Abstract
Background/Objectives: Childhood anemia remains a serious public health issue, negatively affecting cognitive and psychomotor development, with repercussions on school performance and adult productivity. This study aimed to characterize the profile of children aged 6 months to 5 years diagnosed with or at risk of anemia who attended a pediatric hospital in Lisbon, Portugal. Methods: A hospital-based, cross-sectional descriptive study was conducted from September 2023 to September 2024. Descriptive statistics, including frequency distributions and cross-tabulations, summarized participant characteristics and key variables. Results: We observed that 33.3% (74/222) of the children were either anemic or at risk of anemia. Among these, 93.2% (69/74) were confirmed anemic or at risk based on hemoglobin levels. Five children (6.8%) had normal hemoglobin but abnormal red-cell indices, with microcytic (60.0%; 3/5) or normocytic (40.0%; 2/5) patterns. Anemia rates were higher in males (55.1%), children aged 24–59 months, those residing in the Metropolitan Lisbon Area (82.6%), children whose caregivers had only basic or secondary education (58.0%), and those whose mothers were born in foreign countries (48.4%). Microcytic red-cell indices were observed in 63.1% of cases. Serum iron results indicated that 32.0% were pre-anemic and 40.0% anemic. Ferritin levels showed iron-deficiency anemia in 22.2% of tested cases. In addition, 33.3% carried the sickle cell trait, and 35.0% had elevated C-reactive protein, suggesting anemia of inflammation. Conclusions: Anemia is a moderate public health issue, mainly affecting children with less-educated caregivers and migrant mothers. Targeted public health actions, including systematic screening, caregiver education, and multiculturally sensitive interventions, are crucial to address anemia.
1. Introduction
Anemia is a condition where the number of erythrocytes or red blood cells (RBCs) is insufficient to meet metabolic demands [1]. It is a serious public health problem that affects both high- and low-income countries and is associated with adverse health outcomes and reduced quality of life [1,2,3,4,5,6,7]. Globally, anemia is one of the most widespread nutritional disorders, affecting nearly 40.0% of children aged 6 months to 5 years [3,7,8,9].
The causes of childhood anemia are multifactorial. For example, lack of iron, folate, vitamin B12, and vitamin A can cause nutritional anemia [6,7,9]. Iron deficiency is the most common nutritional deficiency. It often occurs when dietary intake is insufficient to meet physiological demands, particularly during life stages when requirements are elevated, such as pregnancy, infancy, and periods of rapid growth and development [10,11].
Deficiencies in several other micronutrients (e.g., vitamin A, B12, folic acid, and riboflavin), which are essential for the normal production of red blood cells, likely contribute to the development of anemia [11]. Nutritional and iron deficiency anemia in children impairs immune function, delays mental, physical, and socioemotional development, and increases the risk of death among infants and young children [7,9]. Non-nutritional causes of anemia include parasitic infections (e.g., malaria, hookworm, schistosomiasis), as well as blood loss, blood-inherited diseases (e.g., thalassemia and sickle-cell), and infectious diseases (e.g., HIV/AIDS) [7,9]. The interaction between anemia and infections is complex: anemia may increase susceptibility to infections, while infections can contribute to anemia through mechanisms such as inflammation, hemolysis, and nutrient malabsorption [12,13]. Parasitic infections impair the intestinal absorption of nutrients, often through the consumption of contaminated water or food, leading to anemia [14]. In addition, sociodemographic and economic factors, child feeding practices, access to health services, and maternal anemia also play a role in the development of childhood anemia [15,16]. Anemia represents a key indicator of poor health and nutrition, acting as a marker of socioeconomic disparities, with children from lower-income households at higher risk [17]. Poverty, limited access to healthcare services, and low caregiver education levels restrict access to nutritious foods and timely medical interventions, thereby increasing the risk of childhood anemia [18]. Few studies [19,20,21] have also shown an association between maternal and fetal anemia.
Anemia is defined quantitatively by hemoglobin (Hb), hematocrit, or red blood cell count levels that fall below the normal age- and sex-specific ranges [1]. Anemia in children aged 6 months to 5 years is characterized by low blood hemoglobin and can be classified into mild, moderate, and severe categories [6,11,22,23]. Anemia symptoms vary according to severity, with mild cases often being asymptomatic and severe cases associated with developmental impairments and increased childhood mortality [16,24,25,26,27]. Anemia is also classified by the mean corpuscular volume (MCV) into microcytic, normocytic, and macrocytic types [1,28,29]. Microcytic anemia is often caused by iron deficiency or thalassemia [30,31,32]. Normocytic anemia may result from chronic infections, systemic diseases, and acquired disorders (e.g., autoimmune hemolytic anemia and microangiopathic hemolysis) [1,31,33]. The premature breakdown of red blood cells that occurs in hemolytic anemia may also increase bilirubin levels [1]. Macrocytic anemia is usually caused by deficiencies in vitamin B12 or folic acid, but it may also result from chronic liver disease, hypothyroidism, myelodysplastic disorders, and bone marrow disorders such as leukemia [1,30,31,34,35].
In 2021, the global anemia prevalence was 24.3%, affecting 1.92 billion people and accounting for 52.0 million years of healthy life lost due to disability (YLDs) [36]. In children aged 6–59 months, the global prevalence was 40.0% in 2019, a modest decline from 48.0% in 2000 [37]. The highest burden remains in Africa, where 60.2% of children under five are affected, compared to 23.0% in Europe [36,37,38,39]. In Portugal, childhood anemia data are scarce. National estimates from 2019 suggest a prevalence of 4.3% in children under five [40], though other studies report that anemia is highly prevalent and largely undiagnosed [41,42]. While studies on childhood anemia in Portugal have been conducted, few were inherently review articles [43,44]. A 2005 study in Braga reported a 19.7% prevalence of iron deficiency anemia in infants [45], but more recent research is limited [46]. Given the implications of anemia on development and productivity, there is an urgent need for updated research. This study aims to characterize the profile of children aged 6 months to 5 years diagnosed with or at risk of anemia attending external pediatric consultations at Dona Estefânia Hospital (HDE) in Lisbon, Portugal. It seeks to provide updated epidemiological evidence to inform public health strategies and improve the management of childhood anemia.
2. Materials and Methods
2.1. Study Setting
Portugal, officially the Portuguese Republic, is the westernmost country in mainland Europe [47,48,49]. This study was conducted at the External Consultations of the Pediatrics Medical Service of Dona Estefânia Hospital (HDE)—Unidade Local de Saúde de São José (ULS São José) in Lisbon. Dona Estefânia Hospital is a reference pediatric center for Southern Portugal and the archipelagos of the Azores and Madeira. It specializes in maternal and child health care [50,51]. In 2024, Dona Estefânia Hospital recorded a total of 59,870 pediatric consultations across all medical specialties. Of these, 4169 were in general pediatrics, with 1046 corresponding to first consultations and 2420 to follow-up consultations, covering a total of 2301 patients [51].
2.2. Study Design, Population, and Sample Universe
This hospital-based, cross-sectional descriptive study was conducted from September 2023 to September 2024. The sample universe was the External General Pediatrics Consultation section (area) of the Dona Estefânia Hospital. During the study period, all children aged 6 months to 5 years who attended the external general pediatric consultations were recorded (census-based approach). From this population, only those meeting the inclusion criteria—children diagnosed with or at risk of anemia—were included in the final study sample.
2.3. Inclusion and Exclusion Criteria
The inclusion criteria were a subset of children aged between 6 months and 5 years, specifically those diagnosed with or at risk of anemia, who attended the External General Pediatrics Consultation section (area) at Dona Estefânia Hospital. This included children referred from the emergency department for evaluation or follow-up by a pediatrician; children referred by family doctors from Health Centers; and children evacuated from Portuguese-speaking African countries due to various pathologies not treatable in their countries of origin. Children with normal hemoglobin levels but altered red-cell indices, including microcytic and normocytic patterns, were also included to capture potential subclinical anemia cases.
