Prognosis of Pediatric Dilated Cardiomyopathy: Nomogram and Risk Score Models for Predicting Death/Heart Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Follow-Up and Grouping
2.4. Statistical Methods
3. Results
3.1. General Information
3.2. Establishment and Test of Prediction Model
3.2.1. Univariate Analysis
3.2.2. Collinearity Diagnosis
3.2.3. Multivariate Analysis and Establishment of Nomogram and Scoring System Models
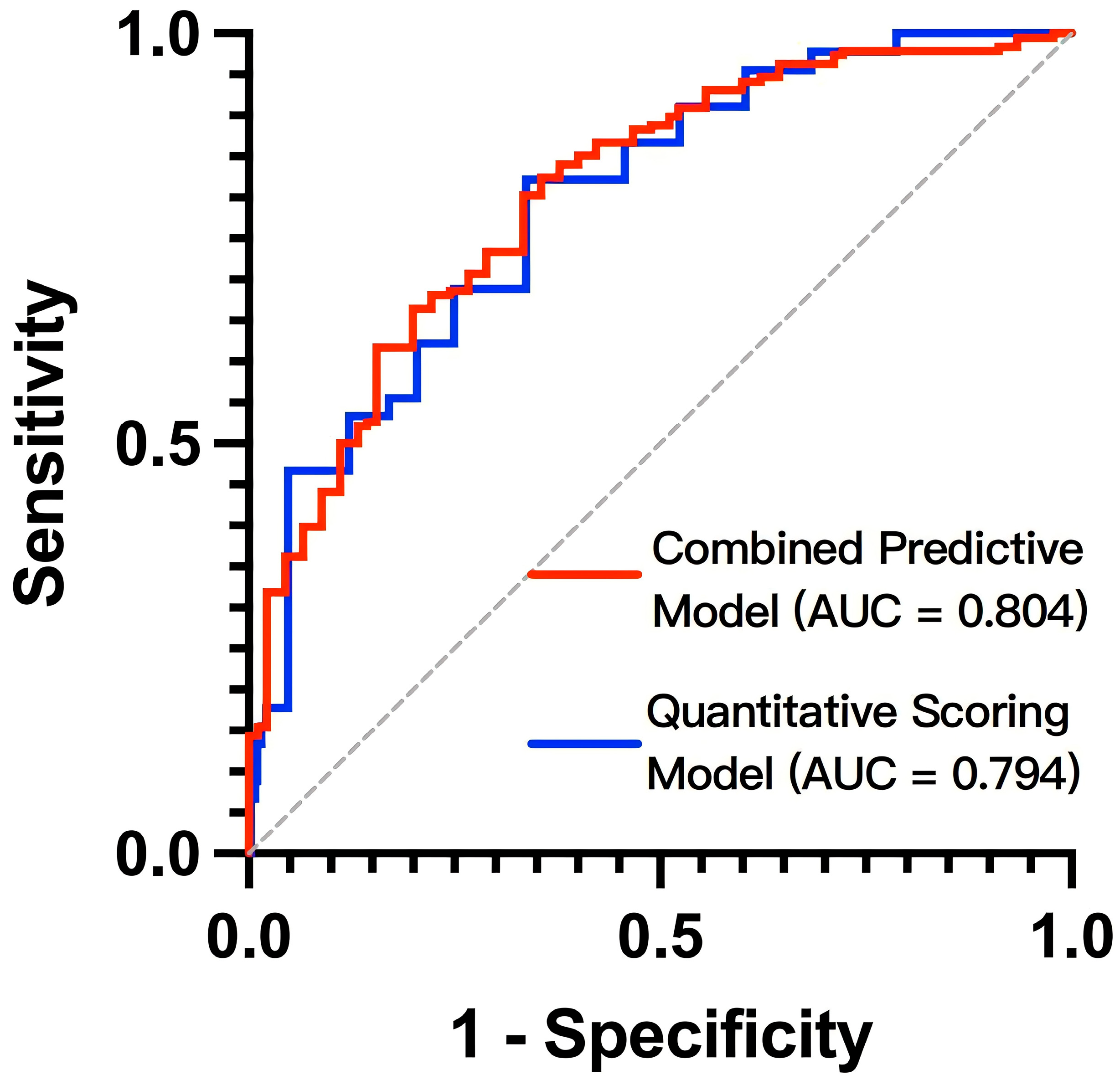
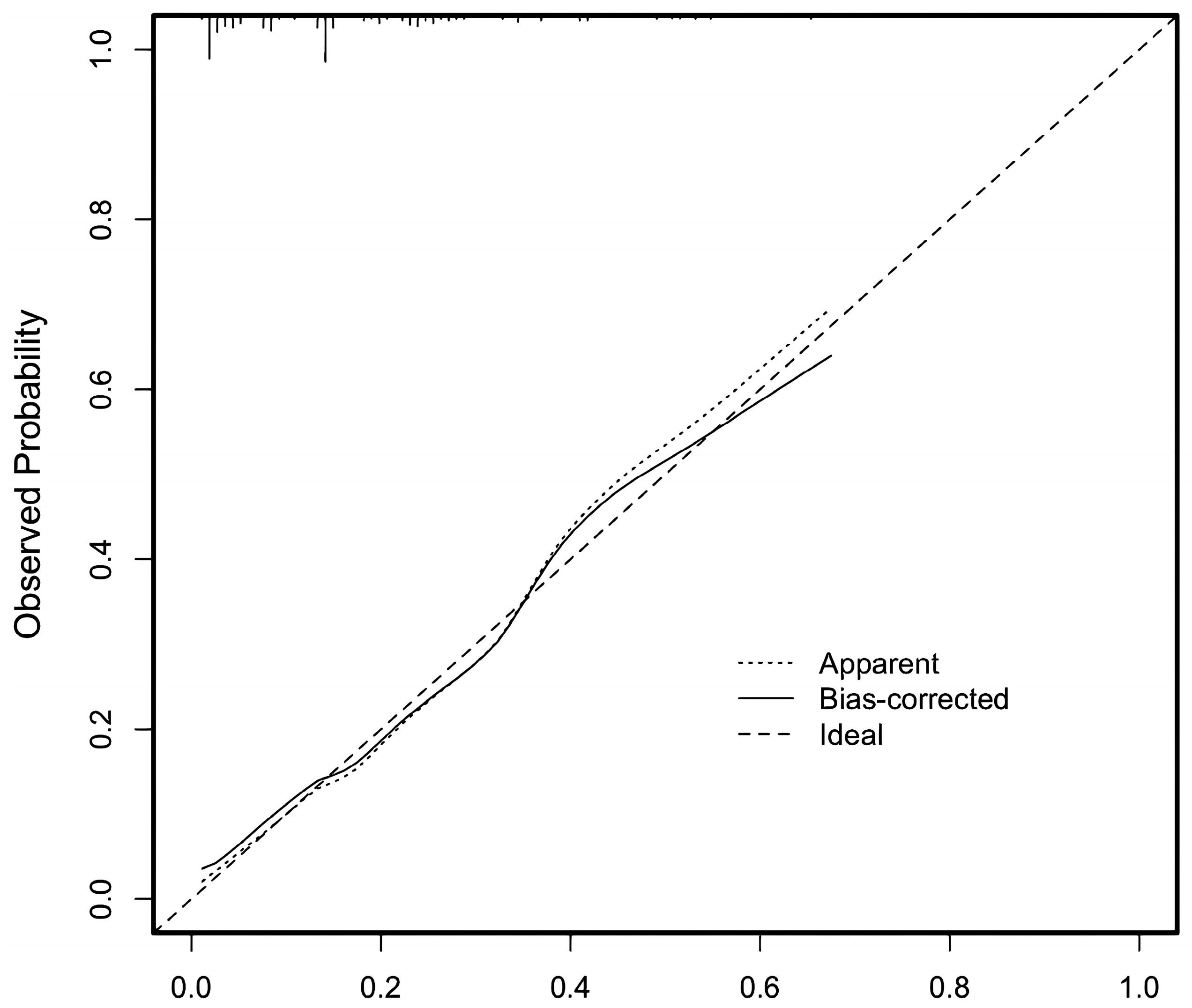
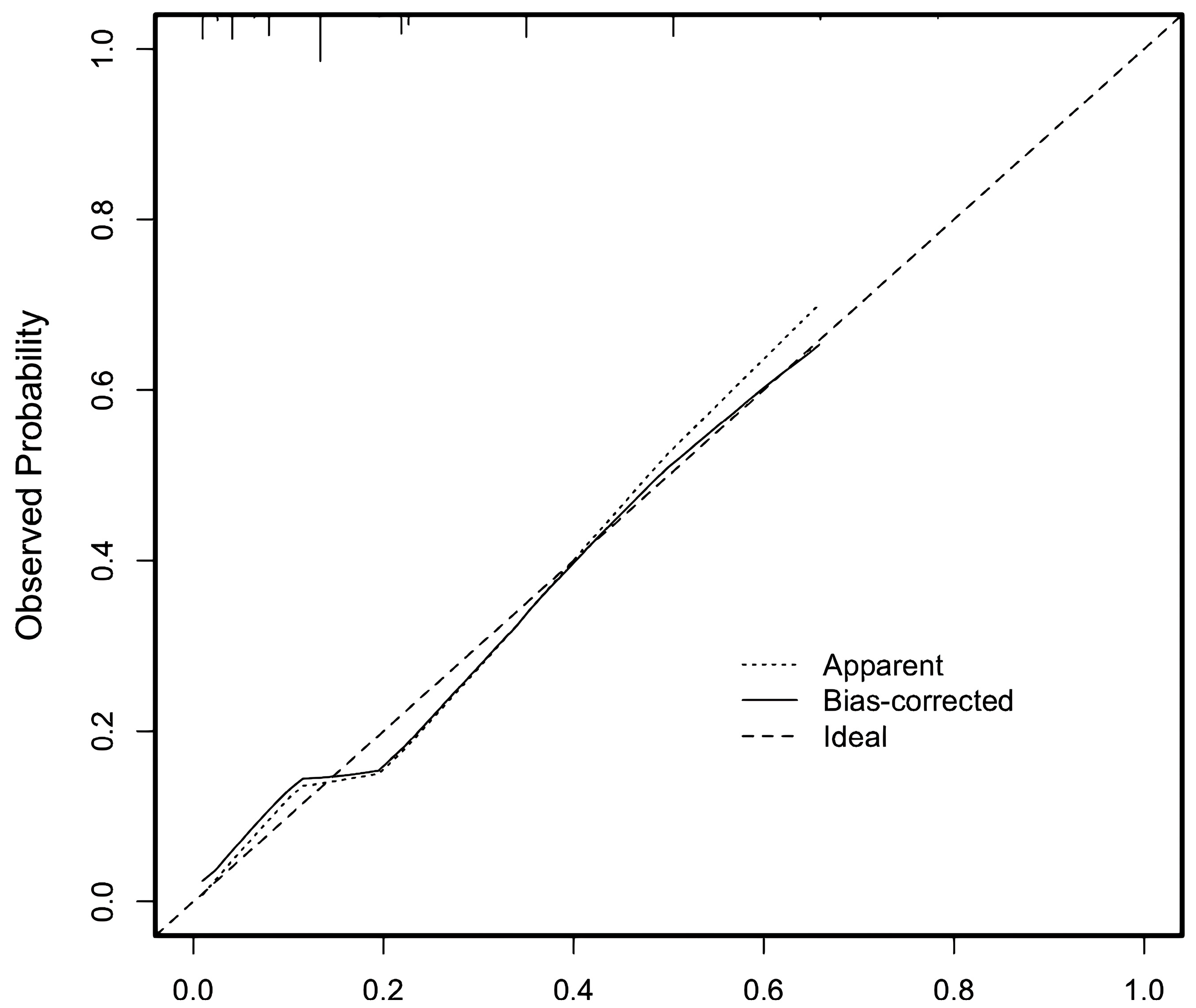
3.2.4. Test of Nomogram Model and Scoring System Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heymans, S.; Lakdawala, N.K.; Tschöpe, C.; Klingel, K. Dilated cardiomyopathy: Causes, mechanisms, and current and future treatment approaches. Lancet 2023, 402, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, R.E.; Hedges, D.J.; Morales, A. Dilated cardiomyopathy: The complexity of a diverse genetic architecture. Nat. Rev. Cardiol. 2013, 10, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Arola, A.; Jokinen, E.; Ruuskanen, O.; Saraste, M.; Pesonen, E.; Kuusela, A.-L.; Tikanoja, T.; Paavilainen, T.; Simell, O. Epidemiology of idiopathic cardiomyopathies in children and adolescents. A nationwide study in Finland. Am. J. Epidemiol. 1997, 146, 385–393. [Google Scholar] [CrossRef]
- Japp, A.G.; Gulati, A.; Cook, S.A.; Cowie, M.R.; Prasad, S.K. The Diagnosis and Evaluation of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2996–3010. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Sleeper, L.A.; Towbin, J.A.; Lowe, A.M.; Orav, E.J.; Cox, G.F.; Lurie, P.R.; McCoy, K.L.; McDonald, M.A.; Messere, J.E.; et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N. Engl. J. Med. 2003, 348, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Nugent, A.W.; Daubeney, P.E.; Chondros, P.; Carlin, J.B.; Cheung, M.; Wilkinson, L.C.; Davis, A.M.; Kahler, S.G.; Chow, C.; Wilkinson, J.L.; et al. The epidemiology of childhood cardiomyopathy in Australia. N. Engl. J. Med. 2003, 348, 1639–1646. [Google Scholar] [CrossRef]
