Abstract
Neonatal respiratory distress is a primary contributor to neonatal morbidity and mortality worldwide. Non-invasive respiratory support such as nasal continuous positive airway pressure (NCPAP) and bubble NCPAP (bNCPAP) are often used as the first line of treatment for neonatal respiratory distress, including respiratory distress syndrome; however, many hospitals in low- and middle-income countries do not have access to advanced respiratory support devices beyond NCPAP. A novel, non-invasive bubble positive pressure ventilation device has been developed as a low-cost, non-electric alternative to providing respiratory support in such scenarios. In this article, we propose evidence-based guidelines for the initiation, titration, and weaning of the new device.
Keywords:
newborn; preterm infant; NCPAP; bubble CPAP; NIPPV; bubble NIPPV; non-invasive ventilation 1. Introduction
Almost 2.5 million infants die every year within the first month of life, with low- and middle-income countries (LMICs) bearing the heaviest burden. The leading causes are prematurity, birth complications, and infections—all of which can lead to respiratory distress and subsequent respiratory failure [1]. Despite a decrease in overall child mortality, current global trends reveal an increase in the proportion of neonatal deaths among all under-five deaths, from 40% in 1990 to 47% in 2021 with South Asia and Sub-Saharan Africa carrying the highest numbers [2]. Over 90% of infants with extreme prematurity (<28 weeks) born in LMICs die within the first few days of life, as opposed to <10% in high-income countries (HICs) [3]. Therefore, improving the management of preterm respiratory distress in LMICs is imperative to reducing neonatal death rates.
Non-invasive respiratory supports, such as nasal continuous positive airway pressure (NCPAP) and bubble nasal CPAP (bNCPAP), have long been accepted to be an effective form of first-line therapy for respiratory distress in neonates, including secondary to respiratory distress syndrome (RDS) in preterm infants. In high-resource settings, invasive mechanical ventilation (IMV) has been associated with the development of lung inflammation, leading to bronchopulmonary dysplasia (BPD) and associated neurological sequelae [4]. In low-resource settings, the additional burden of high patient-to-nurse ratios and limited access to blood gas equipment make invasive ventilation even less desirable. Thus, there is strong motivation to limit the need for invasive ventilation in these settings. Growing evidence supports the use of nasal intermittent positive pressure ventilation (NIPPV) as being superior to NCPAP in primary and post-extubation support for sicker infants, with lower rates of respiratory failure necessitating intubation or post-extubation failure in comparison with NCPAP [5,6]. Some data also show decreased BPD rates with the use of NIPPV [7]. The benefits of NIPPV can be attributed to improved oxygenation due to a higher mean airway pressure and improved ventilation due to the pressure gradient created by the difference in higher and lower pressures [8,9,10,11].
Ventilator-based NIPPV protocols have been established, outlining criteria for initiation, initial parameter selection, adjustment of parameters, and weaning of therapy [12]. It is evident that NIPPV is the preferred mode of primary and secondary respiratory support in infants with RDS, but availability, expense, and lack of appropriate training limit its feasibility in LMICs [13]. Moreover, significant barriers of cost, equipment complexity, access to replacement parts/servicing, and lack of continuous electrical power prevent many resource-constrained settings from adopting ventilator-based NIPPV.
A novel, simple bubble NIPPV (bNIPPV) machine has been developed, which utilizes the same operating principle as bNCPAP, wherein the submerged depth of the circuit’s expiratory limb sets the delivered pressure through a hydrostatic principle [14]. As gas passes through water, it creates bubbles, generating pressure oscillations that facilitate gas exchange and lung alveoli recruitment [15]. This technology is simple, non-electric, and economical. It has thus far been demonstrated to be feasible [16] to use in an under-resourced setting with a similar safety profile to bNCPAP [17]. The efficacy of this mode of non-invasive support has yet to be studied in sufficiently powered randomized clinical trials (RCTs). Given the need to do so, it would be important to provide information and guidelines for the use of bNIPPV. In this article, we review the existing literature for the use of ventilator-based NIPPV and provide guidelines for the use of bNIPPV initiation, adjustment, and weaning as a mode of initial and post-extubation therapy.
2. Nomenclature
NCPAP provides a constant positive end expiratory pressure (PEEP). Pressure is generated by a ventilator, a flow driver, or an underwater seal. When pressure is generated by an underwater seal, or a bubble system, it is referred to as bNCPAP. NIPPV is a system that combines PEEP with intermittent peak inspiratory pressures (PIP), and similarly, bNIPPV utilizes an underwater seal for its pressure generation [18]. There are two major settings in which NIPPV can be used, which are referred to as primary and secondary modes. The primary mode of NIPPV describes its use soon after birth with or without a short period of intubation for the sole purpose of surfactant delivery and subsequent extubation. The secondary mode refers to its use following a longer period of intubation [12]. NIPPV is often confused with BiPAP or bi-level NCPAP, both of which have two pressure settings. The main differences include lower peak pressures with BiPAP and bi-level NCPAP (e.g., P high or PIP of 8 cm H2O, P low or PEEP of 5 cm H2O). Additionally, for the bi-level NCPAP, there is a longer duration at the higher pressure (inspiratory time or Ti) as compared to NIPPV [19,20].
