin, E.S.; Schultz, R.T. The social motivation theory of autism. Trends Cogn. Sci. 2012, 16, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Guillon, Q.; Hadjikhani, N.; Baduel, S.; Rogé, B. Visual social attention in autism spectrum disorder: Insights from eye tracking studies. Neurosci. Biobehav. Rev. 2014, 42, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, E.K.; Gui, A.; Jones, E.J. Social attention: What is it, how can we measure it, and what can it tell us about autism and ADHD? Prog. Brain Res. 2020, 254, 271–303. [Google Scholar] [PubMed]
- Riddiford, J.A.; Enticott, P.G.; Lavale, A.; Gurvich, C. Gaze and social functioning associations in autism spectrum disorder: A systematic review and meta-analysis. Autism Res. 2022, 15, 1380–1446. [Google Scholar] [CrossRef]
- Kasari, C.; Patterson, S. Interventions addressing social impairment in autism. Curr. Psychiatry Rep. 2012, 14, 713–725. [Google Scholar] [CrossRef]
- Lawton, K.; Kasari, C. Teacher-implemented joint attention intervention: Pilot randomized controlled study for preschoolers with autism. J. Consult. Clin. Psychol. 2012, 80, 687. [Google Scholar] [CrossRef]
- Dehghani, Y.; Afshin, S.A.; Keykhosrovani, M. Effectiveness of vestibular stimulation on social development in children with autism spectrum disorder. Q. J. Child Ment. Health 2019, 6, 28–41. [Google Scholar] [CrossRef]
- Rahman, F.S.; Kadar, M.; Harun, D. Ayres Sensory Integration® Implementation in Malaysian Occupational Therapists: Challenges and Limitations. J. Sains Kesihat. Malays. 2022, 20, 117–128. [Google Scholar] [CrossRef]
- McAdams, D.P. What do we know when we know a person? J. Personal. 1995, 63, 365–396. [Google Scholar] [CrossRef]
- Revelle, W. Personality processes. Annu. Rev. Psychol. 1995, 46, 295–328. [Google Scholar] [CrossRef]
- Marshall, P.J.; Fox, N.A.; Henderson, H.A. Temperament as an organizer of development. Infancy 2000, 1, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Hendry, A.; Jones, E.J.; Bedford, R.; Gliga, T.; Charman, T.; Johnson, M.H.; BASIS Team. Developmental change in look durations predicts later effortful control in toddlers at familial risk for ASD. J. Neurodev. Disord. 2018, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Bryson, S.; Garon, N.; McMullen, T.; Brian, J.; Zwaigenbaum, L.; Armstrong, V.; Roberts, W.; Smith, I.; Szatmari, P. Impaired disengagement of attention and its relationship to emotional distress in infants at high-risk for autism spectrum disorder. J. Clin. Exp. Neuropsychol. 2018, 40, 487–501. [Google Scholar] [CrossRef]
- Brock, M.E.; Freuler, A.; Baranek, G.T.; Watson, L.R.; Poe, M.D.; Sabatino, A. Temperament and sensory features of children with autism. J. Autism. Dev. Disord. 2012, 42, 2271–2284. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, R.C.; Mailloux, Z. Clinician’s Guide for Implementing Ayres Sensory Integration: Promoting Participation for Children with Autism; AOTA Press, The American Occupational Therapy Association, Incorporated Bethesda: Bethesda, MD, USA, 2015. [Google Scholar]
- Smith, M.C. Sensory Integration: Theory and Practice; FA Davis: Philadelphia, PA, USA, 2019. [Google Scholar]
- Kranowitz, C. The Out-of-Sync Child Has Fun: Activities for Kids with Sensory Integration Dysfunction; Perigee: New York, NY, USA, 2003. [Google Scholar]
- Shumway-Cook, A.; Woollacott, W.H. Motor Control: Translating Research into Clinical Practice; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Bläsing, B.; Puttke, M.; Schack, T. (Eds.) The Neurocognition of Dance: Mind, Movement and Motor Skills, 2nd ed.; Routledge: London, UK, 2018. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef] [PubMed]
- Ayres, A.J.; Robbins, J. Sensory Integration and the Child: Understanding Hidden Sensory Challenges; Western Psychological Services: Melbourne, VIC, Australia, 2005. [Google Scholar]
- Porges, S.W. The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-Regulation (Norton Series on Interpersonal Neurobiology); WW Norton & Company: New York, NY, USA, 2011. [Google Scholar]
- Rope Therapy Association. Available online: https://www.ropetherapy.org/ (accessed on 13 May 2025).