- Dziewięcka, E.; Gliniak, M.; Winiarczyk, M.; Karapetyan, A.; Wiśniowska-Śmiałek, S.; Karabinowska, A.; Dziewięcki, M.; Podolec, P.; Rubiś, P. Mortality risk in dilated cardiomyopathy: The accuracy of heart failure prognostic models and dilated cardiomyopathy-tailored prognostic model. ESC Heart Fail. 2020, 7, 2455–2467. [Google Scholar] [CrossRef]
- Cannatà, A.; De Angelis, G.; Boscutti, A.; Normand, C.; Artico, J.; Gentile, P.; Zecchin, M.; Heymans, S.; Merlo, M.; Sinagra, G. Arrhythmic risk stratification in non-ischaemic dilated cardiomyopathy beyond ejection fraction. Heart 2020, 106, 656–664. [Google Scholar] [CrossRef]
- Bogle, C.; Colan, S.D.; Miyamoto, S.D.; Choudhry, S.; Baez-Hernandez, N.; Brickler, M.M.; Feingold, B.; Lal, A.K.; Lee, T.M.; Canter, C.E.; et al. Treatment Strategies for Cardiomyopathy in Children: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 174–195. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Law, Y.M.; Asante-Korang, A.; Austin, E.D.; Dipchand, A.I.; Everitt, M.D.; Hsu, D.T.; Lin, K.Y.; Price, J.F.; Wilkinson, J.D.; et al. Cardiomyopathy in Children: Classification and Diagnosis: A Scientific Statement from the American Heart Association. Circulation 2019, 140, e9–e68. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar]
- Colan, S.D. Normal echocardiographic values for cardiovascular structures. In Echocardiography in Pediatric and Congenital Heart Disease: From Fetus to Adult, 2nd ed.; Lai, W.W., Mertens, L.L., Cohen, M.S., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2016; pp. 883–901. [Google Scholar]
- Sluysmans, T.; Colan, S.D. Structural measurements and adjustments for growth. In Echocardiography in Pediatric and Congenital Heart Disease: From Fetus to Adult, 2nd ed.; Lai, W.W., Mertens, L.L., Cohen, M.S., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2016; pp. 61–72. [Google Scholar]
- Tromp, J.; Ouwerkerk, W.; van Veldhuisen, D.J.; Hillege, H.L.; Richards, A.M.; van der Meer, P.; Anand, I.S.; Lam, C.S.; Voors, A.A. A Systematic Review and Network Meta-Analysis of Pharmacological Treatment of Heart Failure with Reduced Ejection Fraction. JACC Heart Fail. 2022, 10, 73–84. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Swedberg, K.; Komajda, M.; Böhm, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L.; on behalf of the SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010, 376, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Tromp, J.; Collins, S.P. Dapagliflozin in heart failure: New frontiers. Eur. J. Heart Fail. 2019, 21, 1412–1414. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Diaz, R.; Felker, G.M.; McMurray, J.J.; Metra, M.; Solomon, S.D.; Adams, K.F.; Anand, I.; Arias-Mendoza, A.; Biering-Sørensen, T.; et al. Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. N. Engl. J. Med. 2021, 384, 105–116. [Google Scholar] [CrossRef]
- Alexander, P.M.; Lee, K.; Daubeney, P.E.; Turner, C.; Robertson, T.; Nugent, A.W.; Davis, A.; Ramsay, J.; Justo, R.; Bharucha, T.; et al. Long-term outcomes of dilated cardiomyopathy diagnosed during childhood: Results from a national population-based study of childhood cardiomyopathy. Circulation 2013, 128, 2039–2046. [Google Scholar] [CrossRef]
- Jammal Addin, M.B.; Young, D.; McCarrison, S.; Hunter, L. Dilated cardiomyopathy in a national paediatric population. Eur. J. Pediatr. 2019, 178, 1229–1235. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Wilkinson, J.D.; Lipshultz, S.E. Outcome Predictors for Pediatric Dilated Cardiomyopathy: A Systematic Review. Prog. Pediatr. Cardiol. 2007, 23, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Everitt, M.D.; Sleeper, L.A.; Lu, M.; Canter, C.E.; Pahl, E.; Wilkinson, J.D.; Addonizio, L.J.; Towbin, J.A.; Rossano, J.; Singh, R.K.; et al. Recovery of echocardiographic function in children with idiopathic dilated cardiomyopathy: Results from the pediatric cardiomyopathy registry. J. Am. Coll. Cardiol. 2014, 63, 1405–1413. [Google Scholar] [CrossRef]
- Lewis, A.B. Late recovery of ventricular function in children with idiopathic dilated cardiomyopathy. Am. Heart J. 1999, 138 Pt 1, 334–338. [Google Scholar] [CrossRef]
- Ahmed, A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am. J. Cardiol. 2007, 99, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Muntwyler, J.; Abetel, G.; Gruner, C.; Follath, F. One-year mortality among unselected outpatients with heart failure. Eur. Heart J. 2002, 23, 1861–1866. [Google Scholar] [CrossRef]