The exclusion criteria were children younger than 6 months, children aged 6 years or older, and children without available hemoglobin or hematocrit measurements. Children being followed in hematology or other specialized consultations, as well as those with pre-existing hematologic or cardiovascular diseases, were excluded from this study.
2.4. Data Collection and Quality Control
The total number of children aged 6 months to 5 years, both with and without anemia, who attended external pediatric consultations during the study period was obtained from daily consultation records. Children diagnosed with anemia or those identified as at risk were selected during each consultation. The study was then explained to the children’s caregivers, and their written informed consent was obtained. A pretested questionnaire, consisting of three sections on sociodemographic characteristics, feeding habits, and health status, was used to collect information. Clinical data, including laboratory test results, were obtained from electronic medical records and children’s health cards. Typically, all children had a complete blood count (CBC) performed. Additional tests—such as serum iron, ferritin, folate, vitamin B12, bilirubin, glucose, uremia, and C-reactive protein (CRP)—were available for a subset of children, depending on clinical assessment. Reticulocyte count and total iron binding capacity (TIBC) were not routinely measured. Other differential diagnoses, including zinc deficiency and bone marrow aspiration, were also not routinely assessed or performed. Sickle cell trait was identified through hemoglobin electrophoresis when available or when clinically justified by the pediatrician’s assessment.
Investigators carefully monitored data collection. To ensure data quality, double data entry verification was conducted, and the information from paper-based forms and electronic questionnaires was compared. Data cleaning was performed to verify frequencies, consistency, and missing values, and any errors identified were corrected.
2.5. Outcome Variable
The outcome variable for this study was the presence of anemia or the risk of developing anemia in children aged 6 months to 5 years. In the context of this study, children with hemoglobin (Hb) concentrations between 11.0 and 11.4 g/dL were classified as being in a borderline or pre-anemic stage—considered at elevated risk of developing anemia [6,11,22,23]. Hemoglobin cut-off values for childhood anemia classification were defined based on the recent WHO guidelines [52,53,54] and were stratified by age group as follows (Table 1):

Table 1.
Hemoglobin cut-off values for anemia in children aged 6 months to 5 years, stratified by age group.
Additional definitions relevant to this study are summarized in Table 2.

Table 2.
Anemia and iron status classification based on MCV, hematocrit, serum iron, and ferritin levels in children aged 6 months to 5 years.
More details on definitions of anemia and the outcome variable can be found in the Supplementary File Table S1.
2.6. Exposure Variables
In this study, exposure variables included sociodemographic characteristics (sex, age, residence area, caregiver’s degree of kinship, caregiver’s education level, country of origin, and parental occupation) and nutritional characteristics (history of breastfeeding, complementary feeding, and intake of food groups). According to the WHO, minimum dietary diversity (MDD) is considered adequate when children aged 6–23 months consume at least five out of eight food groups in a 24 h period [61,62]. However, for children aged 24 months to 5 years, no official WHO threshold exists. Therefore, we applied an adapted cut-off of four or more food groups to define dietary diversity for children aged 6 months to 5 years, following criteria from previous studies [63,64,65,66]. In this study, food consumed the previous day was classified into the following seven food groups, based on WHO and Portuguese dietary guidelines [61,67]: (1) cereals and derivatives and tubers; (2) meat, fish, and eggs; (3) dairy products; (4) fruits; (5) legumes; (6) vegetables; (7) fats and oils. The dietary diversity score (DDS) was calculated as the number of food groups consumed during the previous day, with a DDS ≥ 4 considered “adequate” and <4 considered “inadequate” [63,64,65,66].
Exposure variables also included anthropometric characteristics (weight percentiles) [68] and other characteristics (vomiting or refusal to eat, presence of infections or inflammatory conditions, glucose and bilirubin levels, uremia, hospitalization, and duration of hospitalization). More details on exposure variables can be found in Supplementary File Table S1.
2.7. Data Analysis
Data were analyzed using SPSS 28.0 software (International Business Machine Corporation [IBM Corp] based in Armonk, New York, USA) [69]. Descriptive statistics, including frequency distributions and cross-tabulations, were performed to summarize the characteristics of study participants and key variables. As this was a purely descriptive study aimed at characterizing the profiles of children diagnosed with or at risk of anemia—without the inclusion of a control group—no inferential statistical analyses were conducted. As such, p-values were not reported, as the study did not assess statistical significance.
2.8. Ethical Considerations
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee for Health of the Central Lisbon University Hospital Center (CES-CHULC) (CES 947/2020, first approval on 19 November 2020; with the second approval extension on 3 April 2024). This research is also described as a sub-study that integrates the study protocol on the same topic (PAMC, Ref. Of 0110/CC/2020) approved by the Institutional Committee of Bioethics in Health of the Faculty of Medicine/Maputo Central Hospital (CIBS FM&HCM; HCM/004/2020, first approval on 30 March 2020, with the fourth approval extension granted on 29 February 2024).
3. Results
3.1. Sociodemographic Characteristics
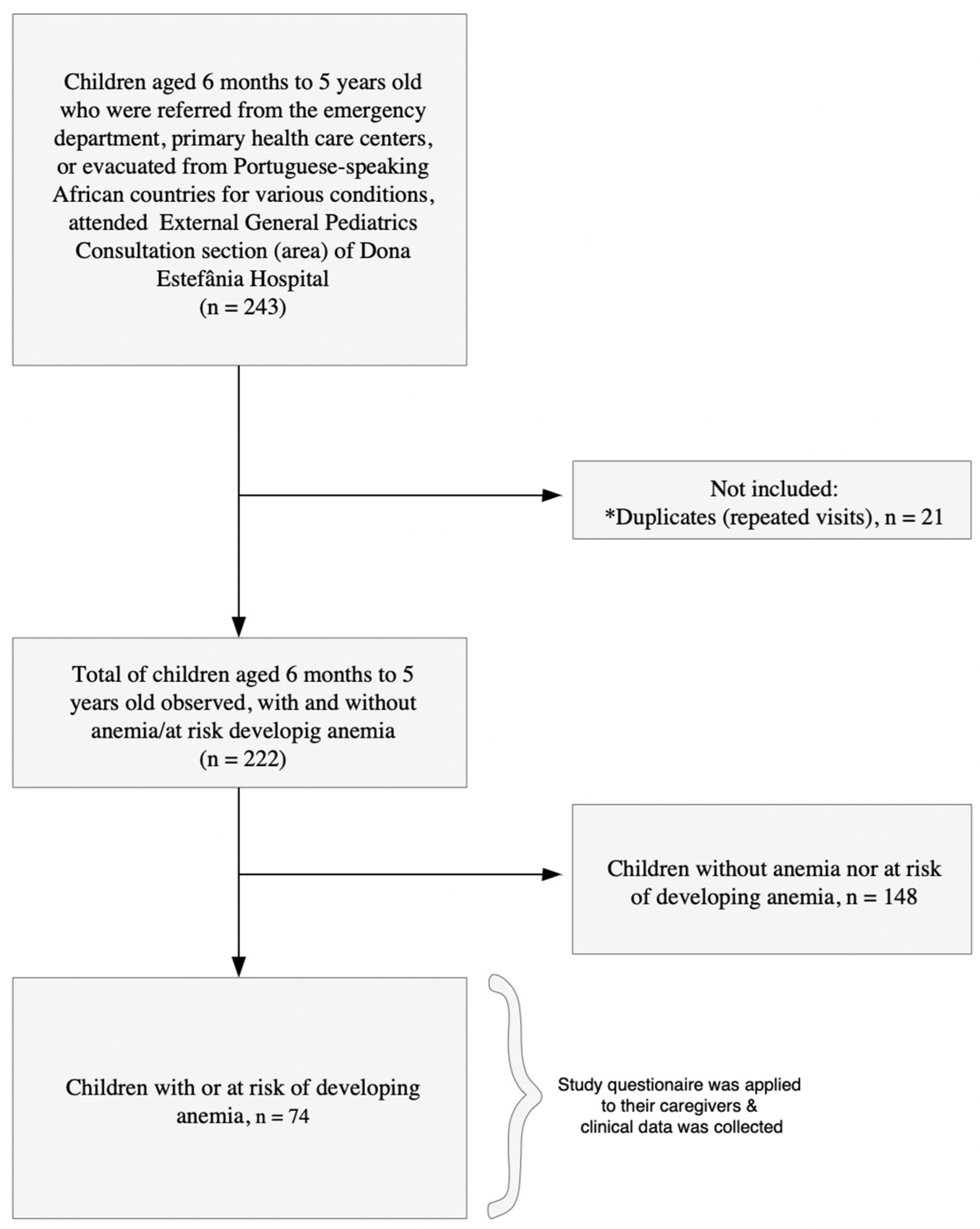
A total of 222 children, aged 6 months to 5 years, who were referred from the emergency department, primary health care centers, or evacuated from Portuguese-speaking African countries for various conditions attended external general pediatric consultations between September 2023 and September 2024. Among them, 33.3% (74/222) children had anemia or were at risk of developing anemia, and their caregivers were invited to participate. All caregivers agreed to participate, resulting in a 100% acceptance rate (Figure 1). The reasons for consultation are detailed in Supplementary Files Tables S2 and S3. Among the 74 children, anemia-related reasons (including anemia combined with other diagnoses) accounted for 60.8% of consultations (45/74). Other clinical presentations (e.g., intestinal failure, abdominal pain, malformations) accounted for the remaining 39.2% (29/74).
