Tetralogy of Fallot: The Burden of Pulmonary Atresia in the NICU Set-Up: Two Case Reports and a Literature Review
Abstract
1. Introduction
2. Preoperative Management
2.1. Delivery Room Stabilization
2.2. Ductal-Dependent Circulation
2.3. Hemodynamic Considerations
2.4. Ventilatory Management
2.5. Preoperative Nutritional Management
3. Operative Timing
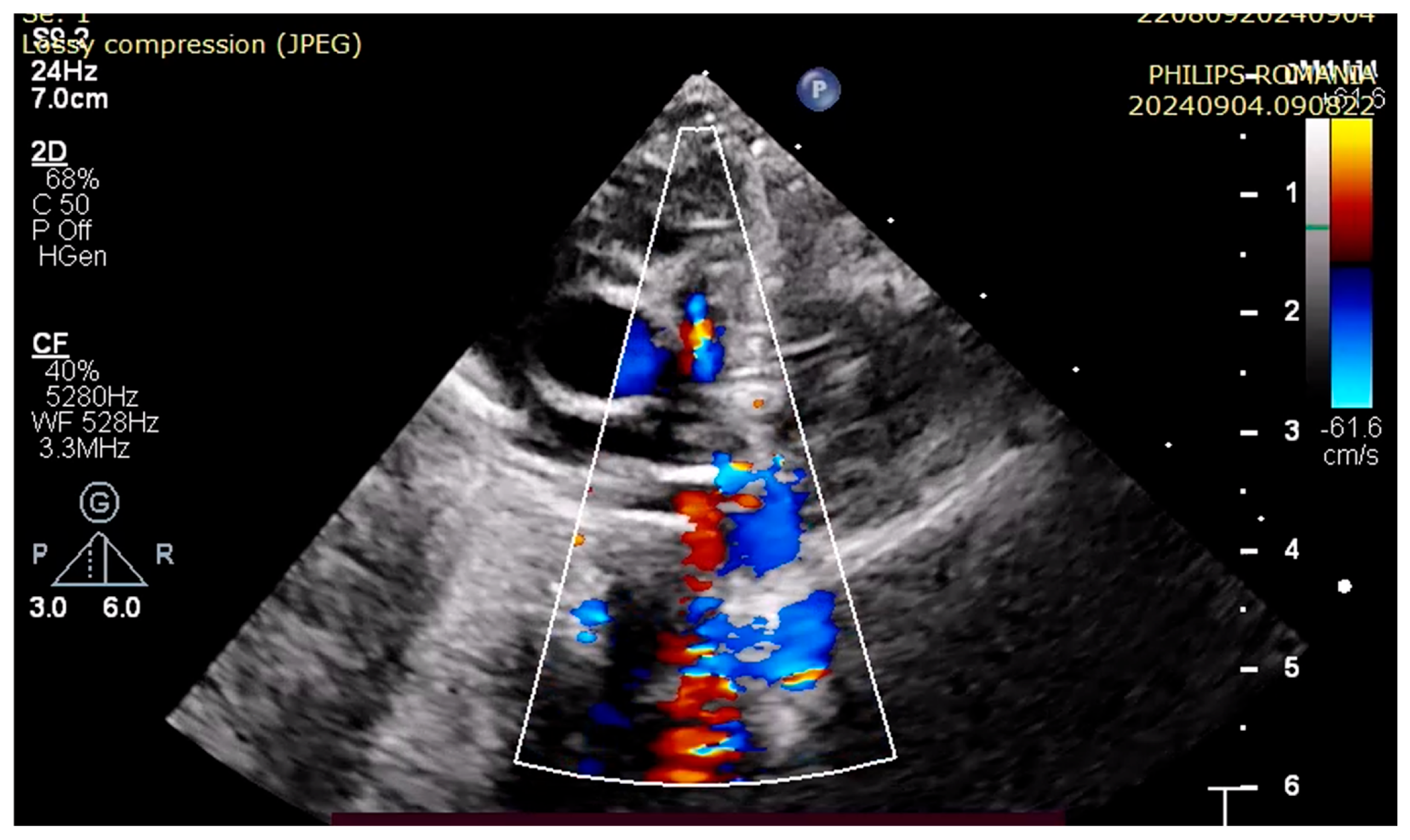
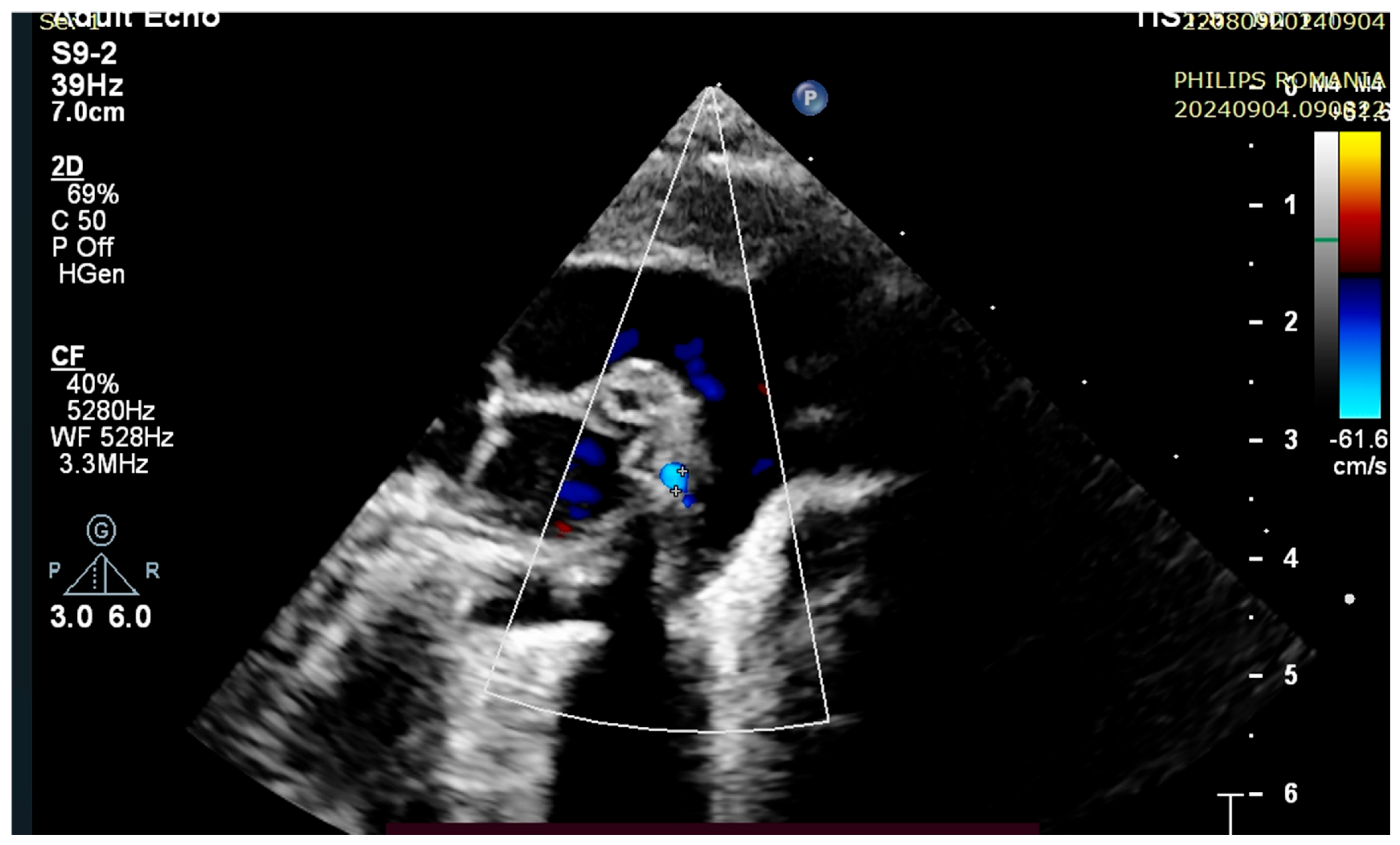
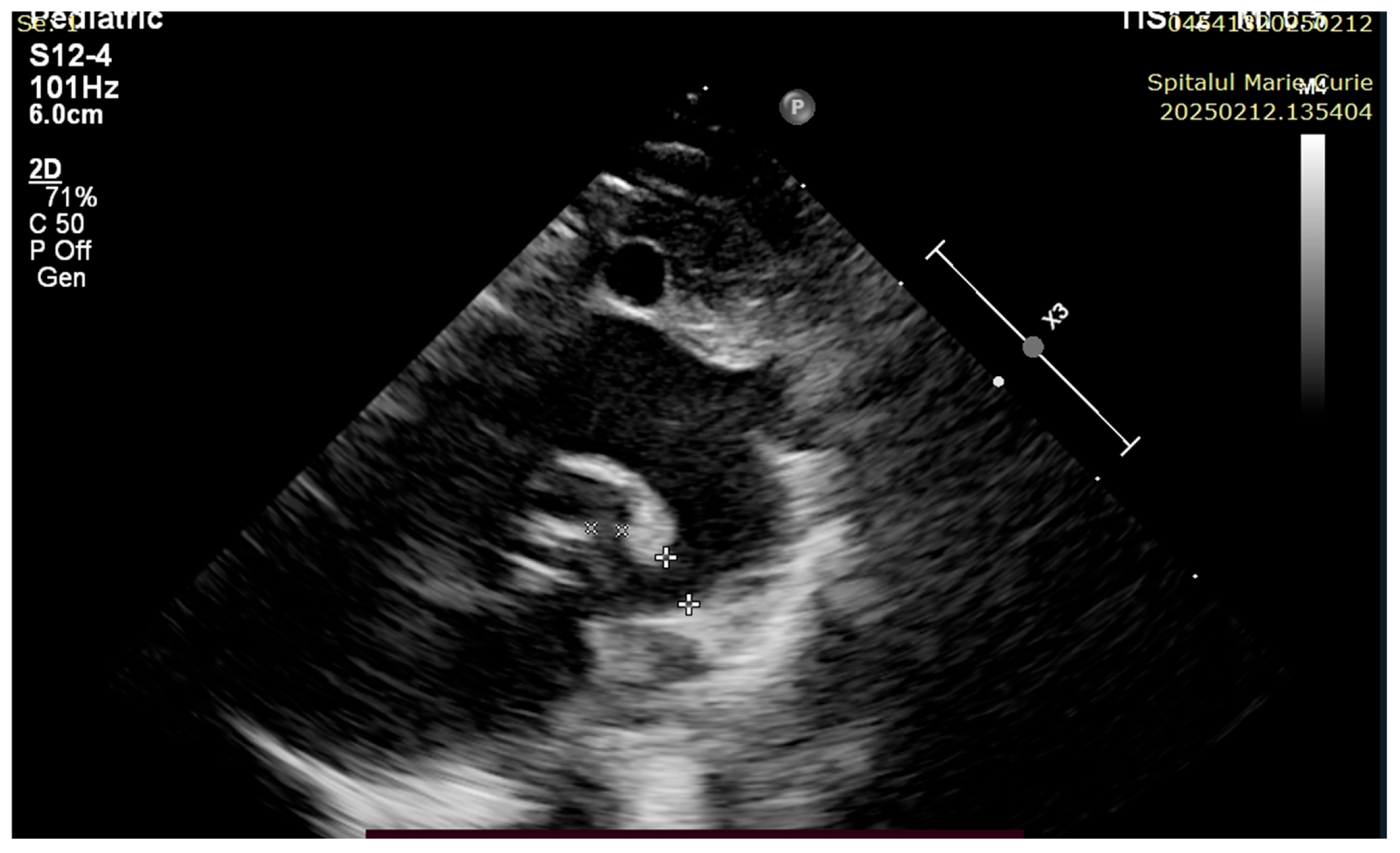
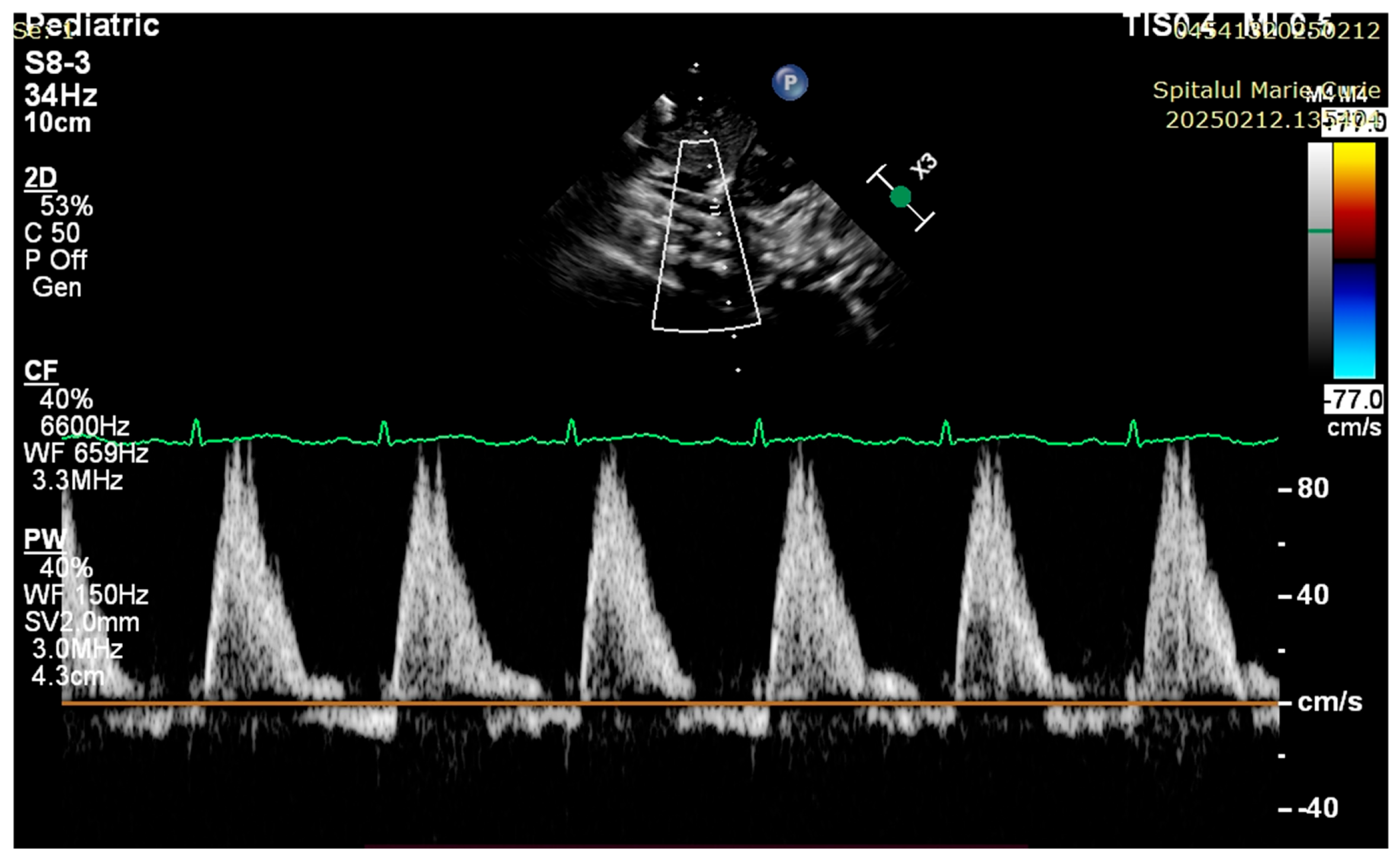
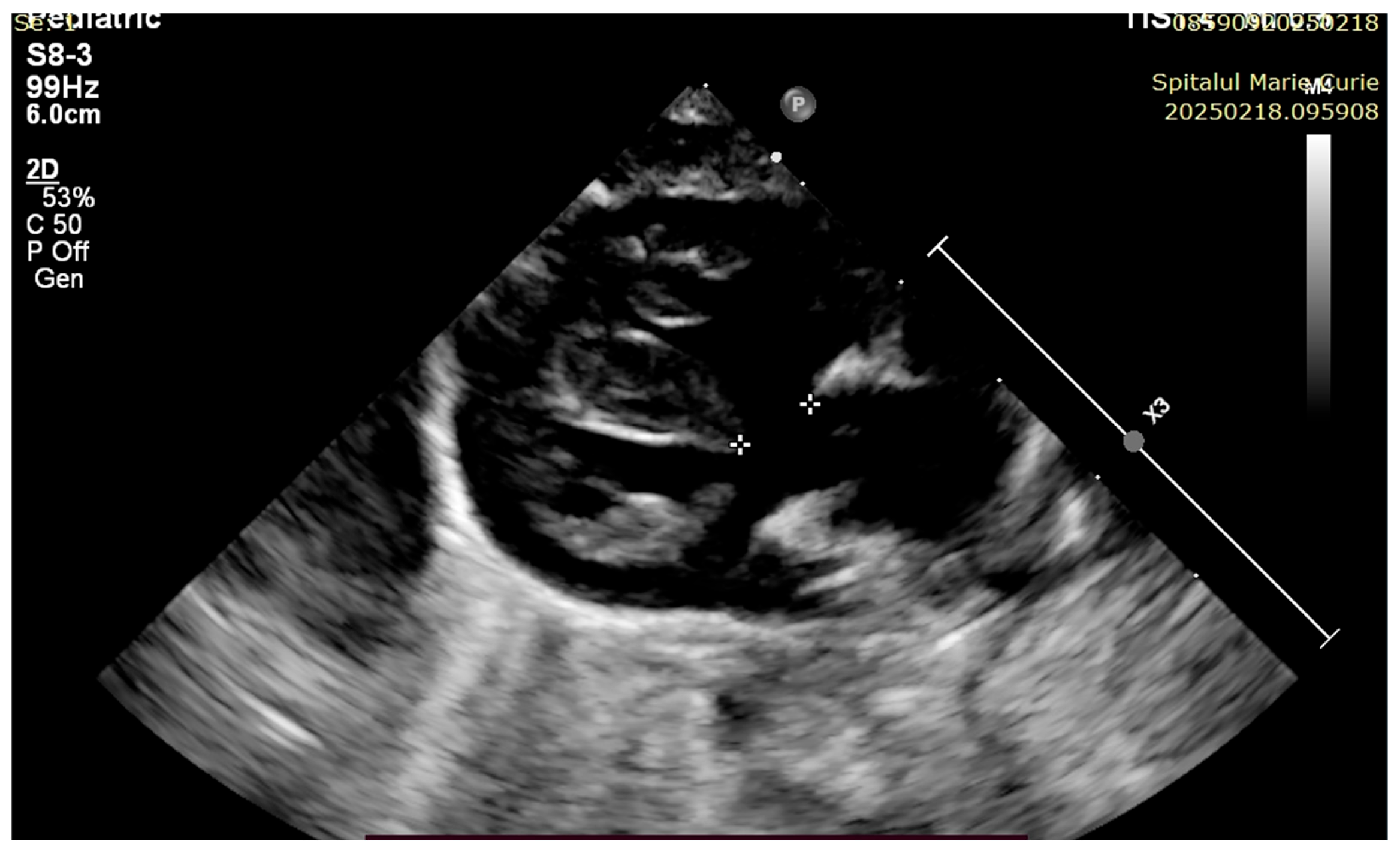
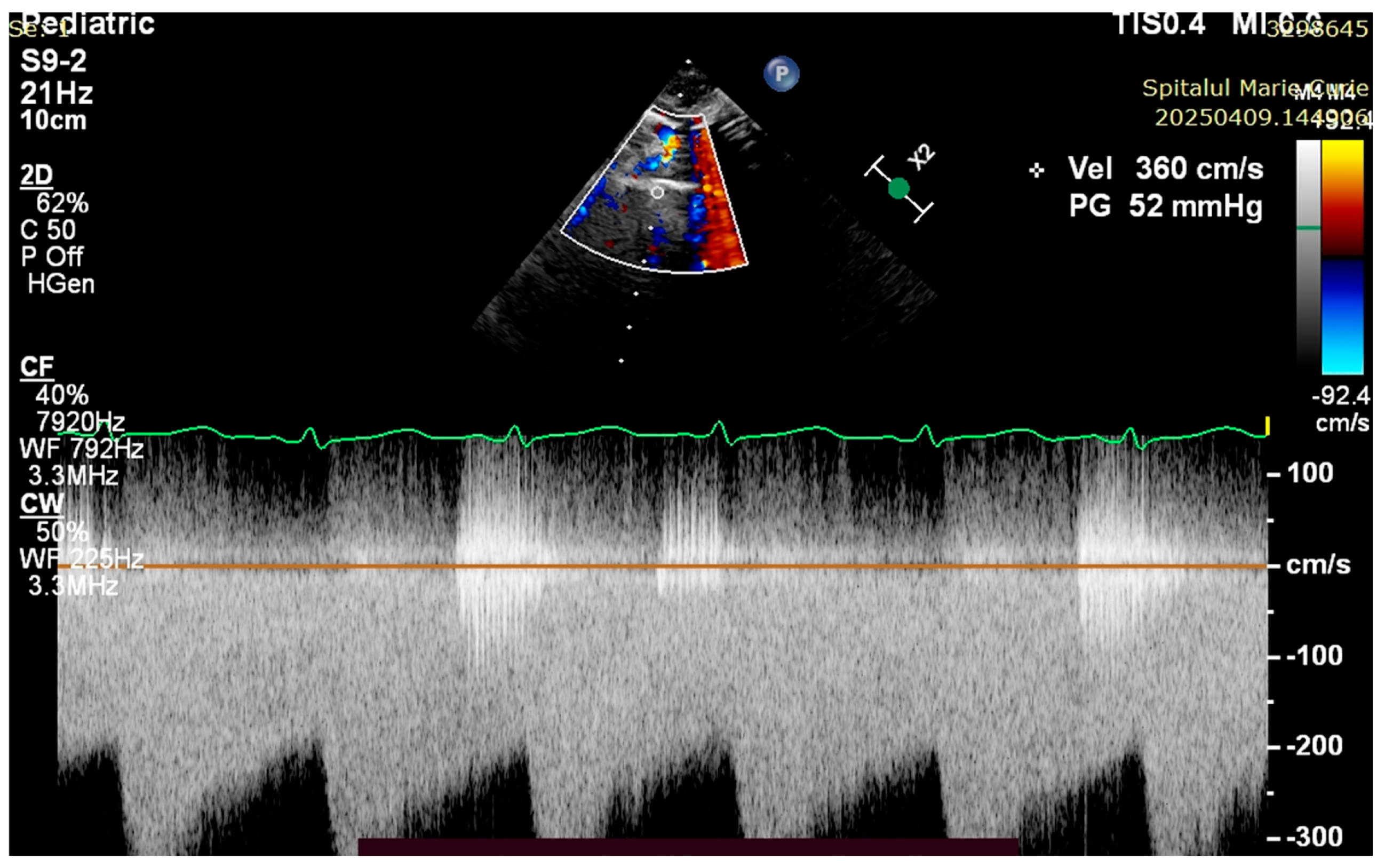
4. Case Reports
5. Discussions
5.1. Clinical Stabilization and Hemodynamics
5.2. Respiratory and Ventilatory Considerations
5.3. Nutritional Strategy
5.4. Surgical Timing and Outcome Considerations
5.5. Need for Standardization and Training
5.6. Article Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Linde, D.; Konings, E.E.M.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.M.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Villafañe, J.; Feinstein, J.A.; Jenkins, K.J.; Vincent, R.N.; Walsh, E.P.; Dubin, A.M.; Geva, T.; Towbin, J.A.; Cohen, M.S.; Fraser, C.; et al. Hot topics in tetralogy of Fallot. J. Am. Coll. Cardiol. 2013, 62, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Bailliard, F.; Anderson, R.H. Tetralogy of Fallot. Orphanet J. Rare. Dis. 2009, 4, 2. [Google Scholar] [CrossRef]
- Smith, C.A.; McCracken, C.; Thomas, A.S.; Spector, L.G.; Louis, J.D.S.; Oster, M.E.; Moller, J.H.; Kochilas, L. Long-term Outcomes of Tetralogy of Fallot. JAMA Cardiol. 2019, 4, 34. [Google Scholar] [CrossRef]
- Blais, S.; Marelli, A.; Vanasse, A.; Dahdah, N.; Dancea, A.; Drolet, C.; Colavincenzo, J.; Vaugon, E.; Dallaire, F. The 30-Year Outcomes of Tetralogy of Fallot According to Native Anatomy and Genetic Conditions. Can. J. Cardiol. 2021, 37, 877–886. [Google Scholar] [CrossRef]
- Haberer, K.; He, R.; McBrien, A.; Eckersley, L.; Young, A.; Adatia, I.; Hornberger, L.K. Accuracy of Fetal Echocardiography in Defining Anatomic Details: A Single-Institution Experience over a 12-Year Period. J. Am. Soc. Echocardiogr. 2022, 35, 762–772. [Google Scholar] [CrossRef]