- Caraballo, C.; Desai, N.R.; Mulder, H.; Alhanti, B.; Wilson, F.P.; Fiuzat, M.; Felker, G.M.; Piña, I.L.; O’COnnor, C.M.; Lindenfeld, J.; et al. Clinical Implications of the New York Heart Association Classification. J. Am. Heart Assoc. 2019, 8, e014240. [Google Scholar] [CrossRef] [PubMed]
- Michels, V.V.; Driscoll, D.J.; Miller, F.A.; Olson, T.M.; Atkinson, E.J.; Olswold, C.L.; Schaid, D.J. Progression of familial and non-familial dilated cardiomyopathy: Long term follow up. Heart 2003, 89, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.L.; Hsiao, S.H.; Wu, C.J.; Kang, P.L.; Chiou, K.R. Treatment strategies for acute coronary syndrome with severe mitral regurgitation and their effects on short- and long-term prognosis. Am. J. Cardiol. 2012, 110, 800–806. [Google Scholar] [CrossRef]
- Goliasch, G.; Bartko, P.E.; Pavo, N.; Neuhold, S.; Wurm, R.; Mascherbauer, J.; Lang, I.M.; Strunk, G.; Hülsmann, M. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur. Heart J. 2018, 39, 39–46. [Google Scholar] [CrossRef]
- Fernandes, F.P.; Manlhiot, C.; McCrindle, B.W.; Mertens, L.; Kantor, P.F.; Friedberg, M.K. Usefulness of mitral regurgitation as a marker of increased risk for death or cardiac transplantation in idiopathic dilated cardiomyopathy in children. Am. J. Cardiol. 2011, 107, 1517–1521. [Google Scholar] [CrossRef]
- Levine, R.A.; Schwammenthal, E. Ischemic mitral regurgitation on the threshold of a solution: From paradoxes to unifying concepts. Circulation 2005, 112, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Drezner, J.A.; Zorzi, A.; Corrado, D. Prevalence and clinical significance of low QRS voltages in healthy individuals, athletes, and patients with cardiomyopathy: Implications for sports pre-participation cardiovascular screening. Eur. J. Prev. Cardiol. 2024, 31, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.Q.; Han, P.; Xu, H.B.; Yu, J.M.; Liu, H.L. Correlation between low tube voltage in dual source CT coronary artery imaging with image quality and radiation dose. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Gerger, D.; Jirak, P.; Wegner, C.; Finsterer, J. Evolution of electrocardiographic abnormalities in association with neuromuscular disorders and survival in left ventricular hypertrabeculation/noncompaction. Ann. Noninvasive Electrocardiol. 2014, 19, 567–573. [Google Scholar] [CrossRef]