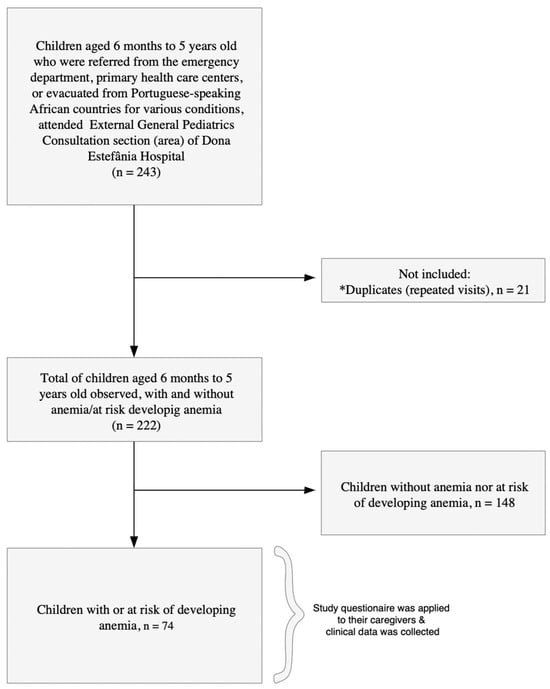
Figure 1.
Flowchart of the study population. (*) Indicates repeated visits by children to the consultations.
Table 3 summarizes the sociodemographic characteristics of children with or at risk of developing anemia. Over half of the participants were male (42/74; 56.8%). The majority of children were aged 24 months to 5 years (46/74; 62.2%), lived in the Metropolitan Lisbon area (61/74; 82.4%), and were accompanied by their mothers to the consultations (63/74; 85.1%).

Table 3.
Sociodemographic characteristics of children aged 6 months–5 years at Dona Estefânia Hospital, PAMC, September 2023–September 2024.
3.2. Childhood Anemia by Sociodemographic Characteristics
Out of the 74 children included in our study, 93.2% (69/74) were confirmed to be anemic or at risk of developing anemia based on hemoglobin (Hb) levels using age-specific cut-offs (Table 4). Five children (6.8%) had Hb values within the normal range. Although they did not meet the criteria for anemia based on Hb, these children exhibited altered red-cell indices, including microcytic (60.0%; 3/5) and normocytic (40.0%; 2/5) patterns (Supplementary File Table S4). Our results indicate that anemia cases in children aged 6 months to 5 years, measured by hemoglobin levels, were slightly higher in males (55.1%; 38/69) (Table 4). The majority of cases occurred in children residing in the Metropolitan Lisbon Area (82.6%; 57/69). Regarding caregiver characteristics, more than half of anemia cases were observed in children whose caregivers had a basic or secondary level of education (58.0%; 40/69). Children whose mothers were from CPLP countries (48.4%; 30/62) accounted for a higher proportion of cases of anemia compared to their peers.

Table 4.
Sociodemographic distribution of anemia cases and hemoglobin (Hb) levels among children aged 6 months to 5 years.
3.3. Childhood Anemia by Nutritional and Clinical Characteristics
Table 5 presents the distribution of anemia cases—based on hemoglobin (Hb) levels—by nutritional and clinical characteristics among children aged 6 months to 5 years. The majority of anemic children had a history of exclusive breastfeeding (82.0%; 50/61) and had received or were currently receiving complementary feeding (85.5%; 59/69). A history of food selectivity behavior—such as refusal to eat certain foods or vomiting after consumption—was reported in 15.9% (11/69) of cases. Most anemic children (81.2%; 56/69) had an adequate dietary diversity score (DDS ≥ 4). Among children with anemia, 87.1% (27/31) had an adequate weight-for-age percentile. Additionally, elevated C-reactive protein (CRP) levels—suggestive of infection or inflammation—were present in 34.9% (15/43) of cases, while 65.1% (28/43) had normal CRP values.

Table 5.
Distribution of anemia cases by nutritional and clinical characteristics among children aged 6 months to 5 years.
3.4. Hematological and Iron Status Characteristics of Anemic Children
Table 6 presents the hematological and iron status characteristics of children who were anemic or at risk based on hemoglobin (Hb) levels. Two-thirds (63.1%; 41/65) of the children presented microcytic indices, particularly those aged 24 months to 5 years. Serum iron levels showed that 32.0% (8/25) of children were in the pre-anemic stage, and 40.0% (10/25) were anemic. Ferritin levels suggested iron deficiency anemia in 22.2% (4/18) of children, especially in those aged 24–59 months. Sickle cell trait was identified in a minority of cases (33.3%; 3/9), all within the older age group.

Table 6.
Hematological and iron status characteristics of children anemic or at risk, by age group.
4. Discussion
This study characterized the profile of children aged 6 months to 5 years who attended the Dona Estefânia Hospital (HDE) in Lisbon, Portugal, and were diagnosed with or considered at risk of anemia. Our results showed that the overall prevalence of anemia among children in this age group was 33.3%, indicating that anemia poses a moderate public health concern [70,71]. Among those with anemia or at risk in our study sample, 93.2% exhibited either established anemia or altered red-cell indices, including microcytic and normocytic patterns, reflecting a substantial burden of disease within this subgroup. The prevalence reported in our study (33.3%) exceeds both the national (14.3%) and broader European region estimates (20.3–22.0%) [37,40]. It is also slightly higher than rates reported by other authors in various regions of Brazil (23.1%) [72] and in Timor-Leste (31.3%) [73]. However, it remains lower than those found in the Community of Portuguese Language Countries (CPLP), namely, in Brazil (38.1–56.6%) [74,75,76], Angola (44.4%) [77], Cape Verde (51.8%) [78], São Tomé and Príncipe (83.0%) [79], Equatorial Guinea (85.0%) [80], and in Mozambique (62.2–83.0%) [81,82,83].
