The Impact of Physical Activity on Clinical Outcomes in Children with Cystic Fibrosis: A Narrative Review
Abstract
1. Introduction
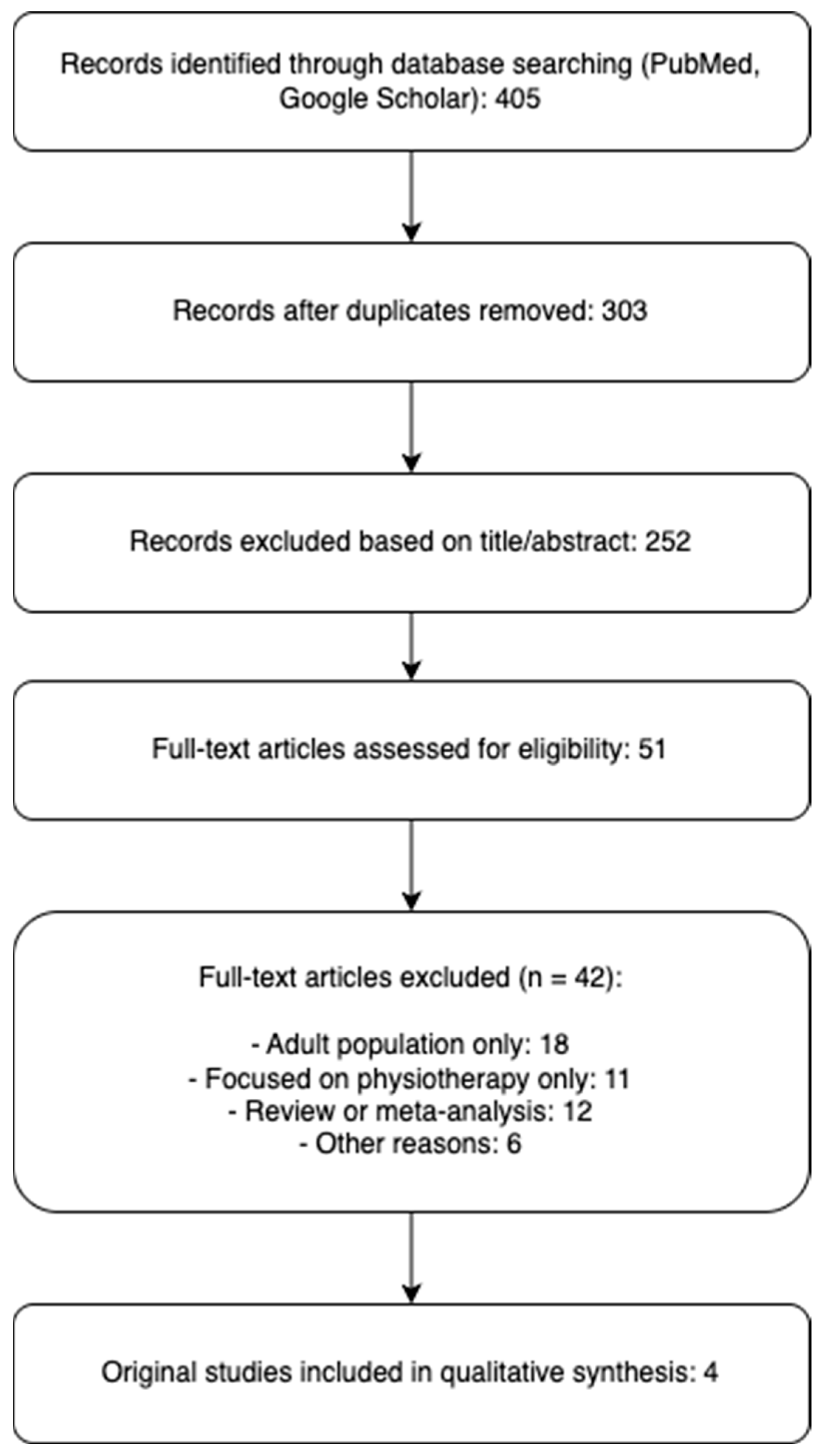
2. Materials and Methods
3. Results
4. Discussion
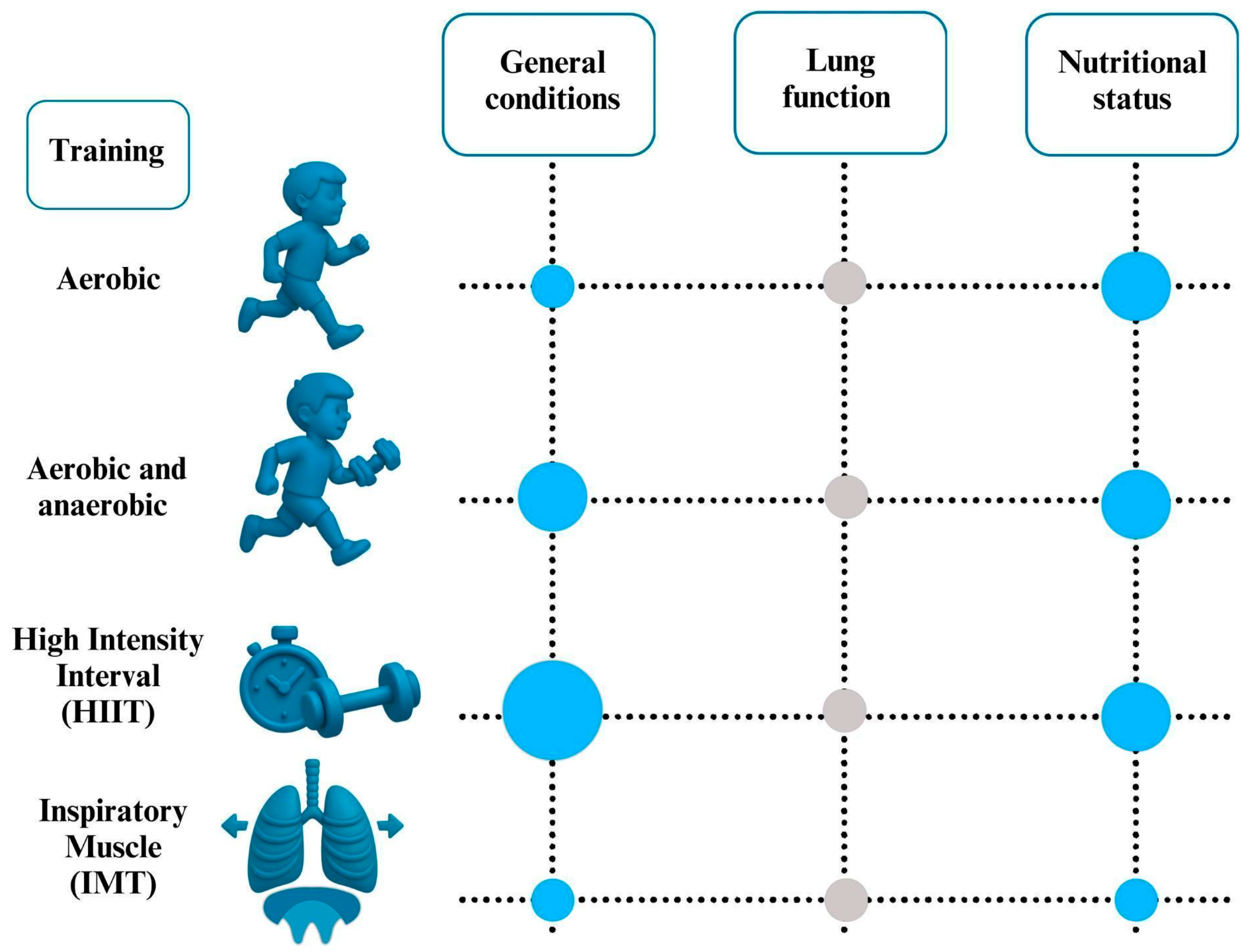
4.1. Aerobic Exercise
4.2. Combination of Aerobic and Strength/Anaerobic Training
4.3. High- Intensity Interval Training
4.4. Inspiratory Muscle Training
4.5. Close Contact and Personalized Approaches
4.6. Forced Expiratory Volume in One Second
4.7. Nutritional Status
5. Limitations
6. Conclusions and Future Perspectives
- Personalized and Tailored Interventions: future directions emphasize the need for more personalized exercise programs [19,21]. This includes considering individual fitness levels, disease severity, preferences, and barriers to participation [24]. Future research should focus on identifying which exercise programs are most effective for specific subgroups of children with CF [23,24].
- Integration of Technology: The use of wearable technology like fitness trackers and step counters, combined with motivational feedback and goal setting, is seen as a promising approach for future physical activity interventions [19,24]. Technology can help monitor activity levels, improve adherence, and provide personalized feedback.