- Lam, W.L.; Chau, K.T.; Wong, C.C.; Low, A.; Chan, M.H.; Chung, C.C.; Kwok, N.Y.; Tsang, L.K. Effects of home-based rope therapy on children with special educational needs. Appl. Psychol. Res. 2024, 3, 1281. [Google Scholar] [CrossRef]
- Amso, D.; Haas, S.; Markant, J. An eye tracking investigation of developmental change in bottom-up attention orienting to faces in cluttered natural scenes. PLoS ONE 2014, 9, e85701. [Google Scholar] [CrossRef]
- Pierce, K.; Conant, D.; Hazin, R.; Stoner, R.; Desmond, J. Preference for geometric patterns early in life as a risk factor for autism. Arch. Gen. Psychiatry 2011, 68, 101–109. [Google Scholar] [CrossRef]
- Kong, X.J.; Wei, Z.; Sun, B.; Tu, Y.; Huang, Y.; Cheng, M.; Yu, S.; Wilson, G.; Park, J.; Feng, Z.; et al. Different Eye Tracking Patterns in Autism Spectrum Disorder in Toddler and Preschool Children. Front. Psychiatry 2022, 13, 899521. [Google Scholar] [CrossRef]
- Fujioka, T.; Inohara, K.; Okamoto, Y.; Masuya, Y.; Ishitobi, M.; Saito, D.N.; Jung, M.; Arai, S.; Matsumura, Y.; Fujisawa, T.X. Gazefinder as a clinical supplementary tool for discriminating between autism spectrum disorder and typical development in male adolescents and adults. Mol. Autism 2016, 7, 19. [Google Scholar] [CrossRef]
- Sasson, N.J.; Touchstone, E.W. Visual Attention to Competing Social and Object Images by Preschool Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2014, 44, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Van Der Schalk, J.; Hawk, S.T.; Fischer, A.H.; Doosje, B. Moving faces, looking places: Validation of the Amsterdam Dynamic Facial Expression Set (ADFES). Emotion 2011, 11, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, C.; Parish-Morris, J.; McVey, A.; Rump, K.M.; Sasson, N.J.; Herrington, J.D.; Schultz, R.T. Measuring social attention and motivation in autism spectrum disorder using eye-tracking: Stimulus type matters. Autism Res. 2015, 8, 620–628. [Google Scholar] [CrossRef]
- Chita-Tegmark, M. Attention allocation in ASD: A review and meta-analysis of eye-tracking studies. Rev. J. Autism Dev. Disord. 2016, 3, 209–223. [Google Scholar] [CrossRef]
- Johnson, T.J. Professions and Power; Routledge Revivals; Routledge: London, UK, 2016. [Google Scholar]
- Taylor, R.M.; Morrison, L.P. Taylor Johnson Temperament Analysis; APA PsycTests: New York, NY, USA, 1966. [Google Scholar]
- Gotham, K.; Unruh, K.; Lord, C. Depression and its measurement in verbal adolescents and adults with autism spectrum disorder. Autism 2015, 19, 491–504. [Google Scholar] [CrossRef]
- Schwartzman, B.C.; Wood, J.J.; Kapp, S.K. Can the Five Factor Model of Personality Account for the Variability of Autism Symptom Expression? Multivariate Approaches to Behavioral Phenotyping in Adult Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 253–272. [Google Scholar] [CrossRef]
- Franchini, M.; Glaser, B.; Wood de Wilde, H.; Gentaz, E.; Eliez, S.; Schaer, M. Social orienting and joint attention in preschoolers with autism spectrum disorders. PLoS ONE 2017, 12, e0178859. [Google Scholar] [CrossRef]
- Schultz, R.T. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. Int. J. Dev. Neurosci. 2005, 23, 125–141. [Google Scholar] [CrossRef]
- Pierce, K.; Marinero, S.; Hazin, R.; McKenna, B.; Barnes, C.C.; Malige, A. Eye tracking reveals abnormal visual preference for geometric images as an early biomarker of an autism spectrum disorder subtype associated with increased symptom severity. Biol. Psychiatry 2016, 79, 657–666. [Google Scholar] [CrossRef]
- Ihaka, R.; Gentleman, R. R: A language for data analysis and graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
- Khodabakhshi, M.K.; Malekpour, M.; Abedi, A. The effect of sensory integration therapy on social interactions and sensory and motor performance in children with autism. Iran. J. Cogn. Educ. 2014, 1, 39–53. [Google Scholar]
- Abdul-Majeed, H.M.; Khodeir, M.S.; El-Din Hafez, N.G. Sensory Integration Therapy as a Management of Sensory Deficits in Autistic Spectrum Disorders. QJM Int. J. Med. 2023, 116 (Suppl. 1), hcad069-255. [Google Scholar] [CrossRef]
- Deng, J.; Lei, T.; Du, X. Effects of sensory integration training on balance function and executive function in children with autism spectrum disorder: Evidence from Footscan and fNIRS [Original Research]. Front. Psychol. 2023, 14, 1269462. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, R.T.; Agrawal, Y. Vestibular involvement in cognition: Visuospatial ability, attention, executive function, and memory. J. Vestib. Res. 2015, 25, 73–89. [Google Scholar] [CrossRef]