The high rates of anemia reported in our study should be interpreted in the light of its unique sociodemographic characteristics. As such, these rates might be partly explained by the considerable portion of children with backgrounds from the CPLP and African regions, where anemia and hemoglobinopathies are more prevalent [84]. Additionally, these variations in anemia prevalence might also be attributed to a combination of geographical disparities (e.g., differences in climate), cultural factors (e.g., food practices), nutritional factors (e.g., dietary habits and food intake), genetic factors (e.g., inherited blood disorders), and socioeconomic context (e.g., caregivers’ education level and access to health facilities) [16,43,85,86,87].
4.1. Sociodemographic Patterns in Childhood Anemia
Our results indicate that anemia rates were slightly higher in male children (55.1%). Consistent with our findings, several studies [16,88,89,90] have shown that boys are generally more susceptible to anemia than girls. This may be attributed to increased prenatal and postnatal growth in males, which elevates their micronutrient requirements—often not fulfilled through diet alone [88,90].
Similar to previous studies [91,92,93], we found higher anemia rates (58.0%) among children whose caregivers had a lower level of education—a factor commonly associated with reduced health literacy and poorer health outcomes. In addition, the predominance of anemia in children whose mothers were from CPLP countries (48.4%) in our study sample reinforces the role of migration and socioeconomic disadvantage. Some studies [43] have shown that anemia plays a more significant role in disability and life imbalances in pregnant women and children under five years of age in CPLP countries than in Portugal. Nonetheless, in recent years, the burden of anemia and hemoglobinopathies in Portugal has been exacerbated by migration from CPLP countries [94]. Some authors [95] state that immigrant mothers often may carry pre-existing conditions common in their countries of origin (e.g., anemia and hemoglobinopathies) that are less frequent in host countries like Portugal. These women often request and receive less antenatal and postnatal care during and after pregnancy, affecting both their own health and that of their children.
In our study, the Metropolitan Lisbon Area (MLA) accounted for 82.6% of anemic cases. This aligns with a study [84] conducted in two of the MLAs (Amadora and Sintra), which reported high rates of anemia (30.1%) among migrant children and adolescents.
Thus, in line with previous studies [84], we emphasize the need for systematic hemoglobin screening among migrant children from African countries, where hemoglobinopathies are common, due to the importance of the disease in the Portuguese population. Broader socioeconomic strategies [96], nutrition education programs targeting caregivers with lower education levels [97], and strengthening the training of healthcare professionals in multicultural care [95] could be crucial for mitigating childhood anemia in this setting. Nationwide strategies for the prevention and management of anemia in Portugal, which integrate nutrition education, particularly for pregnant women and caregivers of young children [41,42], may play a vital role in addressing this public health issue. Further research on the relationship between socioeconomic disparities and childhood anemia is needed to inform future strategies and interventions.
4.2. Nutritional Characteristics in Childhood Anemia
In our study, we observed that 82.0% of anemic children had a history of exclusive breastfeeding up to six months of age, and 85.5% had received or were currently receiving complementary feeding. This highlights a critical window during the weaning period. This result can be explained by the fact that if exclusive breastfeeding occurs alongside low iron stores due to maternal anemia, it might not be sufficient to meet the growing iron requirements of the infant and can lead to iron depletion if not supplemented adequately after six months of age [10,11,98,99].
The quality and timing of complementary feeding may have influenced iron status, as the majority of anemia cases in our study sample (81.2%) occurred despite adequate dietary diversity scores (DDS ≥ 4). This finding suggests that dietary diversity alone may not adequately reflect iron intake, especially if iron-rich or fortified foods are not included in the child’s diet [83,98]. Food selectivity behaviors, reported in 15.9% of cases, may further contribute to inadequate iron intake. Although not predominant, these behaviors align with studies suggesting that feeding difficulties and sensory-related food aversions can hinder nutritional adequacy [15,16,100].
4.3. Clinical and Hematological Parameters in Childhood Anemia
Our findings showed that the majority of children exhibited microcytic red cell indices (63.1%), particularly among those aged 24 months to 5 years. This pattern is often caused by iron deficiency, which was corroborated by serum iron and ferritin levels in our study: 40.0% of children had iron-deficiency anemia based on serum iron, and 22.2% was based on ferritin. Approximately 33.3% of tested children carried the sickle cell trait. In line with previous studies [94], our results emphasize the importance of systematic hemoglobin screening among migrant children from African countries. In addition, 35.0% of anemic children had elevated C-reactive protein (CRP), suggesting that anemia of inflammation may contribute in about one-third of cases. This highlights the need for differential diagnosis, as inflammatory processes can mask iron-deficiency anemia and make difficult the interpretation of ferritin levels [12,13].
4.4. Strengths and Limitations
To our knowledge, this is the first hospital-based study in Lisbon to characterize children aged 6 months to 5 years who are diagnosed with or at risk of anemia. Given the limited existing research on this issue in Portugal, our study can serve as a baseline for future research (whether analytical cross-sectional or longitudinal) focused on the role of feeding practices, dietary habits, nutritional adequacy, and other potential influencing determinants, such as cultural, socioeconomic, and metabolic aspects, on childhood anemia in Portugal. In addition, by providing valuable evidence, our study establishes a foundation for improving the prevention, management, and care of anemia in children living in Portugal.
However, there are some limitations to our study. First, due to constraints from the COVID-19 pandemic, which placed significant pressure on the National Health Service (SNS) and pediatric care in Portugal, the data collection process was impacted. Consequently, this study adopted a purely descriptive design. Our one-year study focused on characterizing the profiles of children diagnosed with or at risk of anemia and did not include children without anemia, limiting the analysis of relationships or associations between various determinants and childhood anemia. Second, our sample universe was not randomly selected, as it consisted of all children aged 6 months to 5 years old who attended the external general pediatric consultations section of Dona Estefânia Hospital and were chosen based on availability during the study period. As such, our findings should not be generalized to all pediatric consultations or patient profiles at the hospital. Third, the sociodemographic composition of our sample included a substantial proportion of anemic children with backgrounds from the Community of Portuguese Language Countries (CPLP), reflecting the multicultural composition of the hospital’s catchment area. Our findings are especially relevant in providing recent evidence on childhood anemia within this specific context in Portugal. Consequently, they cannot be fully generalized to the general pediatric Portuguese population, and some comparisons presented with population-based studies should be interpreted with caution. Fourth, dietary needs differ substantially between a 6-month-old infant beginning complementary feeding and a 5-year-old child. While the WHO provides an age-appropriate threshold for children aged 6–23 months—defining adequate dietary diversity as the consumption of at least five out of eight food groups in the previous day—no official threshold exists for children aged 24 months to 5 years [61,62]. Therefore, similar to previous studies [63,64,65,66], we applied a single threshold across the entire 6-month to 5-year age range as a methodological simplification. This decision was also necessary due to the small sample size, which limited further stratification and has been acknowledged in the interpretation of our findings. Fifth, the lack of complete biochemical data (e.g., ferritin, serum iron levels, folate, vitamin B12), which are not routinely measured, and the cross-sectional descriptive nature of the study prevents causal inference. Nonetheless, the high participation rate and diverse sample might allow generalizability within similar urban pediatric populations in Portugal.
Therefore, future studies aiming at longitudinal monitoring, integrating qualitative data on caregiver beliefs and feeding practices, and exploring anemia associations with various determinants can contribute to providing more generalizable results and enhance our understanding of the factors influencing anemia in children in Portugal.
5. Conclusions
This study describes anemia among children aged 6 months to 5 years attending external general pediatric consultations in Portugal. Based on our findings, anemia poses a moderate public health concern among young children, particularly in populations where caregivers—especially mothers—have lower levels of education and are migrants. While dietary diversity was generally adequate, iron intake may still be insufficient, requiring further investigation.