- Impact of CFTR Modulator Therapies: The advent of highly effective CFTR modulator therapies will likely have a significant impact on how exercise is approached [22,24]. As the nutritional, respiratory, and physical status of children with CF is better preserved due to these therapies, the focus of exercise interventions might evolve to optimize overall fitness and long-term health, rather than solely compensating for disease-related limitations [22]. Research is needed to understand how these therapies influence daily physical activity and exercise behavior [24].
- Focus on Long-Term Adherence and Sustainability: Future efforts will need to prioritize strategies that promote long-term engagement in physical activity and make exercise a part of the patient’s lifestyle [19,22,23]. This may involve incorporating elements of behavior change theory and addressing factors like motivation, self-efficacy, and perceived enjoyment [22,24]. Interventions that are fun and adaptable to daily life, such as video game-based training, might play a larger role [22].
- Combining Different Training Modalities: Future studies should explore the benefits of combining strength exercise with HIIT as these have shown promising results [23] Further research is needed to determine the optimal combination and dosage of aerobic and anaerobic training for children with CF [21].
- Addressing Muscle Function: Given that exercise training can improve peripheral muscle strength in young individuals with CF [22], future research should further investigate the most effective strategies to enhance muscle function and address potential muscle dysfunction from an early age.
- Further High-Quality Research: There is a continued need for high-quality, sufficiently sized randomized controlled trials with longer follow-up periods to comprehensively assess the benefits of different exercise interventions in children with CF [21,22,23,24]. These studies should analyze a wider range of outcomes, including lung function, exercise capacity, muscle strength, nutritional status, and quality of life. Research should also investigate the dose-response relationships between physical activity and clinical outcomes [24].
- The integration of eHealth technologies, such as wearable fitness trackers and app-based feedback systems, shows promising potential in pediatric CF care. Such tools have already proven effective in pediatric asthma management by enhancing motivation, adherence, and health outcomes, as reported by van der Kamp et al. [26]. In CF, these technologies could facilitate the transition toward more autonomous, personalized activity plans. However, as shown by Berghea et al. [27], the adoption of AI-based digital tools may be influenced by parental educational level and access to resources—factors that must be addressed to ensure equitable implementation. Nonetheless, parents of children with chronic diseases like CF may show greater openness to these tools compared to other pediatric populations, representing an opportunity for early integration.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CF | Cystic Fibrosis |
| HIIT | High-Intensity Interval Training |
| IMT | Inspiratory Muscle Training |
| FEV1 | Forced Expiratory Volume in 1 s |
| RCTs | randomized controlled trials |
| BMI | body mass index |
| RET | resistance exercise training |
| FVC | forced vital capacity |
| PEF | peak expiratory flow |
| CPETs | Cardiopulmonary Exercise Tests |
| PAL | physical activity levels |
| VO2 | peak refers to the highest rate of oxygen consumption (VO2) |
| Wpeak | peak workload |
| HRQoL | Health-Related Quality of Life |
| SMD | standardized mean difference |
| low-LPA | low light physical activity |
References
- Wainwright, B.J.; Scambler, P.J.; Schmidtke, J.; Watson, E.A.; Law, H.-Y.; Farrall, M.; Cooke, H.J.; Eiberg, H.; Williamson, R. Localization of CF locus to human chromosome 7cen-q22. Nature 1985, 318, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Savant, A.P.; McColley, S.A. CF year in review 2019: Section 2 pulmonary disease and infections. Pediatr. Pulmonol. 2020, 55, 3236–3242. [Google Scholar] [CrossRef] [PubMed]