2.1. Bubble NIPPV Equipment Description and Use
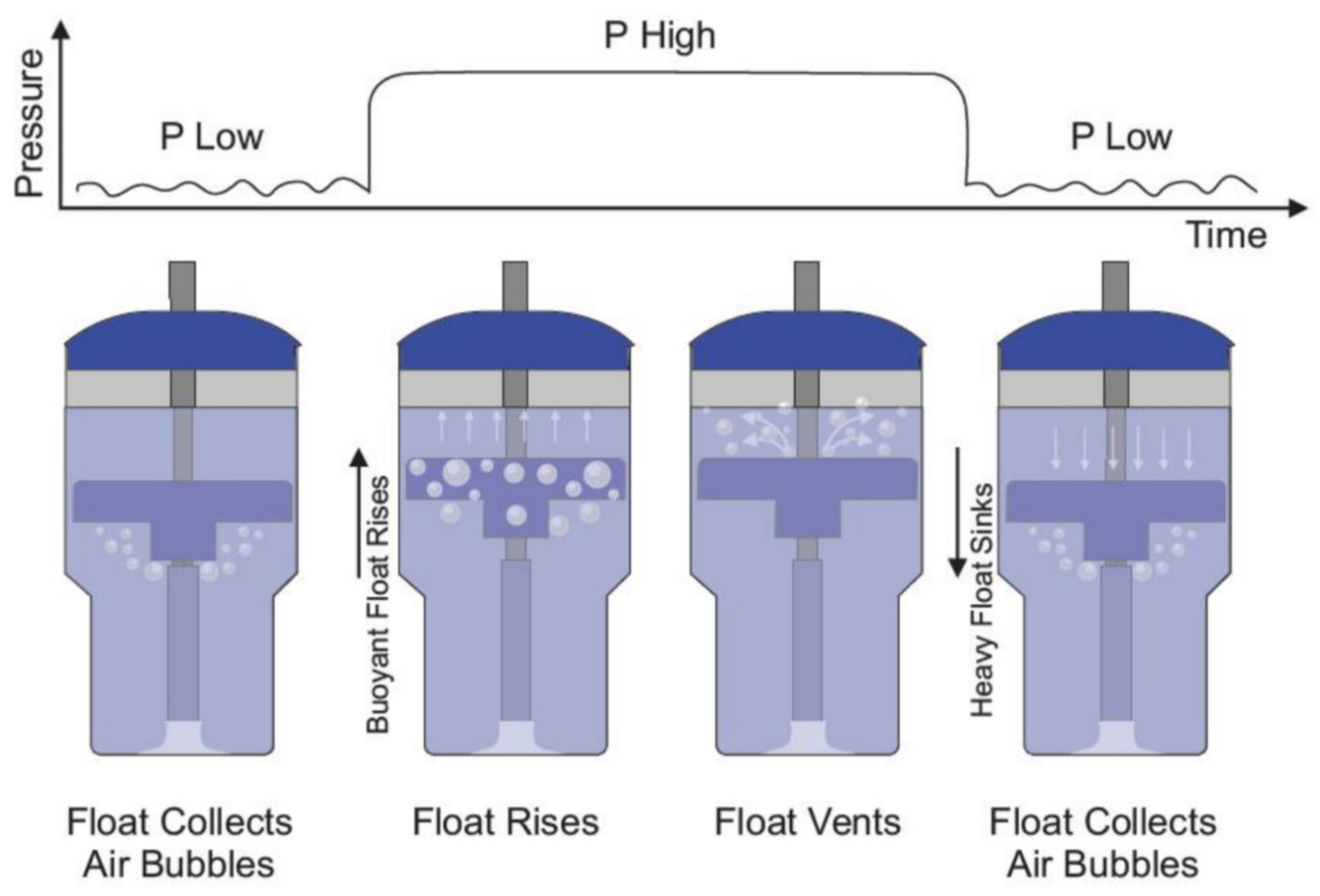
bNCPAP and bNIPPV utilize the same infant respiratory circuit in which a continuous baseline pressure is set by the submerged depth of the circuit’s expiratory limb. In contrast to bNCPAP where a constant level of expiratory pressure is delivered, bNIPPV delivers two different levels of pressure: PIP (8 to 20 cm H2O) and PEEP (5 to 8 cm H2O). To accomplish this, the bNIPPV utilizes a novel and non-electric variable buoyancy float to cycle the level of pressure. Bubbles from the expiratory gas flow are collected in the variable buoyancy float. As the float accumulates these bubbles, it becomes buoyant and rises to the top of the container. This motion blocks further air escape from the bubbling outlet, instead forcing the air to leave the closed circuit through pressure-regulating valves generating PIP. As the float vents the collected bubbles, it loses its buoyancy and sinks, reopening the bubbling outlet producing PEEP and returning the system to its original state (Figure 1). The submerged vertical tube contains distal venting holes, which serve as a safety measure, limiting the maximum delivered pressure in the event of a pressure valve failure.
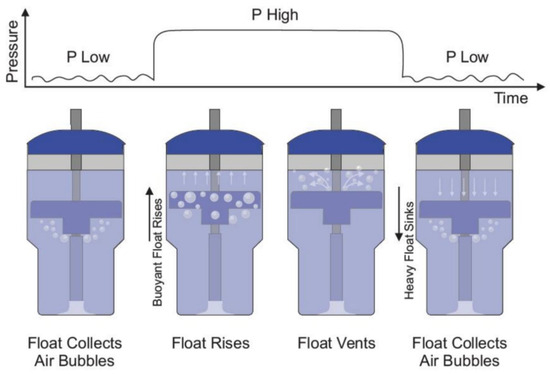
Figure 1.
Bubble NIPPV’s mechanism of action. Figure used with permission from AIM Tech.
The frequency at which the float oscillates is directly proportional to the rate of flow of compressed air/oxygen. In other words, as the flow rate is increased from 5 to 10 LPM, the float fills with air more rapidly and rises sooner, leading to a shorter duration at the low-pressure level and thereby shortening the overall cycle time [21].
bNIPPV is not synchronized with the patient’s efforts. However, anecdotally, patients seem to synchronize their respirations with the delivered pressure cycles, as has also been noted with infants on ventilator-driven non-synchronized NIPPV (nsNIPPV).
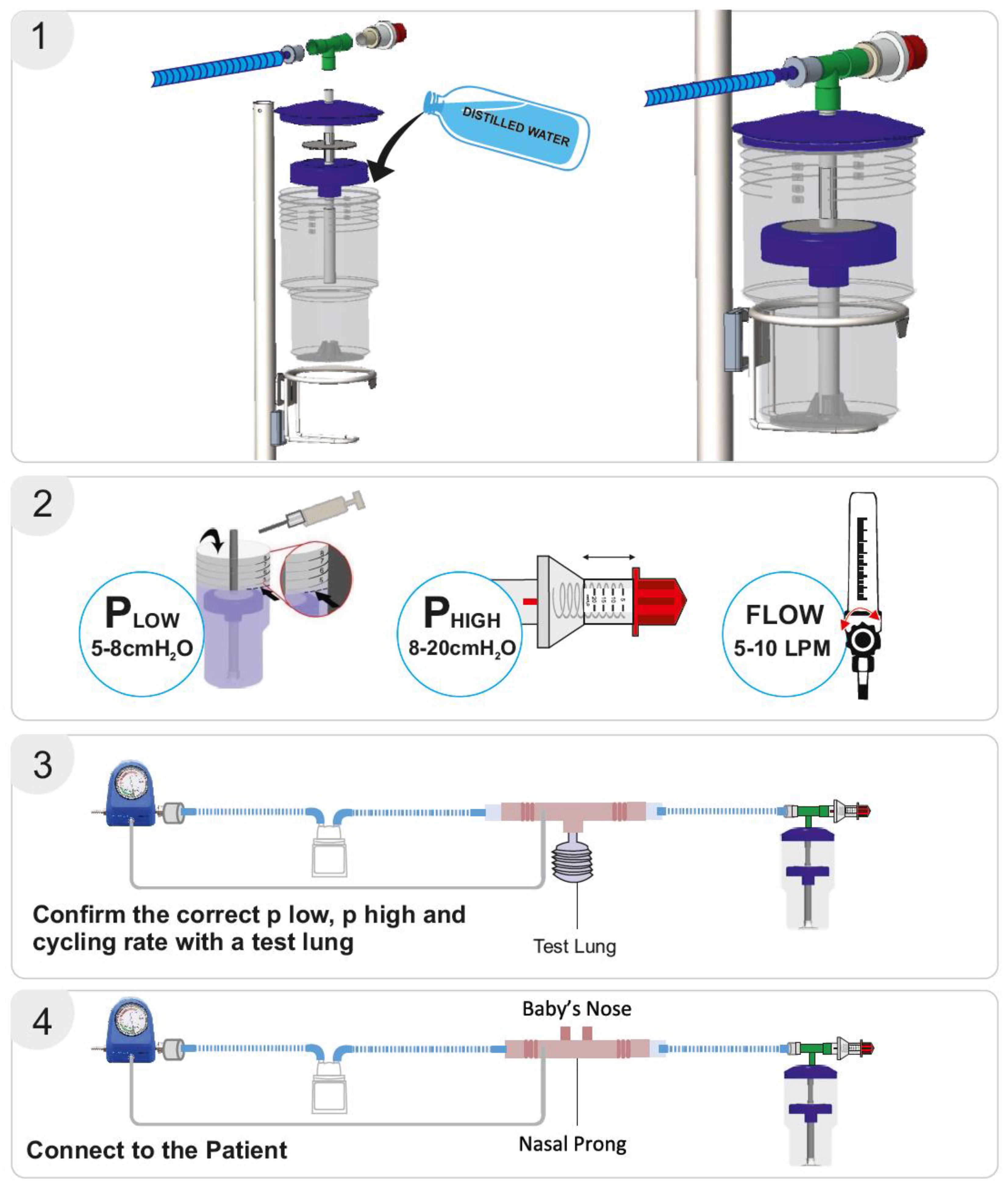
The bNIPPV unit consists of a container, central pipe, float, T-connector, and pressure regulator. The container is filled with distilled water to set the desired low-pressure level as indicated by respective markings on the container. Thereafter, the float is placed on the central pipe, and this pipe is then inserted into the container. The lid is placed on the container, after which the T-connecter is attached to the pressure regulator, central pipe, and respiratory circuit. The high pressure can be set by adjusting this pressure regulator. As in the case of bNCPAP, a blended mixture of compressed air and oxygen is passed through a heated humidifier (if available) to the inspiratory limb of the breathing circuit. The flow of air/oxygen mixture must be set to at least 5–10 L/min (Figure 2). As discussed previously, the expiratory limb of the breathing circuit is connected to the bNIPPV device.