- MERIT-HF Stufy Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999, 353, 2001–2007. [Google Scholar] [CrossRef]
- CIBIS Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study (CIBIS): A randomized trial of beta-blockade in heart failure. Circulation 1994, 90, 1765–1773. [Google Scholar] [CrossRef]
- Joseph, P.; Swedberg, K.; Leong, D.P.; Yusuf, S. The Evolution of β-Blockers in Coronary Artery Disease and Heart Failure (Part 1/5). J. Am. Coll. Cardiol. 2019, 74, 672–682. [Google Scholar] [CrossRef]
- Prakash, A.; Markham, A. Metoprolol: A review of its use in chronic heart failure. Drugs 2000, 60, 647–678. [Google Scholar] [CrossRef]

| Factors Groups | D/HT | Non-D/HT | t/Z/χ2 Value | p-Value | ||
|---|---|---|---|---|---|---|
| General Data and Clinical Manifestations | Number [n (%)] | 45 (19.3%) | 188 (80.7%) | - | - | |
| Sex (M/F) | 24/21 | 95/93 | 0.114 | 0.736 | ||
| Age (months) | 90.5 (38.0, 141.0) | 44.5 (8.0, 101.5) | −3.632 | <0.001 | ||
| BMI (kg/m2) | 17.18 (14.60, 22.13) | 16.06 (14.15, 19.21) | −1.363 | 0.173 | ||
| Diagnosis Time (months) | 1.50 (0.38, 12.0) | 1.00 (0.50, 2.25) | −0.108 | 0.914 | ||
| Dyspnea [n (%)] | 16 (35.6%) | 74 (39.4%) | 0.222 | 0.638 | ||
| Fatigue [n (%)] | 43 (95.6%) | 147 (78.2%) | 7.275 | 0.007 | ||
| Edema [n (%)] | 14 (31.1%) | 37 (19.7%) | 2.775 | 0.096 | ||
| Syncope or Presyncope [n (%)] | 2 (4.4%) | 10 (5.3%) | 0.057 | 0.812 | ||
| Cardiac Arrest [n (%)] | 0 (0%) | 4 (2.1%) | 0.974 | 0.324 | ||
| Growth Retardation [n (%)] | 4 (8.9%) | 14 (7.4%) | 0.106 | 0.745 | ||
| NYHA/Ross Class [n (%)] | I-II | 2 (4.4%) | 64 (34.0%) | 15.667 | <0.001 | |
| III-IV | 43 (95.6%) | 124 (66.0%) | ||||
| Family History of DCM [n (%)] | 9 (20%) | 22 (11.7%) | 2.167 | 0.141 | ||
| Follow-up Time (months) | 43.33 ± 19.21 | 33.90 ± 47.60 | 1.302 | 0.194 | ||
| Blood Analysis Tests | CK (U/L) | 81.50 (50.50, 112.50) | 79.50 (55.75, 143.25) | −0.887 | 0.375 | |
| CK-MB Mass Assay (ng/mL) | 2.41 (1.70, 3.18) | 2.02 (1.08, 3.67) | −1.126 | 0.260 | ||
| hs-cTnI (ng/mL) | 0.029 (0.005, 0.279) | 0.004 (0.001, 0.016) | −2.586 | 0.010 | ||
| Multiple of BNP or NTproBNP Above Upper Limit of Normal | 15.02 (4.07, 21.85) | 2.15 (0.22, 15.72) | −3.376 | 0.001 | ||