- Scaparrotta, A.; Di Pillo, S.; Attanasi, M.; Consilvio, N.P.; Cingolani, A.; Rapino, D.; Mohn, A.; Chiarelli, F. Growth failure in children with cystic fibrosis. J. Pediatr. Endocrinol. Metab. 2012, 25, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.A.; Hartl, D. CFTR: CF and beyond. Eur. Respir. J. 2014, 44, 1042–1054. [Google Scholar] [CrossRef]
- Andersen, D.H. CF of the pancreas and its relation to celiac disease: A clinical and pathologic study. Am. J. Dis. Child. 1938, 56, 344–399. [Google Scholar] [CrossRef]
- MacKenzie, T.; GiSord, A.H.; Sabadosa, K.A.; Quinton, H.B.; Knapp, E.A.; Goss, C.H.; Marshall, B.C. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: Survival analysis of the Cystic Fibrosis Foundation patient registry. Ann. Intern. Med. 2014, 161, 233–241. [Google Scholar] [CrossRef]
- Elborn, J.S. Personalised medicine for CF: Treating the basic defect. Eur. Respir. Rev. 2013, 22, 3–5. [Google Scholar] [CrossRef]
- Scotet, V.; Gutierrez, H.; Farrell, P.M. Newborn screening for CF across the globe–where is it worthwhile? Int. J. Neonatal Screen. 2020, 6, 18. [Google Scholar] [CrossRef]
- Caterini, J.E.; Ratjen, F.; Barker, A.R.; Williams, C.A.; Rendall, K.; Schneiderman, J.E.; Wells, G.D. Exercise intolerance in CF-the role of CFTR modulator therapies. J. Cyst. Fibros. 2022, 21, 282–292. [Google Scholar] [CrossRef]
- Hebestreit, H.; Kieser, S.; Junge, S.; Ballmann, M.; Schindler, C.; Schenk, T.; Posselt, H.-G.; Kriemler, S. Long-term effects of a partially supervised conditioning programme in CF. Eur. Respir. J. 2010, 35, 578–583. [Google Scholar] [CrossRef]
- Dwyer, T.J.; Alison, J.A.; McKeough, Z.J.; Daviskas, E.; Bye, P.T.P. Effects of exercise on respiratory flow and sputum properties in patients with CF. Chest 2011, 139, 870–877. [Google Scholar] [CrossRef] [PubMed]
- García, S.T.; Sánchez, M.A.G.; Cejudo, P.; Gallego, E.Q.; Dapena, J.; Jiménez, R.G.; Luis, P.C.; de Terreros, I.G. Bone health, daily physical activity, and exercise tolerance in patients with CF. Chest 2011, 140, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Prévotat, A.; Godin, J.; Bernard, H.; Perez, T.; le Rouzic, O.; Wallaert, B. Improvement in body composition following a supervised exercise-training program of adult patients with CF. Respir. Med. Res. 2019, 75, 5–9. [Google Scholar]
- Van de Weert-van Leeuwen, P.B.; Arets, H.G.M.; van der Ent, C.K.; Beekman, J.M. Infection, inflammation and exercise in CF. Respir. Res. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Burtin, C.; Hebestreit, H. Rehabilitation in patients with chronic respiratory disease other than chronic obstructive pulmonary disease: Exercise and physical activity interventions in CF and non-CF bronchiectasis. Respiration 2015, 89, 181–189. [Google Scholar] [CrossRef]
- Nixon, P.A.; Orenstein, D.M.; Kelsey, S.F.; Doershuk, C.F. The prognostic value of exercise testing in patients with CF. N. Engl. J. Med. 1992, 327, 1785–1788. [Google Scholar] [CrossRef]
- Zeren, M.; Cakir, E.; Gurses, H.N. Effects of IMT on postural stability, pulmonary function and functional capacity in children with CF: A randomised controlled trial. Respir. Med. 2019, 148, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Elbasan, B.; Tunali, N.; Duzgun, I.; Ozcelik, U. Effects of chest physiotherapy and aerobic exercise training on physical fitness in young children with CF. Ital. J. Pediatr. 2012, 38, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gruber, W.; Stehling, F.; Blosch, C.; Dillenhoefer, S.; Olivier, M.; Koerner-Rettberg, C.; Sutharsan, S.; Mellies, U.; Taube, C.; Welsner, M. Effects of a Long-Term Monitored Exercise Program on Aerobic Fitness in a Small Group of Children with CF. Int. J. Environ. Res. Public Health 2022, 19, 7923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mackintosh, K.A.; Ridgers, N.D.; Evans, R.E.; McNarry, M.A. Physical Activity and Sedentary Time Patterns in Children and Adolescents with CF and Age- and Sex-Matched Healthy Controls. J. Phys. Act. Health 2018, 15, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Benden, C.; Stevens, D.; Radtke, T. Exercise training in children and adolescents with CF: Theory into practice. Int. J. Pediatr. 