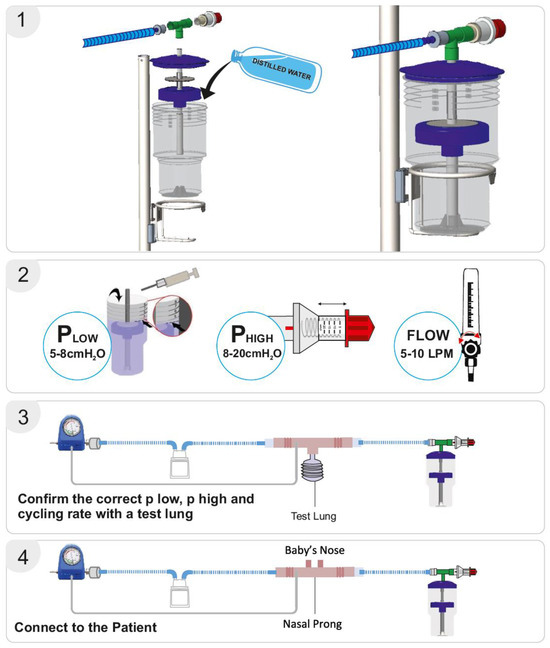
Figure 2.
Bubble NIPPV set up. Figure used with permission from Phoenix Medical Systems. Subfigure (1) illustrates the proper assembly of parts. Subfigure (2) shows how to set the Plow or PEEP, and Phigh or PIP. Subfigures (3,4) depict the whole circuit connected to a test lung or an infant nose respectively, with verification of delivered pressures achieved through the attached pressure monitor. Video link for guidance: https://youtu.be/zoNXS5GUOzs, accessed on 12 May 2025.
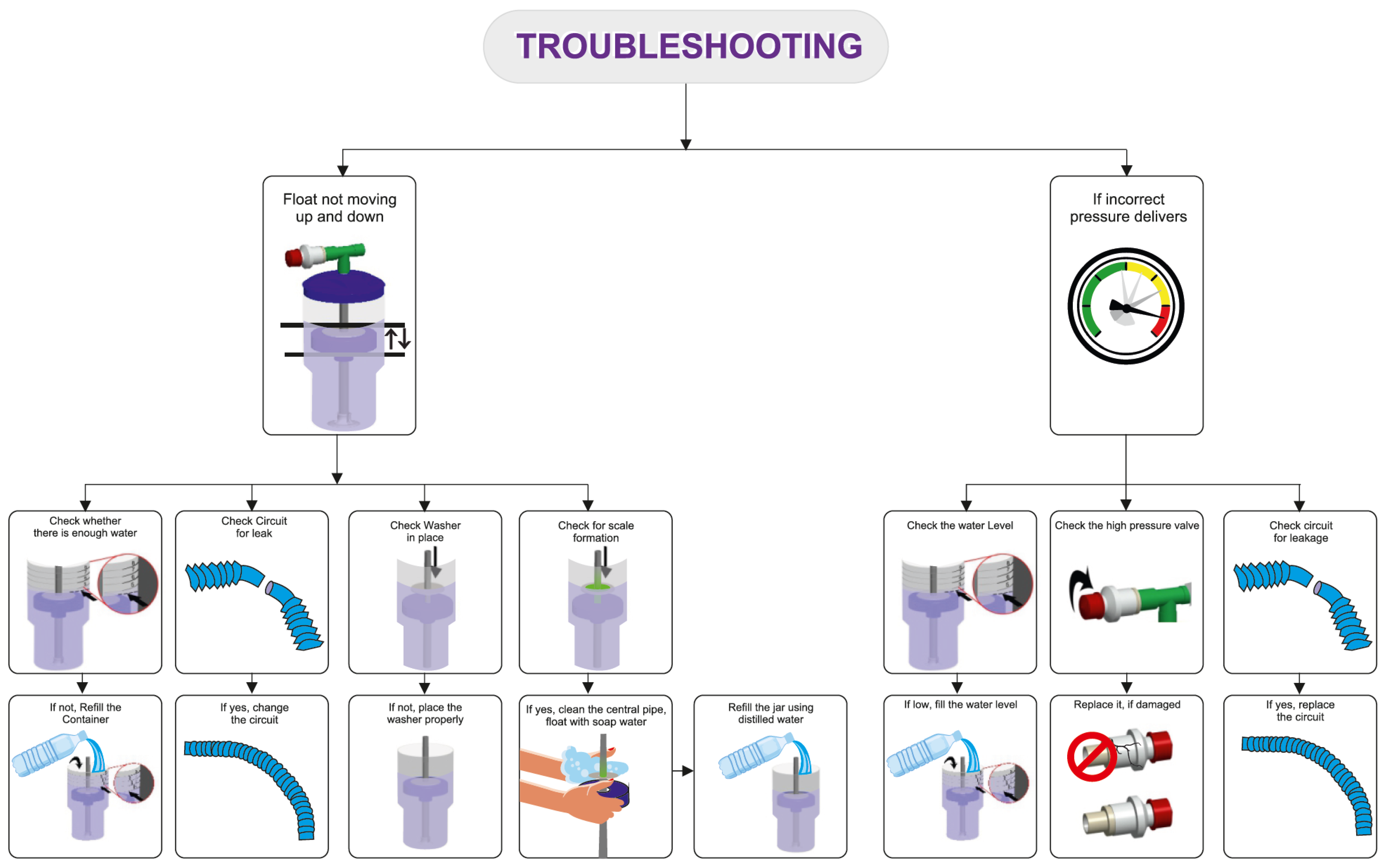
The delivery of the prescribed low and high pressures is first confirmed with a test lung and an in-line pressure transducer prior to connecting the device to the patient [17]. An occlusive nasal interface should be used to ensure adequate delivery of pressure. Hospitals have used the Hudson prong nasal interface, larger-sized RAM cannula, Fisher & Paykel, and Dräger interfaces. If the float does not cycle up and down, there may be a leak in the circuit, inadequate flow of compressed air, or a layer of biofilm between the central pipe/float. If the float is cycling up and down but the prescribed pressures are not delivered, an assessment for an incorrect water fill level, an incorrect setting on the pressure valve, or a leak in the respiratory circuit should be conducted. After the delivery of the prescribed pressures is confirmed with the test lung, the bNIPPV can be connected to the patient. If there is a decrease in delivered pressures to the infant, this likely represents a leak at the nasal interface which should be addressed [22] (Figure 3).