- Kariuki, E.; Sutton, C.; Leone, T.A. Neonatal resuscitation: Current evidence and guidelines. BJA Educ. 2021, 21, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Donofrio, M.T. Delivery room and early postnatal management of neonates with congenital heart disease. Prenat. Diagn. 2024, 44, 915–924. [Google Scholar] [CrossRef]
- Yang, K.C.; te Pas, A.B.; Weinberg, D.D.; Foglia, E.E. Corrective steps to enhance ventilation in the delivery room. Arch. Dis. Child Fetal Neonatal Ed. 2020, 105, 605–608. [Google Scholar] [CrossRef]
- Jonas, R.A. Surgical Management of Absent Pulmonary Valve Syndrome. World J. Pediatr. Congenit. Heart Surg. 2016, 7, 600–604. [Google Scholar] [CrossRef]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rüdiger, M.; Skåre, C.; Szczapa, T.; Pas, A.T.; Trevisanuto, D.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Puri, K. Interpretation of Oxygen Saturation in Congenital Heart Disease: Fact and Fallacy. Pediatr. Rev. 2022, 43, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.T.; Kimura, B.J.; Korcarz, C.E.; Pellikka, P.A.; Rahko, P.S.; Siegel, R.J. Focused Cardiac Ultrasound: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2013, 26, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Addepalli, A.; Guillen, M.; Dreyfuss, A.; Mantuani, D.; Nagdev, A.; Martin, D.A. Point-of-Care Ultrasound Diagnosis of Tetralogy of Fallot Causing Cyanosis: A Case Report. Clin. Pract. Cases Emerg. Med. 2022, 6, 280–283. [Google Scholar] [CrossRef]
- Shlomai, N.O.; Lazarovitz, G.; Koplewitz, B.; Friedman, S.E. Cumulative Dose of Prostaglandin E1 Determines Gastrointestinal Adverse Effects in Term and Near-Term Neonates Awaiting Cardiac Surgery: A Retrospective Cohort Study. Children 2023, 10, 1572. [Google Scholar] [CrossRef]
- Gordon, C.M.; Tan, J.T.; Carr, R.R. Effectiveness of Alprostadil for Ductal Patency. J. Pediatr. Pharmacol. Ther. 2024, 29, 37–44. [Google Scholar] [CrossRef]
- Huang, F.-K.; Lin, C.-C.; Huang, T.-C.; Weng, K.-P.; Liu, P.-Y.; Chen, Y.-Y.; Wang, H.-P.; Ger, L.-P.; Hsieh, K.-S. Reappraisal of the Prostaglandin E1 Dose for Early Newborns with Patent Ductus Arteriosus-Dependent Pulmonary Circulation. Pediatr. Neonatol. 2013, 54, 102–106. [Google Scholar] [CrossRef]