Tailored public health strategies—including systematic screening, particularly among migrant children, caregiver education, and culturally sensitive care—are essential. Further research, including analytical cross-sectional or longitudinal studies, is necessary to better understand the role of feeding practices, dietary habits, nutritional adequacy, and other potential influencing determinants such as cultural, socioeconomic, and metabolic factors in childhood anemia in this setting.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/children12070832/s1, Supplementary File Table S1. Study variables; Supplementary File Table S2. Reasons for consultation in anemic or at-risk children aged 6 months to 5 years at Dona Estefânia Hospital; Supplementary File Table S3. Reasons for consultation among children with a history of food selectivity, refusal to eat, or vomiting after eating; Supplementary File Table S4. Distribution of non-anemic children by age group, mean corpuscular volume, hematocrit, and iron status; Supplementary File Table S5. Distribution of anemia cases among children aged 6 months to 5 years by nutritional characteristics.
Author Contributions
Conceptualization: R.M.C., L.V., and I.C.; methodology: R.M.C., L.V., and I.C.; data collection: R.M.C., A.C., B.M.S., J.V., B.L.V., A.S., M.C., and F.B.C.; coordination of data collection on site: R.M.C.; software: R.M.C.; formal analysis: R.M.C., S.C., and Y.K.; investigation: R.M.C.; resources: R.M.C., L.V., and I.C.; data curation: R.M.C. and S.C.; writing—original draft preparation: R.M.C.; writing—review and editing: R.M.C., A.C., Y.K., S.C., B.M.S., J.V., B.L.V., A.S., M.C., F.B.C., L.V., and I.C.; visualization: R.M.C.; supervision: L.V. and I.C. All authors have read and agreed to the published version of the manuscript.
Funding
R.M.C., L.V., and I.C. were supported by Fundação para a Ciência e a Tecnologia for funds to GHTM-UID/04413/2020 and LA-REAL–LA/P/0117/2020. R.M.C was supported by Fundação para a Ciência e a Tecnologia for funds for her Doctoral Program (Reference UI/BD/151065/2021; DOI: https://doi.org/10.54499/UI/BD/151065/2021). The views expressed in this paper are from the authors and not the official position of the institutions or funder.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee for Health of the Central Lisbon University Hospital Center (CES-CHULC) (CES 947/2020, first approval on 19 November 2020; with the second approval extension on 3 April 2024). This research is also described as a sub-study that integrates the study protocol on the same topic (PAMC, Ref. Of 0110/CC/2020) approved by the Institutional Committee of Bioethics in Health of the Faculty of Medicine/Maputo Central Hospital (CIBS FM&HCM; HCM/004/2020, first approval on 30 March 2020, with the fourth approval extension granted on 29 February 2024).
Informed Consent Statement
Informed consent was obtained from all parents or legal guardians of the subjects involved in this study.
Data Availability Statement
Data from this study are not publicly available due to patients’ privacy and ethical restrictions. However, the data presented in this study are available in this article and its Supplementary Information files.
Acknowledgments
We sincerely thank the children’s caregivers for agreeing to participate in this study. We also thank the doctors and pediatric residents of Dona Estefânia Hospital for their support in data collection: Ana Isabel Carvalho, António Campos, Caroline Lopes, Conceição Neves, Diana Amaral, João Simões, Margarida Almendra, Mafalda Borges, Rita Machado, and Sara Ferreira. We further thank the operational assistants and “Ludoteca” personnel of Dona Estefânia Hospital: Andreia Filipa, Cátia Dias, Fernanda Pires Paulo, Claudia, Rute Gomes, Maria dos Anjos Santos, Sara Fernanda Paiva, Susana Mendes, Isabel Fernandes, and Tomé André. The first author would like to express gratitude to Francisco Merca, Ana Varão, Alexandre Azeredo, Duarte Santos, Eduardo Pedro, Diogo Gonçalves, Rui Ribeiro, Salima Rehemtula and André Gonçalves. Special thanks to Michel Jareski Andrade for his valuable support in formatting the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AIDS | Acquired immunodeficiency syndrome |
| CBC | Complete blood count |
| CPLP | Community of Portuguese Language Countries |
| CRP | C-reactive protein |
| DDS | Dietary diversity score |
| ID | Iron deficiency |
| IDA | Iron deficiency anemia |
| Hb | Hemoglobin |
| HIV | Human immunodeficiency virus |
| HDE | Dona Estefânia Hospital |
| MCV | Mean corpuscular volume |
| MDD | Mininum dietary diversity |
| RCBs | Red blood cells |
| TIBC | Iron binding capacity |
| UI | Uncertainty interval |
| ULS | Unidade Local de Saúde |
| WHO | World Health Organization |
References
- Gallagher, P.G. Anemia in the pediatric patient. Blood 2022, 140, 571–593. [Google Scholar] [CrossRef]
- Ouédraogo, O.; Compaoré, E.W.R.; Kiburente, M.; Dicko, M.H. Prevalence and Associated Factors of Anemia in Children Aged 6 to 59 Months in the Eastern Region of Burkina Faso. Glob. Pediatr. Health 2024, 11, 2333794X241263163. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Anaemia. World Health Organization. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/anaemia (accessed on 30 January 2025).
- Matysiak, M. Anemia in children: A pediatrician’s view. Acta Haematol. Pol. 2021, 52, 402–405. [Google Scholar] [CrossRef]
- Lopes, A.I.; Azevedo, S.; Cabral, J.; Ferreira, M.G.; Sande-Lemos, P.; Ferreira, R.; Trindade, E.; Lima, R.; Antunes, H. Portuguese Consensus on Diagnosis, Treatment, and Management of Anemia in Pediatric Inflammatory Bowel Disease. GE Port. J. Gastroenterol. 2020, 27, 244–254. [Google Scholar] [CrossRef]
- Gelaw, Y.; Getaneh, Z.; Melku, M. Anemia as a risk factor for tuberculosis: A systematic review and meta-analysis. Environ. Health Prev. Med. 2021, 26, 13. [Google Scholar] [CrossRef]
- Thompson, L.; Arnold, C.; Peerson, J.; Long, J.M.; EWestcott, J.L.; Islam, M.M.; Black, R.E.; Krebs, N.F.; McDonald, C.M. Predictors of Anaemia Among Young Children Receiving Daily Micronutrient Powders (MNPs) for 24 Weeks in Bangladesh: A Secondary Analysis of the Zinc in Powders Trial. Matern. Child Nutr. 2025, 21, e13806. [Google Scholar] [CrossRef]
- Braat, S.; Fielding, K.L.; Han, J.; Jackson, V.E.; Zaloumis, S.; Xu, J.X.H.; Moir-Meyer, G.; Blaauwendraad, S.M.; Jaddoe, V.W.V.; Gaillard, R.; et al. Haemoglobin thresholds to define anaemia from age 6 months to 65 years: Estimates from international data sources. Lancet Haematol. 2024, 11, e253–e264. [Google Scholar] [CrossRef]
- King, F.S.; Burgess, A.; Quinn, V.J.; Osei, A.J. Nutrition for Developing Countries, 3rd ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Qiu, Y.; Long, Z.; Long, Z. Epidemiology of dietary iron deficiency in China from 1990 to 2021: Findings from the global burden of disease study 2021. BMC Public Health 2025, 25, 596. [Google Scholar] [CrossRef]
- Chaparro, C.M.; Suchdev, P.S. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef]
- Brittenham, G.M.; Moir-Meyer, G.; Abuga, K.M.; Datta-Mitra, A.; Cerami, C.; Green, R.; Pasricha, S.-R.; Atkinson, S.H. Biology of Anemia: A Public Health Perspective. J. Nutr. 2023, 153, S7–S28. [Google Scholar] [CrossRef]
- USAID Advancing Nutrition. Understanding Anemia and Its Coexisting Factors: A Brief. USAID. 2022. Available online: https://www.usaid.gov/ (accessed on 2 April 2025).