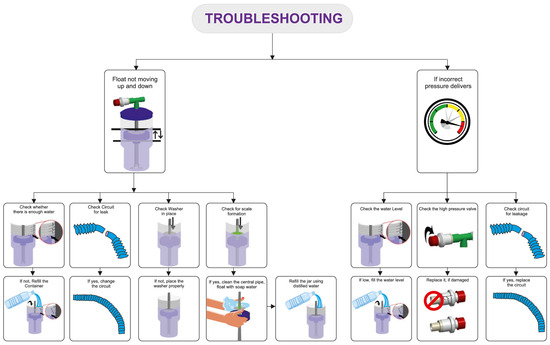
Figure 3.
Troubleshooting common bubble NIPPV issues. Figure used with permission from Phoenix Medical Systems.
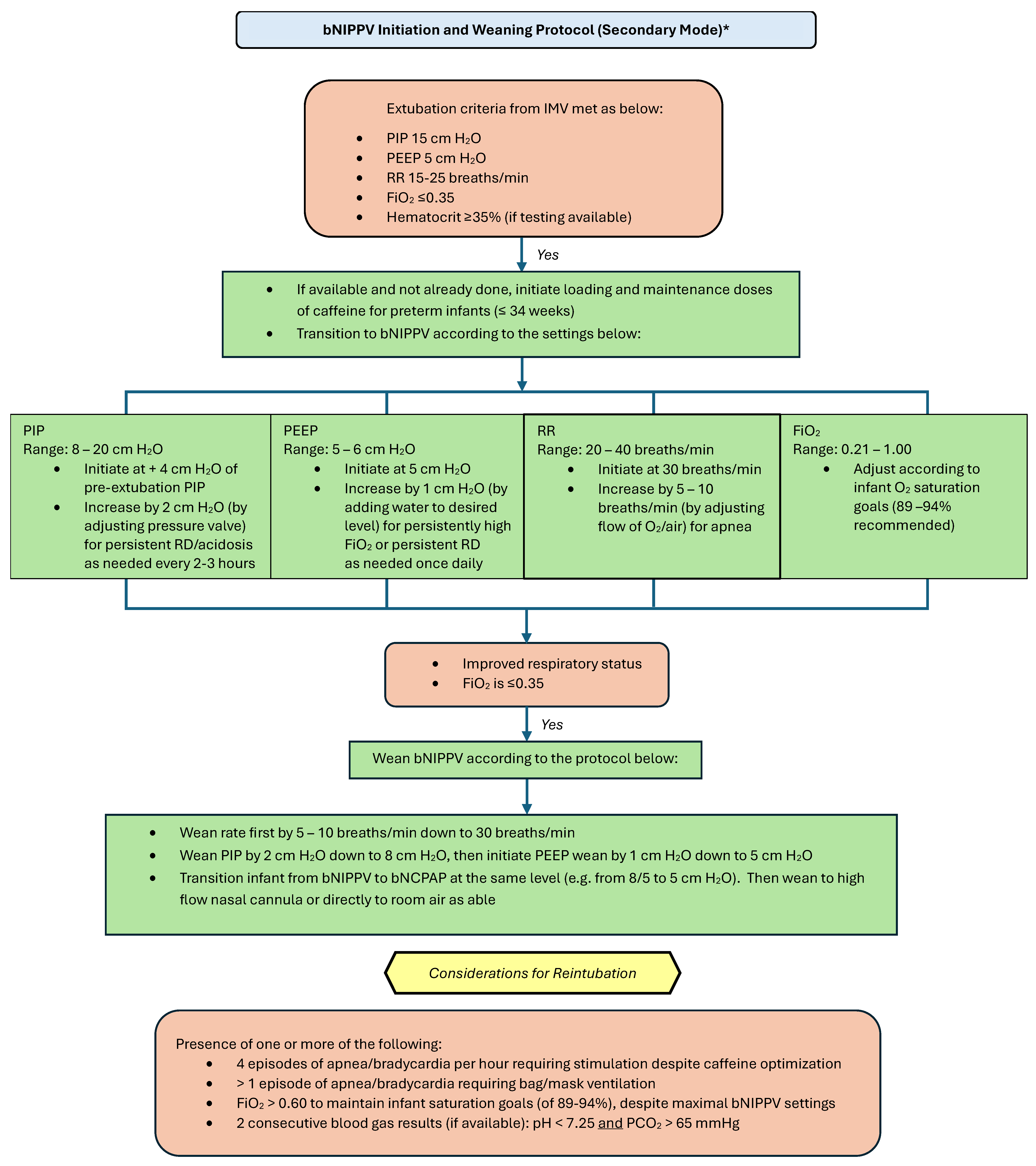
2.2. Proposed Guidelines and Settings
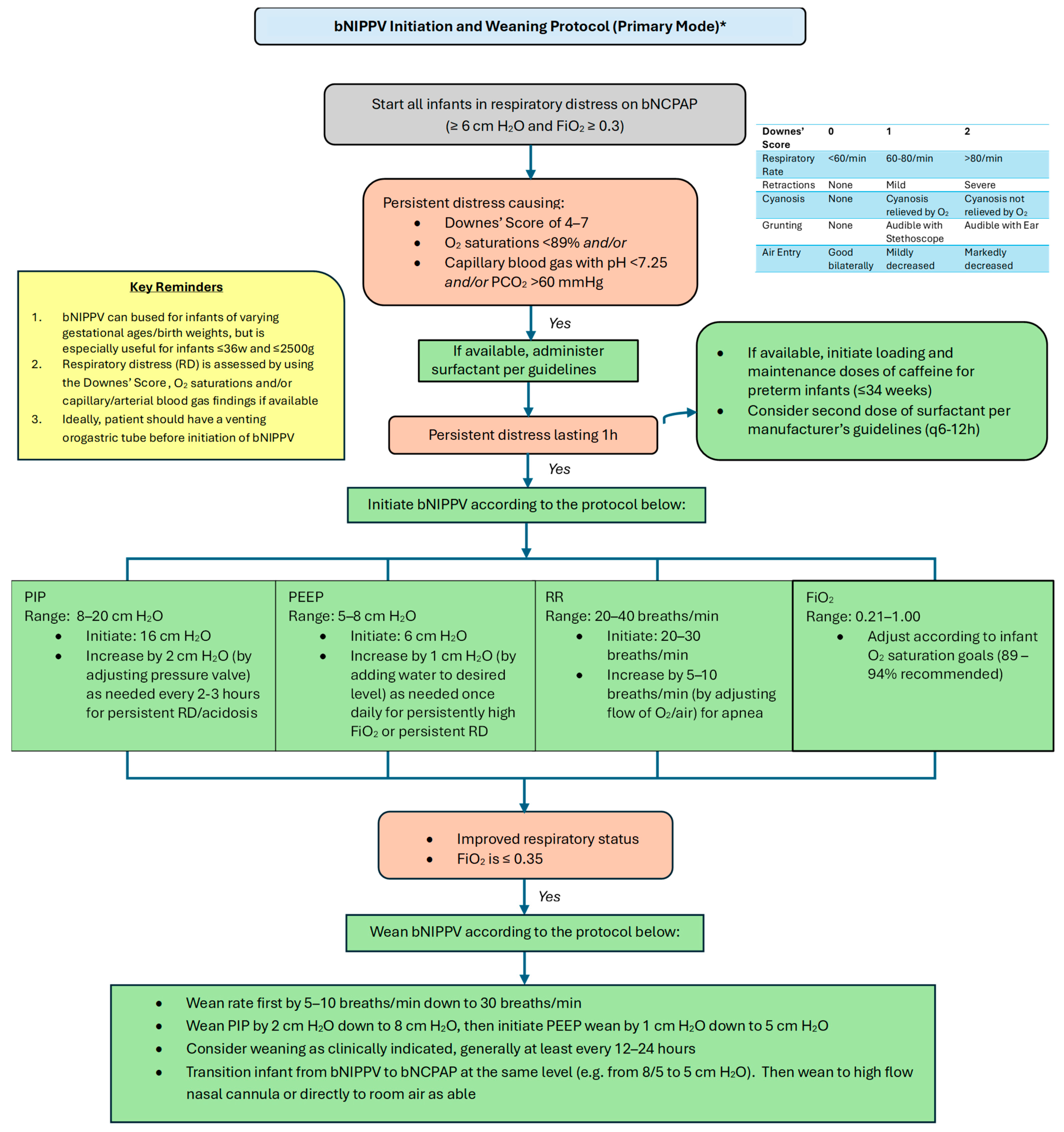
Please refer to Figure 4 and Figure 5 for our proposed guidelines and settings for the usage of bubble NIPPV in the primary and secondary modes, including surfactant administration [23,24].
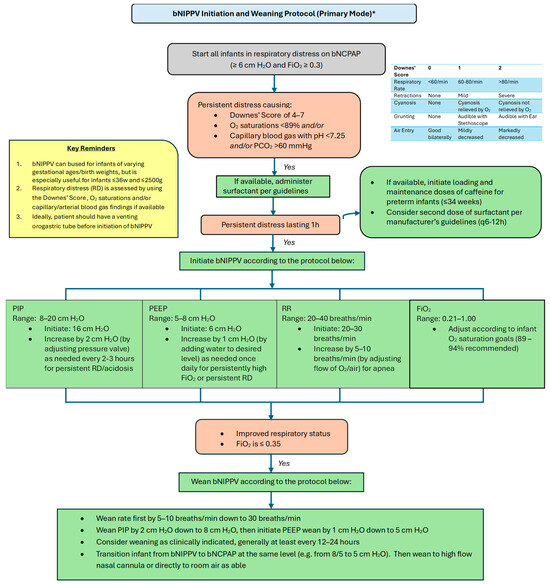
Figure 4.
Bubble NIPPV initiation and weaning protocol (primary mode) *. * Primary mode refers to use soon after birth and/or following a short period of intubation for surfactant delivery. bNIPPV: bubble nasal intermittent positive pressure ventilation; bNCPAP: bubble nasal continuous positive airway pressure; PIP: peak inspiratory pressure; PEEP: positive end expiratory pressure; RR: respiratory ventilation rate; FiO2: fraction of inspired oxygen.
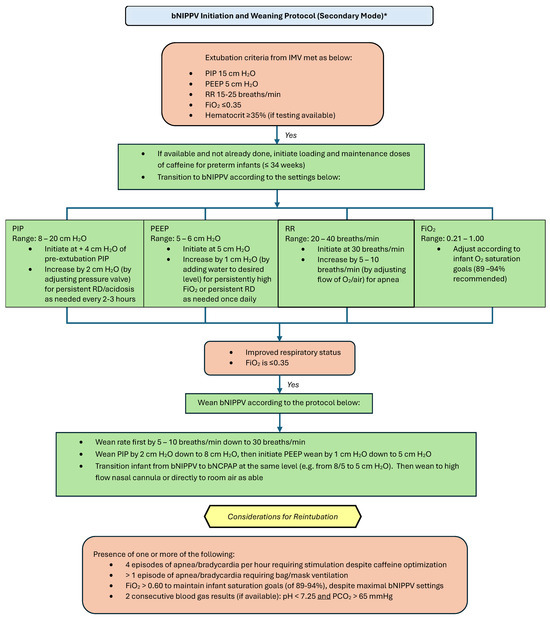
Figure 5.
Bubble NIPPV initiation and weaning protocol (secondary mode) *. * Secondary mode refers to use after a longer period of intubation. IMV: invasive mechanical ventilation; bNIPPV: bubble nasal intermittent positive pressure ventilation; bNCPAP: bubble nasal continuous positive airway pressure; PIP: peak inspiratory pressure; PEEP: positive end expiratory pressure; RR: respiratory (ventilator) rate; FiO2: fraction of inspired oxygen.
3. Discussion