- Cucerea, M.; Simon, M.; Moldovan, E.; Ungureanu, M.; Marian, R.; Suciu, L. Congenital Heart Disease Requiring Maintenance of Ductus Arteriosus in Critically Ill Newborns Admitted at A Tertiary Neonatal Intensive Care Unit. J. Crit. Care Med. 2016, 2, 185–191. [Google Scholar] [CrossRef]
- Higgins, K.L.; Buck, M.L. Caffeine Citrate for the Prevention of Apnea Associated With Alprostadil Infusions. J. Pediatr. Pharmacol. Ther. 2020, 25, 235–240. [Google Scholar] [CrossRef]
- Mitchell, L.J.; Mayer, C.A.; Mayer, A.; Di Fiore, J.M.; Shein, S.L.; Raffay, T.M.; MacFarlane, P.M. Caffeine prevents prostaglandin E1 -induced disturbances in respiratory control in neonatal rats: Implications for infants with critical congenital heart disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R233–R242. [Google Scholar] [CrossRef]
- Chatziantoniou, A.; Rorris, F.-P.; Samanidis, G.; Kanakis, M. Keeping the Ductus Arteriosus Patent: Current Strategy and Perspectives. Diagnostics 2025, 15, 241. [Google Scholar] [CrossRef] [PubMed]
- Won, B.; Kim, M.Y.; Choi, D.Y.; Cho, H.J.; Shim, S.Y.; Son, D.W. Successful Opening of Ductus Arteriosus with Milrinone in a Newborn with Tetralogy of Fallot and Pulmonary Atresia. J. Korean Soc. Neonatol. 2011, 18, 365. [Google Scholar] [CrossRef]
- Pacifici, G.M.; Pharmacology, P. Clinical pharmacology of Milrinone in paediatric patients. J. Drug Des. Res. 2021, 8, 1–5. [Google Scholar] [CrossRef]
- Hillig, K.T. Prostaglandin E1: Administration implications for the care provider in the treatment of neonatal ductal dependent congenital heart disease. J. Neonatal Nurs. 2016, 22, 12–15. [Google Scholar] [CrossRef]
- Glatz, A.C.; Petit, C.J.; Goldstein, B.H.; Kelleman, M.S.; McCracken, C.E.; McDonnell, A.; Buckey, T.; Mascio, C.E.; Shashidharan, S.; Ligon, A.; et al. Comparison Between Patent Ductus Arteriosus Stent and Modified Blalock-Taussig Shunt as Palliation for Infants With Ductal-Dependent Pulmonary Blood Flow. Circulation 2018, 137, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Hammett, O.; Griksaitis, M.J. Management of tetralogy of Fallot in the pediatric intensive care unit. Front. Pediatr. 2023, 11, 1104533. [Google Scholar] [CrossRef]