| HGB (g/L) | 126.93 ± 17.22 | 120.44 ± 17.97 | 2.194 | 0.029 | ||
| SCR (umol/L) | 51.20 (39.30, 64.75) | 32.00 (26.15, 47.68) | −5.007 | <0.001 | ||
| BUN (mmol/L) | 6.76 ± 4.00 | 5.04 ± 2.78 | 3.407 | 0.001 | ||
| UA (umol/L) | 426.40 (285.63, 550.35) | 304.05 (249.50, 412.43) | −3.795 | <0.001 | ||
| ALT (U/L) | 19.00 (13.30, 48.10) | 18.80 (13.25, 31.15) | −2.535 | 0.011 | ||
| AST (U/L) | 33.45 (25.55, 68.90) | 33.85 (23.70, 46.05) | −1.727 | 0.084 | ||
| TBIL (umol/L) | 17.47 ± 8.31 | 17.48 ± 27.71 | −0.003 | 0.998 | ||
| DBIL (umol/L) | 2.21 (1.46, 3.47) | 1.50 (0.96, 2.39) | −3.904 | <0.001 | ||
| IBIL (umol/L) | 13.76 ± 6.63 | 13.55 ± 23.41 | 0.057 | 0.954 | ||
| Serum Potassium (mmol/L) | 4.27 ± 0.50 | 5.11 ± 9.66 | −0.580 | 0.563 | ||
| Serum Sodium (mmol/L) | 136.90 (135.85, 138.00) | 136.35 (134.40, 137.90) | −1.139 | 0.255 | ||
| Serum Calcium (mmol/L) | 2.29 (2.20, 2.42) | 2.39 (2.31, 2.48) | −3.680 | <0.001 | ||
| TSH (mIU/L) | 2.21 (0.90, 3.57) | 2.63 (1.35, 3.54) | −0.118 | 0.906 | ||
| T3 (nmol/L) | 1.93 (0.88, 89.92) | 1.77 (1.37, 65.33) | −0.267 | 0.790 | ||
| T4 (nmol/L) | 97.30 (8.90, 118.28) | 105.70 (10.16, 132.48) | −0.883 | 0.377 | ||
| FT3 (pmol/L) | 4.97 ± 1.03 | 5.30 ± 1.16 | −1.697 | 0.091 | ||
| FT4 (pmol/L) | 15.55 ± 3.75 | 13.26 ± 3.64 | 3.636 | <0.001 | ||
| BG (mmol/L) | 5.71 ± 0.92 | 5.55 ± 1.28 | 0.831 | 0.407 | ||
| Serum Vitamin D (nmol/L) | 51.29 (38.30, 72.66) | 48.51 (37.00, 64.60) | −0.559 | 0.576 | ||
| Hcy (umol/L) | 9.11 ± 3.46 | 9.06 ± 4.41 | 0.052 | 0.958 | ||
| Echocardiography | Z-score Value of IVSd | −2.11 (−2.63, −1.99) | −2.31 (−2.86, −1.96) | −1.412 | 0.158 | |
| IVSE (mm) | 4.55 (3.93, 5.15) | 4.60 (3.48, 5.50) | −1.886 | 0.059 | ||
| Z-score Value of LVEDD | 6.20 ± 2.66 | 5.58 ± 3.47 | 1.323 | 0.190 | ||
| Z-score Value of LVPWd | −2.04 ± 1.54 | −2.10 ± 0.79 | 0.404 | 0.687 | ||
| PWE (mm) | 5.40 ± 2.67 | 5.79 ± 3.03 | −0.789 | 0.431 | ||
| EF (%) | 30.5 ± 14.0 | 39.2 ± 14.8 | −3.560 | <0.001 | ||
| FS (%) | 13.0 (10.0, 21.5) | 19.5 (14.8, 31.0) | −4.023 | <0.001 | ||
| E/A | 1.60 (1.33, 1.98) | 1.50 (1.30, 1.70) | −1.091 | 0.275 | ||
| MR [n (%)] | Mild | 18 (40.0%) | 133 (70.7%) | 15.048 | <0.001 | |
| Moderate or Severe | 27 (60.0%) | 55 (29.3%) | ||||