Respiratory failure is the most common cause of neonatal death worldwide. The European Consensus Guidelines on the Management of Respiratory Distress Syndrome recommend non-invasive ventilation as the ideal support for preterm infants with RDS [25]. (2022 Update). NCPAP is often the first form of support attempted in this setting with failure necessitating invasive ventilation at a rate of 45% to 60% [26]. NIPPV has emerged as a superior mode of respiratory support in preventing the need for IMV, decreasing the incidence of post-extubation failure, and potentially minimizing the risk of BPD [5,27,28].
In a systematic review and network meta-analysis performed by Ramaswamy and colleagues (2020), non-synchronized BiPAP (BiPAP), nsNIPPV, and synchronized (sNIPPV) all decreased the risk for IMV and BPD occurrence compared to NCPAP [28]. In this article, we review RCTs that compare the efficacy of NIPPV, BiPAP, or both modalities with NCPAP in neonates with RDS as utilized in primary and secondary modes. These trials were chosen based on inclusion in two large systematic reviews examining the use of NIPPV in neonatal RDS, one published by Clinics in Perinatology [5] and the other by the Cochrane Collaboration [6,28]. Notably, these trials include significantly different study populations and employ varied settings and equipment to deliver respiratory support as highlighted in Table 1, Table 2 and Table 3.

Table 1.
Randomized controlled trials observing the efficacy of ventilator NIPPV vs. CPAP.

Table 2.
Randomized controlled trials observing the efficacy of BiPAP vs. CPAP.

Table 3.
Randomized controlled trials observing the efficacy of both NIPPV/BiPAP vs. CPAP.