- Wilson, R.; Ross, O.; Griksaitis, M.J. Tetralogy of Fallot. BJA Educ. 2019, 19, 362–369. [Google Scholar] [CrossRef]
- Agakidou, E.; Chatziioannidis, I.; Kontou, A.; Stathopoulou, T.; Chotas, W.; Sarafidis, K. An Update on Pharmacologic Management of Neonatal Hypotension: When, Why, and Which Medication. Children 2024, 11, 490. [Google Scholar] [CrossRef]
- Siefkes, H.M.; Lakshminrusimha, S. Management of Systemic Hypotension in Term Infants with Persistent Pulmonary Hypertension of the Newborn (PPHN)—An Illustrated Review. Arch. Dis. Child Fetal Neonatal Ed. 2021, 106, 446. [Google Scholar] [CrossRef]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin. Infect. Dis. 2011, 52, e162–e193. [Google Scholar] [CrossRef]
- Di Biase, M.; Casani, A.; Orfeo, L. Invasive arterial blood pressure in the neonatal intensive care: A valuable tool to manage very ill preterm and term neonates. Ital. J. Pediatr. 2015, 41, A9. [Google Scholar] [CrossRef]
- Gibson, K.; Smith, A.; Sharp, R.; Ullman, A.; Morris, S.; Esterman, A. Adverse events associated with umbilical vascular catheters in the neonatal intensive care unit: A retrospective cohort study. Aust. Crit. Care 2024, 37, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Mele, R.; Panesar, L.E.; Heyden, M.; Sridhar, S.; Brandon, D. Neonatal Nurse Practitioner Use of Ultrasonography to Verify Umbilical Venous Catheter Placement in the Neonatal Intensive Care Unit. Adv. Neonatal Care 2020, 20, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Rubortone, S.A.; Costa, S.; Perri, A.; D’Andrea, V.; Vento, G.; Barone, G. Real-time ultrasound for tip location of umbilical venous catheter in neonates: A pre/post intervention study. Ital. J. Pediatr. 2021, 47, 68. [Google Scholar] [CrossRef] [PubMed]
- Mullaly, R.; El-Khuffash, A.F. Haemodynamic assessment and management of hypotension in the preterm. Arch. Dis. Child Fetal Neonatal Ed. 2024, 109, 120–127. [Google Scholar] [CrossRef]
- Dunham-Snary, K.J.; Wu, D.; Sykes, E.A.; Thakrar, A.; Parlow, L.R.G.; Mewburn, J.D.; Parlow, J.L.; Archer, S.L. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 2017, 151, 181–192. [Google Scholar] [CrossRef]