- Martins, R.R.; Paixão, F.; Mendes, I.; Schäfer, S.; Monge, I.; Costa, F.; Correia, P. Intestinal Parasitic Infections in Children: A 10-Year Retrospective Study. Cureus 2024, 16, e75862. [Google Scholar] [CrossRef] [PubMed]
- Shimanda, P.P.; Amukugo, H.J.; Norström, F. Socioeconomic factors associated with anemia among children aged 6-59 months in Namibia. J. Public Health Afr. 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Melku, M.; Alene, K.A.; Terefe, B.; Enawgaw, B.; Biadgo, B.; Abebe, M.; Muchie, K.F.; Kebede, A.; Melak, T.; Melku, T. Anemia severity among children aged 6–59 months in Gondar town, Ethiopia: A community-based cross-sectional study. Ital. J. Pediatr. 2018, 44, 107. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, X.; Zha, P. Trends in Socioeconomic Inequalities and Prevalence of Anemia Among Children and Nonpregnant Women in Low- and Middle-Income Countries. JAMA Netw. Open 2018, 1, e182899. [Google Scholar] [CrossRef]
- Osborne, A.; Adeleye, K.; Bangura, C.; Wongnaah, F.G. Trends and inequalities in anaemia prevalence among children aged 6–59 months in Ghana, 2003–2022. Int. J. Equity Health 2024, 23, 231. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, M.; Wu, T.; Li, J.; Shi, H.; Wei, Y. The association between maternal anemia and neonatal anemia: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2024, 24, 677. [Google Scholar] [CrossRef]
- Chandran, V.; Kirby, R.S. An Analysis of Maternal, Social and Household Factors Associated with Childhood Anemia. Int. J. Environ. Res. Public Health 2021, 18, 3105. [Google Scholar] [CrossRef]
- Sowe, A.; Wood, E.; Gautam, S.K. Maternal Anemia as a Predictor of Childhood Anemia: Evidence from Gambian Health Data. Nutrients 2025, 17, 879. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. World Health Organization. 2011. Available online: https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1 (accessed on 2 April 2025).
- Williams, A.M.; Ansai, N.; Ahluwalia, N.; Nguyen, D.T. Anemia Prevalence: United States, August 2021–August 2023 (NCHS Data Brief No. 519). National Center for Health Statistics. 2024. Available online: https://www.cdc.gov/nchs/products/databriefs/db519.htm (accessed on 2 April 2025).
- Leung, A.K.; Lam, J.M.; Wong, A.H.; Hon, K.L.; Li, X. Iron Deficiency Anemia: An Updated Review. Curr. Pediatr. Rev. 2024, 20, 339–356. [Google Scholar] [CrossRef]
- Janus, J.; Moerschel, S.K. Evaluation of anemia in children. Am. Fam. Physician 2010, 81, 1462–1471. [Google Scholar]
- Ngnie-Teta, I.; Receveur, O.; Kuate-Defo, B. Risk Factors for Moderate to Severe Anemia among Children in Benin and Mali: Insights from a Multilevel Analysis. Food Nutr. Bull. 2007, 28, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.K.; Pullum, T.W.; Riese, S.; Milner, E.; Navaneetham, K. Is child anemia associated with early childhood development? A cross-sectional analysis of nine Demographic and Health Surveys. PLoS ONE 2024, 19, e0298967. [Google Scholar] [CrossRef] [PubMed]
- Maner, B.S.; Killeen, R.B.; Moosavi, L. Mean corpuscular volume. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545275/ (accessed on 5 April 2025).
- Turner, J.; Parsi, M.; Badireddy, M. Anemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499994/ (accessed on 5 April 2025).
- Yeboah, F.A.; Bioh, J.; Amoani, B.; Effah, A.; Senu, E.; Mensah, O.S.O.; Agyei, A.; Kwarteng, S.; Agomuo, S.K.S.; Opoku, S.; et al. Iron deficiency anemia and its association with cognitive function among adolescents in the Ashanti Region—Ghana. BMC Public Health 2024, 24, 3209. [Google Scholar] [CrossRef]
- Irwin, J.J.; Kirchner, J.T. Anemia in children. Am. Fam. Physician 2001, 64, 1379–1386. [Google Scholar]
- Chaudhry, H.S.; Kasarla, M.R. Microcytic hypochromic anemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470252/ (accessed on 29 April 2025).
- Sarbay, H.; Ay, Y. Evaluation of children with macrocytosis: Clinical study. Pan Afr. Med. J. 2018, 31, 54. [Google Scholar] [CrossRef]
- Yilmaz, G.; Shaikh, H. Normochromic normocytic anemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK565880/ (accessed on 29 April 2025).
- Moore, C.A.; Killeen, R.B.; Adil, A. Macrocytic anemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459295/ (accessed on 29 April 2025).
- Gardner, W.M.; Razo, C.; McHugh, T.A.; Hagins, H.; Vilchis-Tella, V.M.; Hennessy, C.; Taylor, H.J.; Perumal, N.; Fuller, K.; Cercy, K.M.; et al. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990–2021: Findings from the Global Burden of Disease Study 2021. Lancet Haematol. 2023, 10, e713–e734. [Google Scholar] [CrossRef]
- Stevens, G.A.; Paciorek, C.J.; Flores-Urrutia, M.C.; Borghi, E.; Namaste, S.; Wirth, J.P.; Suchdev, P.S.; Ezzati, M.; Rohner, F.; Flaxman, S.R.; et al. National, regional, and global estimates of anaemia by severity in women and children for 2000–2019: A pooled analysis of population-representative data. Lancet Glob. Health 2022, 10, e627–e639. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. WHO/NMH/NHD/MNM/11.1. 2015. Available online: https://iris.who.int/bitstream/handle/10665/177094/9789241564960_eng.pdf (accessed on 12 March 2025).
- Nunes, A.R.; Mairos, J.; Brilhante, D.; Marques, F.; Belo, A.; Cortez, J.; Fonseca, C. Screening for Anemia and Iron Deficiency in the Adult Portuguese Population. Anemia 2020, 2020, 1048283. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Observatory Data Repository: By Category, Child Malnutrition, Anaemia in Children; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Fonseca, C.; Marques, F.; Nunes, A.R.; Belo, A.; Brilhante, D.; Cortez, J. Prevalence of anaemia and iron deficiency in Portugal: The EMPIRE study. Intern. Med. J. 2016, 46, 470–478. [Google Scholar] [CrossRef]
- Marques, F.; Fonseca, C.; Robalo Nunes, A.; Belo, A.; Brilhante, D.; Cortez, J. Contextualising the High Prevalence of Anaemia in the Portuguese Population: Perception, Characterisation and Predictors: An EMPIRE Sub-Study. Intern. Med. 2016, 23, 26–38. [Google Scholar] [CrossRef]
- Cane, R.M.; Chidassicua, J.B.; Varandas, L.; Craveiro, I. Anemia in Pregnant Women and Children Aged 6 to 59 Months Living in Mozambique and Portugal: An Overview of Systematic Reviews. Int. J. Environ. Res. Public Health 2022, 19, 4685. [Google Scholar] [CrossRef]
- Palaré, M.J.; Ferrão, A.; Carreira, M.; Morais, A. Défice de ferro na criança. Acta Pediátrica Port. 2004, 3, 243–247. [Google Scholar]
- Antunes, H.; Gonçalves, S.; Teixeira-Pinto, A.; Costa-Pereira, A.; Tojo-Sierra, R.; Aguiar, Á. Anemia por deficiência de ferro no lactente: Resultados preliminares do desenvolvimento aos cinco anos. Acta Médica Port. 2005, 18, 261–266. Available online: https://www.actamedicaportuguesa.com/revista/index.php/amp/article/view/1034/702 (accessed on 10 March 2025).