| TR [n (%)] | Mild | 36 (80.0%) | 169 (89.9%) | 3.361 | 0.067 | |
| Moderate or Severe | 9 (20%) | 19 (10.1%) | ||||
| Electrocardiogram | Conduction Block [n (%)] | 20 (44.4%) | 56 (29.8%) | 3.549 | 0.060 | |
| Pathological Q Waves [n (%)] | 4 (8.9%) | 4 (2.1%) | 5.006 | 0.025 | ||
| Prolonged QT Interval [n (%)] | 3 (6.7%) | 16 (8.5%) | 0.165 | 0.685 | ||
| ST/T Abnormalities [n (%)] | 41 (91.1%) | 152 (80.9%) | 2.688 | 0.101 | ||
| Atrial Premature Contraction [n (%)] | 22 (48.9%) | 67 (35.6%) | 2.701 | 0.100 | ||
| Ventricular Premature Contraction [n (%)] | 31 (68.9%) | 88 (46.8%) | 7.084 | 0.008 | ||
| Atrial Tachycardia [n (%)] | 1 (2.2%) | 11 (5.9%) | 0.979 | 0.322 | ||
| Ventricular Tachycardia [n (%)] | 9 (20%) | 17 (9.0%) | 4.397 | 0.036 | ||
| High QRS Voltage [n (%)] | 13 (28.9%) | 61 (32.4%) | 0.212 | 0.645 | ||
| Low QRS Voltage [n (%)] | 9 (20%) | 7 (3.7%) | 15.041 | <0.001 | ||
| Average Heart Rate (bpm) | 98.6 ± 21.0 | 106.3 ± 22.5 | −1.920 | 0.056 | ||
| SDNN (ms) | 98.90 (58.00, 136.25) | 98.50 (74.75, 126.00) | −0.685 | 0.493 | ||
| SDANN (ms) | 84.88 ± 43.47 | 84.02 ± 38.18 | 0.123 | 0.903 | ||
| RMSSD (ms) | 26.50 (13.25, 36.75) | 30.00 (18.00, 45.50) | −1.082 | 0.279 | ||
| pNN50 (%) | 4.77 (0.70, 16.02) | 7.08 (1.62, 20.06) | −0.776 | 0.438 | ||
| Treatment | Digoxin [n (%)] | 41 (91.1%) | 150 (79.8%) | 3.151 | 0.076 | |
| Vasoactive Agents [n (%)] | 32 (71.1%) | 80 (42.6%) | 11.862 | 0.001 | ||
| Diuretics [n (%)] | 42 (93.3%) | 158 (84.0%) | 2.578 | 0.108 | ||
| ACEI/ARB [n (%)] | 44 (97.8%) | 180 (95.7%) | 0.404 | 0.525 | ||
| β-blockers [n (%)] | 5 (11.1%) | 51 (27.1%) | 5.102 | 0.024 | ||
| IVIG [n (%)] | 9 (20.0%) | 49 (26.1%) | 0.714 | 0.398 | ||
| Glucocorticoids [n (%)] | 25 (55.6%) | 97 (51.6%) | 0.228 | 0.633 | ||
| Aspirin [n (%)] | 11 (24.4%) | 41 (21.8%) | 0.146 | 0.703 | ||
| Anticoagulants [n (%)] | 2 (4.4%) | 13 (6.9%) | 0.368 | 0.544 | ||
| Noninvasive or Invasive Respiratory Support [n (%)] | 3 (6.7%) | 24 (12.8%) | 1.318 | 0.251 | ||
| Variable(s) | B | S.E. | Wald | p-Value | OR | 95% CI for OR | Points |
|---|---|---|---|---|---|---|---|
| Age | −0.008 | 0.004 | 4.362 | 0.037 | 0.993 | 0.986–1.000 | 1 |
| NYHA/ROSS Class | 2.821 | 1.268 | 4.951 | 0.026 | 16.796 | 1.399–201.581 | 10 |