- Archer, S.L.; Snary, K.J.D.; Bentley, R.E.T.; Alizadeh, E.; Weir, E.K. Hypoxic Pulmonary Vasoconstriction: An Important Component of the Homeostatic Oxygen Sensing System. Physiol. Res. 2024, 73 (Suppl. 2), S493. [Google Scholar] [CrossRef]
- Mitra, S.; Altit, G. Inhaled nitric oxide use in newborns. Paediatr. Child Health 2023, 28, 119–122. [Google Scholar] [CrossRef]
- Bronicki, R.A.; Benitz, W.E.; Buckley, J.R.; Yarlagadda, V.V.; Porta, N.F.M.; Aganga, D.O.; Kim, M.; Costello, J.M. Respiratory Care for Neonates With Congenital Heart Disease. Pediatrics 2022, 150 (Suppl. 2), e2022056415H. [Google Scholar] [CrossRef]
- Keszler, M.; Abubakar, M.K. Volume-targeted ventilation. Semin. Perinatol. 2024, 48, 151886. [Google Scholar] [CrossRef]
- Zhang, H.; Keszler, M. Mechanical ventilation in special populations. Semin. Perinatol. 2024, 48, 151888. [Google Scholar] [CrossRef] [PubMed]
- Centeno-Malfaz, F.; Moráis-López, A.; Caro-Barri, A.; Peña-Quintana, L.; Gil-Villanueva, N.; Redecillas-Ferreiro, S.; Marcos-Alonso, S.; Ros-Arnal, I.; Tejero, M.Á.; Sánchez, C.S.; et al. Nutrition in congenital heart disease: Consensus document. An. Pediatría Engl. Ed. 2023, 98, 373–383. [Google Scholar] [CrossRef]
- Mills, K.I.; Kim, J.H.; Fogg, K.; Goldshtrom, N.; Graham, E.M.; Kataria-Hale, J.; Osborne, S.W.; Figueroa, M. Nutritional Considerations for the Neonate With Congenital Heart Disease. Pediatrics 2022, 150 (Suppl. 2), e2022056415G. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.M.; Caldarone, C.A.; Romano, J.C.; Chai, P.J.; Mascio, C.E.; Glatz, A.C.; Petit, C.J.; McCracken, C.E.; Kelleman, M.S.; Nicholson, G.T.; et al. Comparison of management strategies for neonates with symptomatic tetralogy of Fallot and weight <2.5 kg. J. Thorac. Cardiovasc. Surg. 2022, 163, 192–207.e3. [Google Scholar] [CrossRef]
- Curzon, C.L.; Milford-Beland, S.; Li, J.S.; O’Brien, S.M.; Jacobs, J.P.; Jacobs, M.L.; Welke, K.F.; Lodge, A.J.; Peterson, E.D.; Jaggers, J. Cardiac surgery in infants with low birth weight is associated with increased mortality: Analysis of the Society of Thoracic Surgeons Congenital Heart Database. J. Thorac. Cardiovasc. Surg. 2008, 135, 546–551. [Google Scholar] [CrossRef]