Table 1 summarizes RCTs that study the efficacy of ventilator-driven NIPPVs in comparison with NCPAP in primary and secondary modes. In general, more favorable outcomes were observed in the NIPPV arms without any notable adverse events. Table 2 details RCTs that examine BiPAP vs. NCPAP efficacy in primary and secondary modes. These studies overall found less significant differences between the two modalities, which may be attributable to the lower pressures used in BiPAP mode when compared to NIPPV. Table 3 contains RCTs, which incorporate both NIPPV and BiPAP in the experimental group as opposed to a singular mode. The largest multi-centered RCT led by Kirpalani et al. on the comparison of NCPAP and NIPPV in the primary and secondary modes concluded that there was no difference in the rate of intubation, re-intubation, and survival to 36 weeks postmenstrual age without BPD in the very-low-birth-weight infants. This is perhaps reflective of the varied settings and modalities used in the experimental arm, as ~53% of this arm employed BiPAP settings.
Of all the trials analyzed, none found a notable advantage for NCPAP. None of these trials observed a difference in the safety profile of NIPPV and NCPAP. There were initial concerns of gastrointestinal (GI) perforation, although two systematic reviews of the Cochrane Collaboration on NIPPV in the primary and secondary mode have suggested similar rates of GI complications with both NIPPV and NCPAP usage [6]. It is a recommended standard practice to place an abdominal venting device (an 8 or 9 Fr oro-gastric tube, with the proximal part of a 10cc syringe attached to it (plunger removed), kept open to air and at a higher level than the baby) to minimize abdominal distension.
In settings where effective NCPAP has failed and further ventilator-based resources are limited or unavailable, the novel bNIPPV device offers a reliable alternative. Its existing literature has been promising and has been proven to be clinically safe and feasible to use. In infant manikins, the ability to consistently cycle between a set lower pressure and higher pressure with bNIPPV was established [21]. In the IngMar ASL 5000 infant lung simulator, the delivered pressure and volumes of bNIPPV were compared with those of the conventional ventilator-driven NIPPV with lung resistance and compliance set to approximate values of infants with RDS, transient tachypnea of the newborn, and pneumonia [14]. In an animal model, the ability of bNIPPV to normalize arterial blood gas values in sedated rabbits was compared with that of a conventional ventilator [53].
The feasibility of bNIPPV in comparison to bNCPAP was established in a cross-over case series, in which patients were randomized to four hours of bNCPAP vs. bNIPPV, followed by the alternate treatment [16]. This was followed by a formal study of safety, in which 60 infants in respiratory distress were pragmatically allocated to bNCPAP vs. bNIPPV (30 infants in each arm). A similar rate of complications, including septal necrosis, pneumothorax, and gastric distention, was observed in both arms [17]. Notably, this safety study utilized a lower level of PIP (similar to what is used in BiPAP); however, the proposed guidelines are grounded on existing literature surrounding ventilator generated NIPPV usage in the primary and secondary modes as referenced in Table 1, Table 2 and Table 3. While the PIPs recommended for bNIPPV use in this protocol have not been studied in an RCT, the PIPs recommended are still lower based on our experience of >25 years of using ventilator-generated NIPPV and those reported in other studies [10,27,54]. It is important to use such pressures, if needed, in preterm infants with respiratory distress in resource-limited countries (with little or no access to exogenous surfactant), as avoiding intubation and IMV is an important goal because of its potential life-threatening complications, such as sepsis.
In summary, most studies on NIPPV have confirmed its superiority over NCPAP in terms of preventing extubation failure. This aspect of NIPPV is of critical importance in low-resource settings where access to IMV is limited, in addition to the other known complications of IMV (for, e.g., ventilator associated pneumonia and sepsis). Hence, in this scenario where the NCPAP is not effective, the NIPPV has the potential to provide enhanced non-invasive support. Higher initial NIPPV settings may be required to ascertain a greater effect on the prevention of initial IMV as well as the prevention of reintubation following extubation. The novel bNIPPV device—with its associated benefits of being low-cost, nonelectric, and simple to use—offers those infants without access to advanced respiratory support something that bNCPAP alone often fails to do—a chance at survival. These guidelines for the use of bNIPPV provide a thorough and important resource for medical personnel taking care of neonates in LMICs.
Author Contributions
Conceptualization, M.M., V.B., S.J., T.S.; Writing—original draft preparation, M.M., V.B., S.J.; Writing—review and editing, M.M., V.B., S.J., T.S. All authors have read and agreed to the published version of the manuscript.
Funding
Research reported in this publication was supported by the Thrasher Foundation and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1-TR002494.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
S.J. is a co-inventor of bubble NIPPV technology and co-founder of AIM Tech Health.
References
- Kamath, B.D.; Macguire, E.R.; McClure, E.M.; Goldenberg, R.L.; Jobe, A.H. Neonatal mortality from respiratory distress syndrome: Lessons for low-resource countries. Pediatrics 2011, 127, 1139–1146. [Google Scholar] [CrossRef]
- United Nations Children’s Fund. Neonatal Mortality. Available online: https://data.unicef.org/topic/child-survival/neonatal-mortality/ (accessed on 4 June 2025).