| MR | 0.950 | 0.446 | 4.526 | 0.033 | 2.585 | 1.078–6.201 | 2.5 |
| Low QRS Voltage | 1.447 | 0.714 | 4.113 | 0.043 | 4.252 | 1.050–17.225 | 4 |
| Digoxin | −2.380 | 1.254 | 3.604 | 0.058 | 0.093 | 0.008–1.080 | - |
| Vasoactive Agents | 1.020 | 0.502 | 4.130 | 0.042 | 2.773 | 1.037–7.417 | 3 |
| Constant | −0.502 | 0.797 | 0.398 | 0.528 | 0.605 | - | - |
| Variable(s) | AUC | 95% CI | p-Value | Cut-off Value | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Age | 0.674 | 0.594–0.755 | <0.001 | ≥58.5 months | 66.7% | 63.3% |
| NYHA/ROSS Class | 0.648 | 0.570–0.726 | 0.002 | ≥0.5 | 95.6% | 34.0% |
| MR | 0.654 | 0.562–0.745 | 0.001 | ≥0.5 | 60.0% | 70.7% |
| Low QRS Voltage | 0.581 | 0.482–0.681 | 0.090 | ≥0.5 | 20.0% | 96.3% |
| Vasoactive Agents | 0.643 | 0.555–0.731 | 0.003 | ≥0.5 | 71.1% | 57.4% |
| Combined Predictive Model | 0.804 | 0.734–0.874 | - | - | 80.3% | 66.7% |
| Quantitative Scoring Model | 0.794 | 0.724–0.864 | <0.001 | ≥13.25 | 68.9% | 73.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Yuan, Y.; Gao, L.; Wang, Z.; Lv, Z.; Yu, W.; Jin, H.; Zhen, Z.; Zhao, Z.; Na, J.; et al. Prognosis of Pediatric Dilated Cardiomyopathy: Nomogram and Risk Score Models for Predicting Death/Heart Transplantation. Children 2025, 12, 880. https://doi.org/10.3390/children12070880
Xu B, Yuan Y, Gao L, Wang Z, Lv Z, Yu W, Jin H, Zhen Z, Zhao Z, Na J, et al. Prognosis of Pediatric Dilated Cardiomyopathy: Nomogram and Risk Score Models for Predicting Death/Heart Transplantation. Children. 2025; 12(7):880. https://doi.org/10.3390/children12070880
Chicago/Turabian StyleXu, Bowen, Yue Yuan, Lu Gao, Zhiyuan Wang, Zhenyu Lv, Wen Yu, Hongfang Jin, Zhen Zhen, Zhihui Zhao, Jia Na, and et al. 2025. "Prognosis of Pediatric Dilated Cardiomyopathy: Nomogram and Risk Score Models for Predicting Death/Heart Transplantation" Children 12, no. 7: 880. https://doi.org/10.3390/children12070880
APA StyleXu, B., Yuan, Y., Gao, L., Wang, Z., Lv, Z., Yu, W., Jin, H., Zhen, Z., Zhao, Z., Na, J., Hu, A., & Xiao, Y. (2025). Prognosis of Pediatric Dilated Cardiomyopathy: Nomogram and Risk Score Models for Predicting Death/Heart Transplantation. Children, 12(7), 880. https://doi.org/10.3390/children12070880