- Tarca, A.; Peacock, G.; McKinnon, E.; Andrews, D.; Saundankar, J. A Single-Centre Retrospective Review of Modified Blalock-Taussig Shunts: A 22-Year Experience. Heart Lung Circ. 2023, 32, 405–413. [Google Scholar] [CrossRef]
- Al Nasef, M.; Shahbah, D.A.; Batlivala, S.P.; Darwich, R.; Qureshi, A.M.; Breatnach, C.R.; Linnane, N.; Walsh, K.P.; Oslizlok, P.; McCrossan, B.; et al. Short- and medium-term outcomes for patent ductus arteriosus stenting in neonates ≤2.5 kg with duct-dependent pulmonary circulation. Catheter. Cardiovasc. Interv. 2022, 100, 596–605. [Google Scholar] [CrossRef]
- Dib, N.; Chauvette, V.; Diop, M.S.; Bouhout, I.; Hadid, M.; Vô, C.; Khairy, P.; Poirier, N. Tetralogy of Fallot in Low- and Middle-Income Countries. CJC Pediatr. Congenit. Heart Dis. 2024, 3, 67–73. [Google Scholar] [CrossRef]

| Category | Standard | Intervention |
|---|---|---|
| Thermal control | Monitor and record infant temperature after birth Maintain temperature between 36.5 and 37.5 °C Avoid hypothermia (<36.5 °C) or hyperthermia (>38.5 °C) | Environment temperature between 23 and 25 °C. Skin-to-skin care if no resuscitation is needed. Using radiant warmer with servo-control if resuscitation is performed. |
| Umbilical cord | Delay cord clamping for at least 60 s if feasible | If there is no need for resuscitation, clamping should be delayed after lung aeration. If adequate thermal care and resuscitation is possible, these interventions can be performed with intact cord. Consider milking cord if delayed cord clamping is not feasible in infants > 28 weeks. |
| Initial assessment | Assess heart rate, tone and breathing at birth and every 30 s | Initial assessment can be performed via observation and auscultation. |
| Initial steps | Positioning Drying and stimulating Suctioning | Place infant’s head in neutral position. Face is horizontal. Neck is neither flexed nor extended. Start drying and tactilely stimulating infant after birth. Perform suctioning only if visible obstruction is present. Perform suctioning before PPV is delivered. |
| PPV | Start PPV if heart rate remains <100 bpm or if infant is apneic, gasping or not breathing effectively Assess chest movement and heart rate Use pulse oximetry to assess SpO2 and heart rate | PPV should be delivered using “T” piece resuscitator. Initial peak pressure 30 cm H2O for term infants and 25 cm H2O for preterm infants. Use PEEP 6–8 cm H2O, if possible. FiO2 21% for term infants and 21–30% for preterm infants. Adjust FiO2 accordingly with SpO2. |