- Virella, D.; Pina, M.J. Prevalence of iron deficiency in early infancy. Acta Médica Port. 1998, 11, 607–613. [Google Scholar]
- Ministério dos Negócios Estrangeiros. Sobre Portugal. Portal Diplomático. 2025. Available online: https://portaldiplomatico.mne.gov.pt/sobre-portugal (accessed on 19 February 2025).
- European Union. Portugal. 2024. Available online: https://european-union.europa.eu/principles-countries-history/eu-countries/portugal_en (accessed on 6 February 2025).
- European Parliament. Economic, Social and Territorial Situation of Portugal: Briefing Requested by the REGI Committee. 2019. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2019/629190/IPOL_BRI(2019)629190_EN.pdf (accessed on 6 February 2025).
- Serviço Nacional de Saúde. Unidade Local de Saúde de São José. 2025. Available online: https://www.sns.gov.pt/entidades-de-saude/unidade-local-de-saude-de-sao-jose/ (accessed on 20 February 2025).
- Hospital Dona Estefânia. Contexto Regional e Nacional da Instituição; ULS São José: Lisbon, Portugal, 2025. [Google Scholar]
- World Health Organization. Guideline on Haemoglobin Cutoffs to Define Anaemia in Individuals and Populations. 2024. Available online: https://www.who.int/publications/i/item/9789240088542 (accessed on 27 May 2025).
- World Health Organization. Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations. 2023. Available online: https://www.who.int/tools/elena/interventions/ferritin-concentrations (accessed on 27 May 2025).
- World Health Organization. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations (WHO/NMH/NHD/EPG/20.1). 2020. Available online: https://iris.who.int/bitstream/handle/10665/331505/9789240000124-eng.pdf?sequence=1 (accessed on 27 May 2025).
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. Morb. Mortal. Wkly. Rep. 1998, 47, 1–29. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00051880.htm (accessed on 27 May 2025).
- UpToDate. Approach to the Child with Anemia. 2025. Available online: https://www.uptodate.com/contents/approach-to-the-child-with-anemia?search=Approach%20to%20the%20child%20with%20anemia&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 27 May 2025).
- North Bristol NHS Trust. Children’s Reference Ranges for Routine Haematology Tests [PDF]. 2025. Available online: https://www.nbt.nhs.uk/sites/default/files/Childrens%20FBC%20Reference%20Ranges.pdf (accessed on 27 May 2025).
- Staples, A.O.; Wong, C.S.; Smith, J.M.; Gipson, D.S.; Filler, G.; Warady, B.A.; Martz, K.; Greenbaum, L.A. Anemia and Risk of Hospitalization in Pediatric Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 48–56. [Google Scholar] [CrossRef]
- World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention and Control: A Guide for Programme Managers. WHO/NHD/01.3. World Health Organization. 2001. Available online: https://www.who.int/publications/m/item/iron-children-6to23--archived-iron-deficiency-anaemia-assessment-prevention-and-control (accessed on 27 May 2025).
- University of Iowa Health Care. Pediatric Reference Ranges. University of Iowa Health Care. 2025. Available online: https://www.healthcare.uiowa.edu/path_handbook/appendix/heme/pediatric_normals.html (accessed on 27 May 2025).
- World Health Organization. Prevalence of Anemia Among Children Under 5 Years (%). Global Health Observatory (GHO) Data. 2025. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/7042 (accessed on 27 May 2025).
- Raru, T.B.; Merga, B.T.; Mulatu, G.; Deressa, A.; Birhanu, A.; Negash, B.; Gamachu, M.; Regassa, L.D.; Ayana, G.M.; Roba, K.T. Minimum Dietary Diversity Among Children Aged 6–59 Months in East Africa Countries: A Multilevel Analysis. Int. J. Public Health 2023, 68, 1605807. [Google Scholar] [CrossRef]
- Paulo, H.A.; Andrew, J.; Luoga, P.; Omary, H.; Chombo, S.; Mbishi, J.V.; Addo, I.Y. Minimum dietary diversity behaviour among children aged 6 to 24 months and their determinants: Insights from 31 Sub-Saharan African (SSA) countries. BMC Nutr. 2024, 10, 160. [Google Scholar] [CrossRef]
- Lencha, F.M.; Zaza, Z.J.; Digesa, L.E.; Ayana, T.M. Minimum dietary diversity and associated factors among children under the age of five attending public health facilities in Wolaita Soddo town, Southern Ethiopia, 2021: A cross-sectional study. BMC Public Heal 2022, 22, 2368. [Google Scholar] [CrossRef]
- Bliznashka, L.; Perumal, N.; Yousafzai, A.; Sudfeld, C. Diet and development among children aged 36–59 months in low-income countries. Arch. Dis. Child. 2021, 107, 719–725. [Google Scholar] [CrossRef]
- Woldegebriel, A.G.; Desta, A.A.; Gebreegziabiher, G.; Berhe, A.A.; Ajemu, K.F.; Woldearegay, T.W. Dietary Diversity and Associated Factors among Children Aged 6-59 Months in Ethiopia: Analysis of Ethiopian Demographic and Health Survey 2016 (EDHS 2016). Int. J. Pediatr. 2020, 2020, 3040845. [Google Scholar] [CrossRef] [PubMed]
- Direção-Geral da Saúde. Roda dos Alimentos. Programa Nacional para a Promoção da Alimentação Saudável. 2024. Available online: https://alimentacaosaudavel.dgs.pt/roda-dos-alimentos (accessed on 27 May 2025).
- World Health Organization. Child Growth Standards. World Health Organization. 2025. Available online: https://www.who.int/tools/child-growth-standards/standards (accessed on 15 March 2025).
- IBM Corp. IBM SPSS Statistics for Windows, Version 28.0; IBM Corp: Armonk, NY, USA, 2021. [Google Scholar]
- World Health Organization. Haemoglobin Cutoffs to Define Anaemia in Individuals and Populations. Guideline Central. 2024. Available online: https://www.guidelinecentral.com/guideline/3534081/#section-3534102 (accessed on 20 March 2025).
- World Health Organization. Nutrition Landscape Information System (Nlis). Anaemia. Nutrition and Nutrition-Related Health and Development Data. Geneva, Switzerland: WHO. 2023. Available online: https://www.who.int/data/nutrition/nlis/info/anaemia (accessed on 20 March 2025).