2010, 2010, 670640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thorel, A.; Machefert, M.; Gillot, T.; Gravier, F.E.; Bonnevie, T.; Le Roux, P.; Medrinal, C.; Prieur, G.; Combret, Y. Effects of Exercise Training on Peripheral Muscle Strength in Children and Adolescents with CF: A Meta-Analysis. Healthcare 2022, 10, 2520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García-Pérez-de-Sevilla, G.; Yvert, T.; Blanco, Á.; Sosa Pedreschi, A.I.; Thuissard, I.J.; Pérez-Ruiz, M. Effectiveness of Physical Exercise Interventions on Pulmonary Function and Physical Fitness in Children and Adults with CF: A Systematic Review with Meta-Analysis. Healthcare 2022, 10, 2205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Radtke, T.; Smith, S.; Nevitt, S.J.; Hebestreit, H.; Kriemler, S. Physical activity and exercise training in CF. Cochrane Database Syst. Rev. 2022, 8, CD002768. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nicolson, W.B.; Bailey, J.; Alotaibi, N.Z.; Krick, S.; Lowman, J.D. Effects of Exercise on Nutritional Status in People with CF: A Systematic Review. Nutrients 2022, 14, 933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van der Kamp, M.R.; Hengeveld, V.S.; Brusse-Keizer, M.G.J.; Thio, B.J.; Taba, M. eHealth Technologies for Monitoring Pediatric Asthma at Home: Scoping Review. J. Med. Internet Res. 2023, 25, e45896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berghea, E.C.; Ionescu, M.D.; Gheorghiu, R.M.; Tincu, I.F.; Cobilinschi, C.O.; Craiu, M.; Bălgrădean, M.; Berghea, F. Integrating Artificial Intelligence in Pediatric Healthcare: Parental Perceptions and Ethical Implications. Children 2024, 11, 240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Outcomes and Conclusions | Objective | Type of Training | Study Population | Study Design | Year | Authors |
|---|---|---|---|---|---|---|
| IMT group showed greater increase in inspiratory muscle strength. IMT may benefit those with respiratory muscle weakness. | Assess IMT addition to standard physiotherapy. | Chest physiotherapy ± Inspiratory Muscle Training | N = 36, aged 8–18 years ys | RCT | 2019 | Zeren et al. [17] |
| Improved thoracic mobility, muscle endurance, and cardiovascular performance. | Evaluate impact on thoracic mobility and physical fitness. | Aerobic exercise + chest physiotherapy | N = 16, aged 5–14 years | CT | 2012 | Elbasan et al. [18] |
| Slight aerobic capacity improvements; maintained post-monitoring. Importance of therapist contact for adherence. | Investigate aerobic training effects over 12 months. | Monitored aerobic exercise program | N = 6, aged 6–14 years (mean FEV1 102%) | One-group pre-post intervention study | 2022 | Gruber et al. [19] |
| Similar activity patterns; underscores need for promoting physical activity among CF patients. | Compare activity levels between CF patients and healthy peers. | Free-living activity pattern analysis | N = 18 CF children and N = 18 controls, mean age ~12 years | CT | 2018 | Mackintosh et al. [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosolia Capasso, C.; Miniato, A.L.; Di Filippo, P.; Di Ludovico, A.; Di Pillo, S.; Chiarelli, F.; Sferrazza Papa, G.F.; Attanasi, M. The Impact of Physical Activity on Clinical Outcomes in Children with Cystic Fibrosis: A Narrative Review. Children 2025, 12, 831. https://doi.org/10.3390/children12070831
Rosolia Capasso C, Miniato AL, Di Filippo P, Di Ludovico A, Di Pillo S, Chiarelli F, Sferrazza Papa GF, Attanasi M. The Impact of Physical Activity on Clinical Outcomes in Children with Cystic Fibrosis: A Narrative Review. Children. 2025; 12(7):831. https://doi.org/10.3390/children12070831
Chicago/Turabian StyleRosolia Capasso, Chiara, Antonio Luca Miniato, Paola Di Filippo, Armando Di Ludovico, Sabrina Di Pillo, Francesco Chiarelli, Giuseppe Francesco Sferrazza Papa, and Marina Attanasi. 2025. "The Impact of Physical Activity on Clinical Outcomes in Children with Cystic Fibrosis: A Narrative Review" Children 12, no. 7: 831. https://doi.org/10.3390/children12070831
APA StyleRosolia Capasso, C., Miniato, A. L., Di Filippo, P., Di Ludovico, A., Di Pillo, S., Chiarelli, F., Sferrazza Papa, G. F., & Attanasi, M. (2025). The Impact of Physical Activity on Clinical Outcomes in Children with Cystic Fibrosis: A Narrative Review. Children, 12(7), 831. https://doi.org/10.3390/children12070831