| Alternate airway | Orotracheal intubation in delivery room Consider laryngeal mask in infants > 1500 g or when intubation is not possible | Consider alternative airway when adequate ventilation is not achieved after corrective steps are performed. Consider alternative airway when performing chest compressions. |
| Chest compressions | Consider chest compressions when heart rate is <60 bpm when good-quality ventilation is delivered | Use synchronous technique. Provide chest compression to ventilation 3:1. Increase FiO2 to 100%. |
| Medication | Consider intravenous access Intraosseous access can be alternative for emergency access | iv Adrenaline 10–30 micrograms/kg if heart rate remains <60 bpm after 30 s of chest compressions and ventilation. Consider iv bolus glucose 250 mg/kg (2.5 mL/kg of glucose 10%) in prolonged resuscitation. Consider volume replacement (10 mL/kg O Rh negative blood or isotonic crystalloid) if volume depletion or unresponsive shock is suspected. |
| Parameter | Set-Up and Adjustment | Rationale |
|---|---|---|
| Tidal volume | VT 4.5–5.5 mL/kg Titrate VT in steps of 0.5 mL/kg based on blood gas analysis and work of breathing | Maintain PaCO2 within normal ranges Tachypnea may develop when lower tidal volumes are used If Ph is normal, moderate hypercapnia (PaCO2 45–60 mmHg) may be tolerated If Ph is normal, mild hypocapnia (PaCO2 30–35 mmHg) may be tolerated |
| Pressure support | Set pressure support to deliver at least 4 mL/kg Titrate in steps of 1 cm H2O | Insufficient pressure support may deliver a lower tidal volume that leads to the ventilation of dead space Increase the level of support to avoid distress and maintain a normal respiratory rate |
| PEEP | Set initial PEEP based on SpO2 and FiO2 4–6 cm H2O may be a good start Titrate in steps of 0.5 cm H2O | Optimal PEEP promotes good lung recruitment and prevents overdistension Optimal PEEP allows for lower FiO2 Overdistension increases PVR, limiting PBF |