- da Silva, L.L.S.; Fawzi, W.W.; Cardoso, M.A.; ENFAC Working Group; Connor, J.R. Factors associated with anemia in young children in Brazil. PLoS ONE 2018, 13, e0204504. [Google Scholar] [CrossRef] [PubMed]
- Agho, K.E.; Dibley, M.J.; D’Este, C.; Gibberd, R. Factors Associated with Haemoglobin Concentration among Timor-Leste Children Aged 6–59 Months. J. Health Popul. Nutr. 2008, 26, 200–209. [Google Scholar]
- Ferreira, H.S.; Vieira, R.C.S.; Livramento, A.R.S.; Dourado, B.L.L.; Silva, G.F.; Calheiros, M.S.C. Prevalence of anaemia in Brazilian children in different epidemiological scenarios: An updated meta-analysis. Public Health Nutr 2020, 24, 2171–2184. [Google Scholar] [CrossRef]
- dos Santos, R.F.; Gonzalez, E.S.C.; de Albuquerque, E.C.; de Arruda, I.K.G.; Diniz, A.d.S.; Figueroa, J.N.; Pereira, A.P.C. Prevalence of anemia in under five-year-old children in a children's hospital in Recife, Brazil. Rev. Bras. Hematol. Hemoter. 2010, 33, 100–104. [Google Scholar] [CrossRef]
- Oliveira, C.S.M.; Cardoso, M.A.; Araújo, T.S.; Muniz, P.T. Anemia em crianças de 6 a 59 meses e fatores associados no Município de Jordão, Estado do Acre, Brasil. Cad. Saúde Pública 2011, 27, 1008–1020. [Google Scholar] [CrossRef]
- Fançony, C.; Lavinha, J.; Brito, M.; Barros, H. Anemia in preschool children from Angola: A review of the evidence. Porto Biomed. J. 2020, 5, e60. [Google Scholar] [CrossRef]
- Semedo, R.M.; Santos, M.M.; Baião, M.R.; Luiz, R.R.; Da Veiga, G.V. Prevalence of Anaemia and Associated Factors among Children below Five Years of Age in Cape Verde, West Africa. J. Health Popul. Nutr. 2014, 32, 646–657. [Google Scholar]
- Silva, C.C.; Catarino, E. Malnutrition and anemia in children aged 6 to 59 months in the Autonomous Region of Príncipe and its relation to maternal health. Popul. Med. 2023, 5, 164213. [Google Scholar] [CrossRef]
- Ncogo, P.; Romay-Barja, M.; Benito, A.; Aparicio, P.; Nseng, G.; Berzosa, P.; Santana-Morales, M.A.; Riloha, M.; Valladares, B.; Herrador, Z.; et al. Prevalence of anemia and associated factors in children living in urban and rural settings from Bata District, Equatorial Guinea, 2013. PLoS ONE 2017, 12, e0176613. [Google Scholar] [CrossRef]
- Aly, M.M.; Berti, C.; Chemane, F.; Macuelo, C.; Marroda, K.R.; La Vecchia, A.; Agostoni, C.; Baglioni, M. Prevalence of anemia among children aged 6–59 months in the Ntele camp for internally displaced persons (Cabo Delgado, Mozambique): A preliminary study. Eur. J. Clin. Nutr. 2024, 79, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Cane, R.M.; Keita, Y.; Lambo, L.; Pambo, E.; Gonçalves, M.P.; Varandas, L.; Craveiro, I. Prevalence and factors related to anaemia in children aged 6–59 months attending a quaternary health facility in Maputo, Mozambique. Glob. Public Health 2023, 18, 2278876. [Google Scholar] [CrossRef] [PubMed]
- Muhajarine, N.; Adeyinka, D.A.; Matandalasse, M.; Chicumbe, S. Inequities in childhood anaemia at provincial borders in Mozambique: Cross-sectional study results from multilevel Bayesian analysis of 2018 National Malaria Indicator Survey. BMJ Open 2021, 11, e051395. [Google Scholar] [CrossRef]
- Ribeiro, L.C.-B.; Paixão, F.; Costa, F.; Correia, P. Migrant Pathology Screening in the Pediatric Population: A Five-Year Retrospective Study From a Level II Hospital. Cureus 2024, 16, e53770. [Google Scholar] [CrossRef]
- Akkermans, M.D.; van der Horst-Graat, J.M.; Eussen, S.R.; van Goudoever, J.B.; Brus, F. Iron and Vitamin D Deficiency in Healthy Young Children in Western Europe Despite Current Nutritional Recommendations. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 635–642. [Google Scholar] [CrossRef]
- van der Merwe, L.F.; Eussen, S.R. Iron status of young children in Europe. Am. J. Clin. Nutr. 2017, 106, 1663S–1671S. [Google Scholar] [CrossRef]
- Leal, L.P.; Filho, M.B.; de Lira, P.I.C.; Figueiroa, J.N.; Osório, M.M. Prevalence of anemia and associated factors in children aged 6-59 months in Pernambuco, Northeastern Brazil. Rev. Saude Publica 2011, 45, 457–466. [Google Scholar] [CrossRef]
- Aheto, J.M.K.; Alhassan, Y.; Puplampu, A.E.; Boglo, J.K.; Sedzro, K.M. Anemia prevalence and its predictors among children under-five years in Ghana. A multilevel analysis of the cross-sectional 2019 Ghana Malaria Indicator Survey. Health Sci. Rep. 2023, 6, e1643. [Google Scholar] [CrossRef]
- Belachew, A.; Tewabe, T. Under-five anemia and its associated factors with dietary diversity, food security, stunted, and deworming in Ethiopia: Systematic review and meta-analysis. Syst. Rev. 2020, 9, 31. [Google Scholar] [CrossRef]
- Tadesse, S.E.; Zerga, A.A.; Mekonnen, T.C.; Tadesse, A.W.; Hussien, F.M.; Feleke, Y.W.; Anagaw, M.Y.; Ayele, F.Y.; Toma, A. Burden and Determinants of Anemia among Under-Five Children in Africa: Systematic Review and Meta-Analysis. Anemia 2022, 2022, 1382940. [Google Scholar] [CrossRef]
- Mboya, I.B.; Mamseri, R.; Leyaro, B.J.; George, J.; Msuya, S.E.; Mgongo, M. Prevalence and factors associated with anemia among children under five years of age in Rombo district, Kilimanjaro region, Northern Tanzania. F1000Research 2023, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Torres, V.; Torres, N.; Davis, J.A.; Corrales-Medina, F.F. Anemia and Associated Risk Factors in Pediatric Patients. Pediatr. Health Med. Ther. 2023, 14, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Sunardi, D.; Bardosono, S.; Basrowi, R.W.; Wasito, E.; Vandenplas, Y. Dietary Determinants of Anemia in Children Aged 6–36 Months: A Cross-Sectional Study in Indonesia. Nutrients 2021, 13, 2397. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.N.; Madeira, S.; Sobral, M.A.; Delgadinho, G. Hemoglobinopatias em Portugal e a intervenção do médico de família. Rev. Port. Med. Geral E Familiar 2016, 32, 416–424. [Google Scholar] [CrossRef]
- Estrela, P. A saúde dos imigrantes em Portugal. Rev. Port. Clínica Geral 2009, 25, 45–55. [Google Scholar] [CrossRef]
- Raju, A.A.; Boddu, A.B.; Raju, D.S.S.K.; Surabhi, U.S. The prevalence and predictors of iron deficiency anemia in toddlers: A population-based study. J. Adv. Med. Pharm. 2023, 6, 648–651. [Google Scholar]
- Omer, A.; Hailu, D.; Nigusse, G.; Mulugeta, A. Magnitude and morphological types of anemia differ by age among under five children: A facility-based study. Heliyon 2022, 8, e10494. [Google Scholar] [CrossRef]
- Fatima, T.; Faridi, M.M.A.; Srivastava, G. Iron status of exclusively breastfed low-birth-weight infants born to anemic mothers and effect of maternal iron supplementation for 3 versus 6 months: A randomized double-blind placebo control trial. Front. Pediatr. 2022, 10, 880431. [Google Scholar] [CrossRef]
- Meinzen-Derr, J.K.; Guerrero, M.L.; Altaye, M.; Ortega-Gallegos, H.; Ruiz-Palacios, G.M.; Morrow, A.L. Risk of Infant Anemia Is Associated with Exclusive Breast-Feeding and Maternal Anemia in a Mexican Cohort. J. Nutr. 2006, 136, 452–458. [Google Scholar] [CrossRef]
- Caldwell, A.R.; Krause, E.K. Mealtime behaviours of young children with sensory food aversions: An observational study. Aust. Occup. Ther. J. 2021, 68, 336–344. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).