- Ekhaguere, O.A.; Okonkwo, I.R.; Batra, M.; Hedstrom, A.B. Respiratory distress syndrome management in resource limited settings-Current evidence and opportunities in 2022. Front. Pediatr. 2022, 10, 961509. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Carroll, C.; Bhandari, V. BPD Following Preterm Birth: A Model for Chronic Lung Disease and a Substrate for ARDS in Childhood. Front. Pediatr. 2016, 4, 60. [Google Scholar] [CrossRef]
- Ruegger, C.M.; Owen, L.S.; Davis, P.G. Nasal Intermittent Positive Pressure Ventilation for Neonatal Respiratory Distress Syndrome. Clin. Perinatol. 2021, 48, 725–744. [Google Scholar] [CrossRef] [PubMed]
- Lemyre, B.; Deguise, M.O.; Benson, P.; Kirpalani, H.; Ekhaguere, O.A.; Davis, P.G. Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants. Cochrane Database Syst. Rev. 2023, 7, CD005384. [Google Scholar] [CrossRef]
- Bhandari, V.; Gavino, R.G.; Nedrelow, J.H.; Pallela, P.; Salvador, A.; Ehrenkranz, R.A.; Brodsky, N.L. A randomized controlled trial of synchronized nasal intermittent positive pressure ventilation in RDS. J. Perinatol. 2007, 27, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, A.; Abdul Wahab, M.G.; Razak, A.; Rempel, E.; Patel, W.; Mondal, T.; Beck, J. High CPAP vs. NIPPV in preterm neonates—A physiological cross-over study. J. Perinatol. 2021, 41, 1690–1696. [Google Scholar] [CrossRef]
- Owen, L.S.; Morley, C.J.; Davis, P.G. Neonatal nasal intermittent positive pressure ventilation: What do we know in 2007? Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F414–F418. [Google Scholar] [CrossRef]
- Shi, Y.; Muniraman, H.; Biniwale, M.; Ramanathan, R. A Review on Non-invasive Respiratory Support for Management of Respiratory Distress in Extremely Preterm Infants. Front. Pediatr. 2020, 8, 270. [Google Scholar] [CrossRef]
- Boel, L.; Broad, K.; Chakraborty, M. Non-invasive respiratory support in newborn infants. Paediatr. Child Health 2018, 28, 6–12. [Google Scholar] [CrossRef]
- Bhandari, V. Nasal intermittent positive pressure ventilation in the newborn: Review of literature and evidence-based guidelines. J. Perinatol. 2010, 30, 505–512. [Google Scholar] [CrossRef]
- Kumar, J.; Kumar, P.; Bhandari, V. Noninvasive ventilation strategies in neonates. Indian Pediatr. 2025, 62, 451–460. [Google Scholar] [CrossRef]
- John, S.C.; John, A.V.; Moss, A.W.; Gustafson, P.A.; Fernando-Silva, L.; John, S.P. Bench Testing of a Bubble Noninvasive Ventilation Device in an Infant Lung Simulator. Respir. Care 2020, 65, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Poletto, S.; Trevisanuto, D.; Ramaswamy, V.V.; Seni, A.H.A.; Ouedraogo, P.; Dellaca, R.L.; Zannin, E. Bubble CPAP respiratory support devices for infants in low-resource settings. Pediatr. Pulmonol. 2023, 58, 643–652. [Google Scholar] [CrossRef]
- John, S.C.; Adhikari, B.R.; John, A.V.; Cheng, E.O.; Weiner, G.M.; John, S.P. Feasibility of bubble non-invasive positive pressure ventilation, a first-in-human study. J. Trop. Pediatr. 2022, 68, fmac095. [Google Scholar] [CrossRef]
- John, S.C.; Garg, M.; Muttineni, M.; Brearley, A.M.; Rao, P.; Bhandari, V.; Slusher, T.; Murki, S. Safety of bubble nasal intermittent positive pressure ventilation (NIPPV) versus bubble nasal continuous positive airway pressure (NCPAP) in preterm infants with respiratory distress. J. Perinatol. 2024, 44, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.; Donaldsson, S.; Drevhammar, T. Infant CPAP for low-income countries: An experimental comparison of standard bubble CPAP and the Pumani system. PLoS ONE 2018, 13, e0196683. [Google Scholar] [CrossRef]
- Bhandari, V. NIPPV in the neonate: Answers to FAQs—A personal perspective. J. Neonatol. 2017, 31, 31–34. [Google Scholar] [CrossRef]
- Owen, L.S.; Manley, B.J. Nasal intermittent positive pressure ventilation in preterm infants: Equipment, evidence, and synchronization. Semin. Fetal Neonatal Med. 2016, 21, 146–153. [Google Scholar] [CrossRef]
- John, S.C.; Barnett, J.D.; Habben, N.D.; Le, H.T.; Cheng, E.; John, S.P.; Gustafson, P.A. Development and Testing of a Bubble Bi-Level Positive Airway Pressure System. Respir. Care 2017, 62, 1131–1136. [Google Scholar] [CrossRef]
- John, S.C.; Cheng, E.O.; John, S.P. The BCPAP Score: Five Questions to Assess the Effectiveness of a Bubble CPAP Circuit. J. Trop. Pediatr. 2020, 66, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Downes, J.J.; Vidyasagar, D.; Boggs, T.R.; Morrow, G.M. Respiratory distress syndrome of newborn infants. I. New clinical scoring system (RDS score) with acid–base and blood-gas correlations. Clin. Pediatr. 1970, 9, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.; Black, R.; Gandhi, B.; Hogue, S.; Kakkilaya, V.; Mikhael, M.; Moya, F.; Pezzano, C.; Read, P.; Roberts, K.D.; et al. RDS-NExT workshop: Consensus statements for the use of surfactant in preterm neonates with RDS. J. Perinatol. 2023, 43, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Carnielli, V.P.; Greisen, G.; Hallman, M.; Klebermass-Schrehof, K.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology 2023, 120, 3–23. [Google Scholar] [CrossRef]
- Wright, C.J.; Sherlock, L.G.; Sahni, R.; Polin, R.A. Preventing Continuous Positive Airway Pressure Failure: Evidence-Based and Physiologically Sound Practices from Delivery Room to the Neonatal Intensive Care Unit. Clin. Perinatol. 2018, 45, 257–271. [Google Scholar] [CrossRef]
- Ramaswamy, V.V.; More, K.; Roehr, C.C.; Bandiya, P.; Nangia, S. Efficacy of noninvasive respiratory support modes for primary respiratory support in preterm neonates with respiratory distress syndrome: Systematic review and network meta-analysis. Pediatr. Pulmonol. 2020, 55, 2940–2963. [Google Scholar] [CrossRef]
- Lemyre, B.; Deguise, M.O.; Benson, P.; Kirpalani, H.; De Paoli, A.G.; Davis, P.G. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst. Rev. 2023, 7, CD003212. [Google Scholar] [CrossRef]
- Bisceglia, M.; Belcastro, A.; Poerio, V.; Raimondi, F.; Mesuraca, L.; Crugliano, C.; Corapi, U.P. A comparison of nasal intermittent versus continuous positive pressure delivery for the treatment of moderate respiratory syndrome in preterm infants. Minerva Pediatr. 2007, 59, 91–95. [Google Scholar]
- Kishore, M.S.; Dutta, S.; Kumar, P. Early nasal intermittent positive pressure ventilation versus continuous positive airway pressure for respiratory distress syndrome. Acta Paediatr. 2009, 98, 1412–1415. [Google Scholar] [CrossRef]
- Meneses, J.; Bhandari, V.; Alves, J.G.; Herrmann, D. Noninvasive ventilation for respiratory distress syndrome: A randomized controlled trial. Pediatrics 2011, 127, 300–307. [Google Scholar] [CrossRef]