| Pmax | Set Pmax above 25–30% of working PIP | Alerts the clinician about changes in patient status Alerts the clinician about leaks or endotracheal tube displacement |
| Respiratory rate | Aim for a moderate rate of 25–35 breaths/min Adjust in steps of 3–5 breaths/min | Allows the infant to trigger spontaneous breaths while maintaining the mandatory rate for lung recruitment Adjust to maintain PH < 7.35 when the weaning process is started; PH is more responsible for respiratory drive than PCO2 |
| Inspiratory time | Set initial inspiratory time based on the pulmonary disease and gestational age Adjust in steps of 0.1 s based on the ventilatory waveforms | Lower inspiratory times are needed in homogenous pulmonary disease (e.g., RDS) Longer inspiratory times may be needed in heterogenous pulmonary disease (e.g., BPD, MAS) A short inspiratory time might deliver insufficient volume A long inspiratory time might result in insufficient expiratory time and air trapping |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragomir, I.; Vasilescu, D.I.; Dan, A.M.; Voicu, D.; Vasilescu, S.L.; Stefan, L.A.; Nicolescu, A.; Cîrstoiu, M.M. Tetralogy of Fallot: The Burden of Pulmonary Atresia in the NICU Set-Up: Two Case Reports and a Literature Review. Children 2025, 12, 780. https://doi.org/10.3390/children12060780
Dragomir I, Vasilescu DI, Dan AM, Voicu D, Vasilescu SL, Stefan LA, Nicolescu A, Cîrstoiu MM. Tetralogy of Fallot: The Burden of Pulmonary Atresia in the NICU Set-Up: Two Case Reports and a Literature Review. Children. 2025; 12(6):780. https://doi.org/10.3390/children12060780
Chicago/Turabian StyleDragomir, Ion, Diana Iulia Vasilescu, Adriana Mihaela Dan, Diana Voicu, Sorin Liviu Vasilescu, Laura Andreea Stefan, Alin Nicolescu, and Monica Mihaela Cîrstoiu. 2025. "Tetralogy of Fallot: The Burden of Pulmonary Atresia in the NICU Set-Up: Two Case Reports and a Literature Review" Children 12, no. 6: 780. https://doi.org/10.3390/children12060780
APA StyleDragomir, I., Vasilescu, D. I., Dan, A. M., Voicu, D., Vasilescu, S. L., Stefan, L. A., Nicolescu, A., & Cîrstoiu, M. M. (2025). Tetralogy of Fallot: The Burden of Pulmonary Atresia in the NICU Set-Up: Two Case Reports and a Literature Review. Children, 12(6), 780. https://doi.org/10.3390/children12060780