- Salamati, A.; Hosseini, S.A.; Haghgo, H.; Rostami, R. Vestibular Stimulation and Auditory Perception in children with Attention Deficit Hyperactivity Disorder. Iran. Rehabil. J. 2014, 12, 39–42. [Google Scholar]

| Stratification | Levels | SIT Group (n = 12) | RT Group (n = 14) | p |
|---|---|---|---|---|
| Age | Mean ± SD | 7.3 ± 1.4 | 8.9 ± 3.5 | 0.134 |
| Gender | Female | 2 (16.7%) | 1 (7.1%) | 0.887 |
| Male | 10 (83.3%) | 13 (92.9%) | ||
| Comorbidity | ADHD | 2 (16.7%) | 4 (28.6%) | 0.733 |
| Developmental Delay | 3 (25%) | 4 (28.6%) | ||
| Intellectual Disability | 1 (8.3%) | 1 (7.1%) | ||
| ADHD/Developmental Delay | 1 (8.3%) | 0 (0%) | ||
| ADHD/Intellectual Disability | 1 (8.3%) | 0 (0%) | ||
| No Comorbidity | 4 (33.3%) | 5 (35.7%) |
| Temperament Trait Pattern | SIT Group (n = 12) | RT Group (n = 12) |
|---|---|---|
| None | 6 | 5 |
| Anxiety, Dependent/Hostile | 2 | 0 |
| Anxiety, Emotionally Repressed | 2 | 2 |
| Anxiety, Dominant/Hostile/Subjective/Impulsive/Indifferent | 1 | 1 |
| Anxiety, Emotionally Repressed, Dependent/Hostile | 1 | 0 |
| Withdraw, Emotionally Repressed | 0 | 1 |
| Anxiety | 0 | 1 |
| Dominant/Hostile/Subjective/Impulsive/Indifferent | 0 | 1 |
| Emotionally Repressed | 0 | 1 |
| 1st Assessment | 2nd Assessment | 3rd Assessment |
|---|---|---|
| None | Emotionally Repressed | None |
| None | Emotionally Repressed | Emotionally Repressed |
| None | None | None |
| None | Anxiety, Emotionally Repressed | Anxiety |
| None | Withdrawal, Emotionally Repressed | None |
| Anxiety, Dominant/Hostile/Subjective/Impulsive/Indifferent | Anxiety, Dominant/Hostile/Subjective/Impulsive/Indifferent | None |
| Anxiety, Dependent/Hostile | Anxiety | Dominant/Hostile/Indifferent |
| Anxiety, Dependent/Hostile | None | None |
| Anxiety, Emotionally Repressed, Dependent/Hostile | Anxiety, Emotionally Repressed | Anxiety, Emotionally Repressed |
| Anxiety, Emotionally Repressed | Anxiety, Emotionally Repressed | Anxiety |
| Anxiety, Emotionally Repressed | None | None |
| None | None | None |
| 1st Assessment | 2nd Assessment | 3rd Assessment |
|---|---|---|
| Withdraw, Emotionally Repressed | Emotionally Repressed | None |
| Anxiety | None | None |
| None | None | None |
| None | None | None |
| None | None | None |
| Dominant/Hostile/Subjective/Impulsive/Indifferent | None | Dominant/Hostile/Impulsive/Indifferent |
| Anxiety, Dominant/Hostile/Subjective/Impulsive/Indifferent | Anxiety, Dominant/Hostile/Subjective/Impulsive/Indifferent | Anxiety, Dominant/Hostile/Subjective/Impulsive/Indifferent |
| None | None | None |
| None | Dominant/Hostile/Subjective/Impulsive/Indifferent | None |
| Anxiety, Emotionally Repressed | None | Anxiety |
| Anxiety, Emotionally Repressed | Anxiety | Anxiety, Emotionally Repressed, Dominant/Hostile/Inhibited/Subjective/Impulsive/Indifferent |
| Emotionally Repressed | Withdrawal, Emotionally Repressed | Emotionally Repressed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Hung, K.; Wong, M.C.-C.; Chau, T.K.-T.; Lam, B.W.-L.; Chu, C.Y.-C.; Gu, J.; Dai, J.; Chow, D.H.-K. Effects of Rope Therapy on Social Attention and Temperament Traits in Autistic Children. Children 2025, 12, 881. https://doi.org/10.3390/children12070881
Zhou M, Hung K, Wong MC-C, Chau TK-T, Lam BW-L, Chu CY-C, Gu J, Dai J, Chow DH-K. Effects of Rope Therapy on Social Attention and Temperament Traits in Autistic Children. Children. 2025; 12(7):881. https://doi.org/10.3390/children12070881
Chicago/Turabian StyleZhou, Mi, Kevin Hung, Marco Chun-Cheong Wong, Tony Keng-Tou Chau, Benny Wai-Lun Lam, Cecilia Yuen-Ching Chu, Jialiang Gu, Jiawen Dai, and Daniel Hung-Kay Chow. 2025. "Effects of Rope Therapy on Social Attention and Temperament Traits in Autistic Children" Children 12, no. 7: 881. https://doi.org/10.3390/children12070881
APA StyleZhou, M., Hung, K., Wong, M. C.-C., Chau, T. K.-T., Lam, B. W.-L., Chu, C. Y.-C., Gu, J., Dai, J., & Chow, D. H.-K. (2025). Effects of Rope Therapy on Social Attention and Temperament Traits in Autistic Children. Children, 12(7), 881. https://doi.org/10.3390/children12070881