- Ramanathan, R.; Sekar, K.C.; Rasmussen, M.; Bhatia, J.; Soll, R.F. Nasal intermittent positive pressure ventilation after surfactant treatment for respiratory distress syndrome in preterm infants <30 weeks’ gestation: A randomized, controlled trial. J. Perinatol. 2012, 32, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Armanian, A.M.; Badiee, Z.; Heidari, G.; Feizi, A.; Salehimehr, N. Initial Treatment of Respiratory Distress Syndrome with Nasal Intermittent Mandatory Ventilation versus Nasal Continuous Positive Airway Pressure: A Randomized Controlled Trial. Int. J. Prev. Med. 2014, 5, 1543–1551. [Google Scholar]
- Oncel, M.Y.; Arayici, S.; Uras, N.; Alyamac-Dizdar, E.; Sari, F.N.; Karahan, S.; Canpolat, F.E.; Oguz, S.S.; Dilmen, U. Nasal continuous positive airway pressure versus nasal intermittent positive-pressure ventilation within the minimally invasive surfactant therapy approach in preterm infants: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F323–F328. [Google Scholar] [CrossRef] [PubMed]
- Sabzehei, M.K.; Basiri, B.; Shokouhi, M.; Naser, M. A comparative study of treatment response of respiratory distress syndrome in preterm infants: Early nasal intermittent positive pressure ventilation versus early nasal continuous positive airway pressure. Int. J. Pediatr. 2018, 6, 8339–8346. [Google Scholar] [CrossRef]
- Skariah, T.A.; Lewis, L.E. Early nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for respiratory distress syndrome in infants of 28–36 weeks gestational age: A randomized controlled trial. Iran. J. Neonatol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Shi, Y.; Tang, S.; Zhao, J.; Shen, J. A prospective, randomized, controlled study of NIPPV versus nCPAP in preterm and term infants with respiratory distress syndrome. Pediatr. Pulmonol. 2014, 49, 673–678. [Google Scholar] [CrossRef]
- Khorana, M.; Paradeevisut, H.; Sangtawesin, V.; Kanjanapatanakul, W.; Chotigeat, U.; Ayutthaya, J.K. A randomized trial of non-synchronized Nasopharyngeal Intermittent Mandatory Ventilation (nsNIMV) vs. Nasal Continuous Positive Airway Pressure (NCPAP) in the prevention of extubation failure in pre-term <1500 grams. J. Med. Assoc. Thai. 2008, 91 (Suppl. 3), S136–S142. [Google Scholar]
- Kahramaner, Z.; Erdemir, A.; Turkoglu, E.; Cosar, H.; Sutcuoglu, S.; Ozer, E.A. Unsynchronized nasal intermittent positive pressure versus nasal continuous positive airway pressure in preterm infants after extubation. J. Matern. Fetal Neonatal Med. 2014, 27, 926–929. [Google Scholar] [CrossRef]
- Jasani, B.; Nanavati, R.; Kabra, N.; Rajdeo, S.; Bhandari, V. Comparison of non-synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as post-extubation respiratory support in preterm infants with respiratory distress syndrome: A randomized controlled trial. J. Matern. Fetal Neonatal Med. 2016, 29, 1546–1551. [Google Scholar] [CrossRef]
- Komatsu, D.F.; Diniz, E.M.; Ferraro, A.A.; Ceccon, M.E.; Vaz, F.A. Randomized controlled trial comparing nasal intermittent positive pressure ventilation and nasal continuous positive airway pressure in premature infants after tracheal extubation. Rev. Assoc. Med. Bras. (1992) 2016, 62, 568–574. [Google Scholar] [CrossRef]
- Ribeiro, S.N.S.; Fontes, M.J.F.; Bhandari, V.; Resende, C.B.; Johnston, C. Noninvasive Ventilation in Newborns </=1500 g after Tracheal Extubation: Randomized Clinical Trial. Am. J. Perinatol. 2017, 34, 1190–1198. [Google Scholar] [CrossRef]
- Estay, A.S.; Mariani, G.L.; Alvarez, C.A.; Milet, B.; Agost, D.; Avila, C.P.; Roldan, L.; Abdala, D.A.; Keller, R.; Galletti, M.F.; et al. Randomized Controlled Trial of Nonsynchronized Nasal Intermittent Positive Pressure Ventilation versus Nasal CPAP after Extubation of VLBW Infants. Neonatology 2020, 117, 193–199. [Google Scholar] [CrossRef]
- Kong, L.K.; Kong, X.Y.; Li, L.H.; Dong, J.Y.; Shang, M.X.; Chi, J.H.; Huang, R.X.; Zheng, Y.; Ma, J.E.; Chen, X.C.; et al. Comparative study on application of Duo positive airway pressure and continuous positive airway pressure in preterm neonates with respiratory distress syndrome. Zhongguo Dang Dai Er Ke Za Zhi Chin. J. Contemp. Pediatr. 2012, 14, 888–892. [Google Scholar]
- Aguiar, T.; Macedo, I.; Voutsen, O.; Silva, P.; Nona, J.; Araujo, C.; Imaginário, J.; Mauricio, A.; Barroso, R.; Tomé, T.; et al. Nasal bilevel versus continuous positive airway pressure in preterm infants: A randomized controlled trial. J. Clin. Trials 2015, 5, 221. [Google Scholar] [CrossRef]
- Sadeghnia, A.; Barekateyn, B.; Badiei, Z.; Hosseini, S.M. Analysis and comparison of the effects of N-BiPAP and Bubble-CPAP in treatment of preterm newborns with the weight of below 1500 grams affiliated with respiratory distress syndrome: A randomised clinical trial. Adv. Biomed. Res. 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Chen, G.Y.; Wang, J.; Zhou, Z.X.; Zhang, P.Y.; Chang, L.W.; Rong, Z.H. Bi-level Nasal Positive Airway Pressure (BiPAP) versus Nasal Continuous Positive Airway Pressure (CPAP) for Preterm Infants with Birth Weight Less Than 1500 g and Respiratory Distress Syndrome Following INSURE Treatment: A Two-center Randomized Controlled Trial. Curr. Med. Sci. 2021, 41, 542–547. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Campbell, C.; Brown, L.; Wenger, L.; Shah, V. Infant flow biphasic nasal continuous positive airway pressure (BP- NCPAP) vs. infant flow NCPAP for the facilitation of extubation in infants’ </=1250 grams: A randomized controlled trial. BMC Pediatr. 2012, 12, 43. [Google Scholar] [CrossRef]
- Victor, S.; Roberts, S.A.; Mitchell, S.; Aziz, H.; Lavender, T.; Extubate Trial, G. Biphasic Positive Airway Pressure or Continuous Positive Airway Pressure: A Randomized Trial. Pediatrics 2016, 138, e20154095. [Google Scholar] [CrossRef]
- Manjunatha, C.M.; Kalyanasundaram, S.; Ibhanesebhor, S.E.; Vigni, D.; Robertson, C. Prospective randomized controlled trial comparing the use of biphasic positive airway pressure (BiPAP) with nasal continuous positive airway pressure (n-CPAP) following extubation of preterm babies. EC Paediatr. 2019, 8, 525–532. [Google Scholar]
- Kirpalani, H.; Millar, D.; Lemyre, B.; Yoder, B.A.; Chiu, A.; Roberts, R.S.; Group, N.S. A trial comparing noninvasive ventilation strategies in preterm infants. N. Engl. J. Med. 2013, 369, 611–620. [Google Scholar] [CrossRef]
- El-Farrash, R.A.; DiBlasi, R.M.; Abd, E.L.A.E.A.; El-Tahry, A.M.; Eladawy, M.S.; Tadros, M.A.; Koriesh, M.A.; Farid, J.V.; AbdElwahab, R.S.; Elsayed, M.A.; et al. Postextubation Noninvasive Ventilation in Respiratory Distress Syndrome: A Randomized Controlled Trial. Am. J. Perinatol. 2022, 29, 1577–1585. [Google Scholar] [CrossRef]
- John, S.C.; Mohammed, A.; Church, J.T.; John, A.V.; Perkins, E.M.; McLeod, J.S.; Carr, B.D.; Smith, S.; Barnett, J.H.; Gustafson, P.A.; et al. Bubble bilevel ventilation facilitates gas exchange in anesthetized rabbits. Pediatr. Res. 2021, 89, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Badiee, Z.; Nekooie, B.; Mohammadizadeh, M. Noninvasive positive pressure ventilation or conventional mechanical ventilation for neonatal continuous positive airway pressure failure. Int. J. Prev. Med. 2014, 5, 1045–1053. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).