Abstract
Objectives: This review summarizes current evidence on robotic-assisted upper airway and neck surgery in pediatric patients, highlighting clinical indications, outcomes, limitations, and areas for future research. Methods: A systematic review was conducted in accordance with PRISMA guidelines, including studies on robotic surgery for pediatric patients (≤18 years) with upper airway conditions and cervical pathologies. Data on study characteristics, patient demographics, surgical details, outcomes, and robotic system advantages or limitations were extracted. Results: Twenty studies met inclusion criteria, comprising 104 pediatric patients who underwent 110 robotic procedures, mostly transoral robotic surgery (TORS) for base of tongue, laryngeal, and cervical pathologies. The Da Vinci Si was the most used system. The mean operative time was ~74 min, with minimal blood loss and no intra/post operative tracheostomies. Reported advantages included enhanced visualization, precision, and reduced morbidity. Limitations involved size mismatches, limited working space, and high costs. Follow-up (mean 11.4 months) revealed no recurrences, confirming feasibility and safety in selected pediatric cases. Conclusions: Robotic-assisted surgery appears to be a feasible and safe option for managing pediatric upper airway and neck conditions, offering promising functional and aesthetic outcomes with low complication rates. However, its use is currently limited by anatomical constraints, high costs, and the need for surgeon training. Long-term prospective studies with larger cohorts are needed to confirm its efficacy and define its role compared to traditional techniques.
1. Introduction
Transoral robotic surgery (TORS) has become an established technique in the treatment of oropharyngeal, hypopharyngeal, and laryngeal pathologies in adults, with numerous studies demonstrating its feasibility and efficacy in this population [1,2]. In contrast, the adoption of robotic-assisted procedures in pediatric otolaryngology remains relatively limited, although robotic surgery has already gained traction in pediatric abdominal, thoracic, urological, and gynecological procedures due to its precision and minimally invasive nature [3]. The first reported application of TORS in children was in 2007, when Rahbar et al. [4] described the use of robotic assistance for laryngeal cleft repair in five pediatric patients. However, the study highlighted the limitations of the robotic platform at that time, particularly regarding the size of the robotic arms and limited surgical exposure, which prevented completion in three of the five cases due to inadequate visualization and restricted instrument [4]. Since then, technological advancements, including improved optics, miniaturization of instruments, and better patient selection, have contributed to the gradual increase in reported pediatric TORS procedures.
Several case reports and small case series have demonstrated the feasibility of robotic-assisted approaches in pediatric head and neck surgery, including sleep apnea interventions, airway reconstructions, and excision of pharyngeal masses [5,6,7]. Notably, the literature also includes rare cases of robot-assisted procedures for pediatric neck masses using alternative approaches, such as transhairline incisions [8] or retroauricular access [9], which allow mass removal through small, well-concealed skin incisions that minimize visible scarring. These minimally invasive approaches are gaining popularity for their favorable surgical, functional, and cosmetic outcomes.
Recent evidence has emphasized the importance of careful patient selection, preoperative planning, and a multidisciplinary approach to ensure safety and optimize outcomes in children [10]. Furthermore, emerging data suggest that the integration of robotic techniques may reduce surgical morbidity, hospital stay, and recovery time, while improving functional outcomes, particularly in complex airway procedures [11].
Nonetheless, the literature remains sparse, with most publications describing isolated experiences rather than large-scale or comparative studies. The pediatric application of robotic surgery faces important challenges: the bulky system, high costs, steep learning curve, and need for adaptation to smaller, delicate anatomy remain significant barriers [10,12]. As Johnston et al. [12] suggest, the potential of robotic surgery to expand pediatric otolaryngology indications depends on ongoing technological improvements and the development of pediatric-specific training.
This systematic review aims to summarize current evidence on the use of robotic-assisted surgery for upper airway and neck procedures in pediatric patients, with a focus on clinical indications, surgical outcomes, and limitations, while also identifying areas that require further investigation.
2. Materials and Methods
After registering with the PROSPERO database (ID CRD420251059669), this systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13].
A comprehensive literature search was performed using PubMed, Scopus, and Web of Science databases, employing the following search string: “robotic surgery” [All Fields] AND (“TORS” [All Fields] OR “otorhinolaryngology” [All Fields] OR “upper airways” [All Fields] OR “adenotonsillar hypertrophy” [All Fields] OR “tonsillar hypertrophy” [All Fields] OR “laryngology” [All Fields] OR “pediatrics” [All Fields] OR “children” [All Fields]). Reference lists of the included studies were also manually screened to identify additional relevant articles. All titles and abstracts published up to 23 April 2025 were independently reviewed by two authors (I.C.V. and M.R.). Disagreements regarding study inclusion were resolved through discussion.
The inclusion criteria were original articles and case reports written in English, describing pediatric patients (aged ≤ 18 years) with congenital or acquired upper airway and neck pathologies—either benign or malignant—who underwent robotic surgery, with an available abstract.
Exclusion criteria comprised non-original publications (e.g., reviews, meta-analyses, editorials, letters to the editor, consensus statements, conference abstracts, “how I do it” articles), studies not related to robotic surgery, or those involving mixed populations of pediatric and adult patients. Also, cases in which the robotic approach failed and it was converted were excluded from the final analysis.
Full texts of the eligible studies were reviewed, and their reference lists were examined to identify additional eligible articles. The extracted data included general study characteristics, demographic and clinical information about the pediatric patients, the type of surgical procedure, robotic system used, complications, follow-up duration, recurrence, and the reported advantages or limitations of the robotic approach.
3. Results
The study selection process is illustrated in the PRISMA flow diagram (Figure 1).
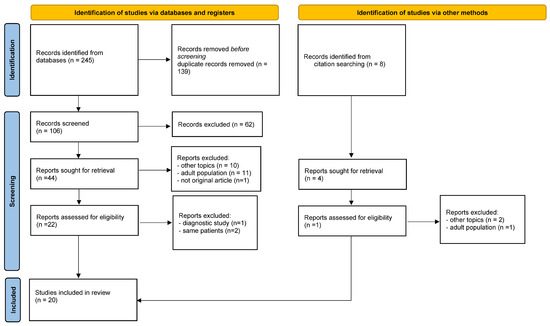
Figure 1.
Prisma flow diagram of the review [13].
A total of 20 studies met the inclusion criteria: 10 case reports, 5 retrospective studies, 4 case series, and 1 prospective study. In total, 104 pediatric patients underwent 110 robotic procedures (Table 1). Geographically, 11 studies originated from the United States, 6 from Europe (Turkey, Italy, and France), and 3 from Asia (India and China). The study with the largest sample size was conducted by Worden et al. [14] encompassing 40 patients.

Table 1.
Overview of the included studies and robotic procedures in pediatric airway and neck surgery. BOT, base of tongue; OSA, obstructive sleep apnea; LTGDC, lingual thyroglossal duct cyst; TORS, transoral robotic surgery; TBR, tongue base reduction; TLM, transoral laser microsurgery; FAMM, facial artery musculomucosal flap; SD, standard deviation; n.a., not available.
The most common pathological site was the base of the tongue (60/110, 54.55%), followed by the larynx (26/110, 23.64%), the cervical region (22/110, 20.00%), and the oropharynx (2/110, 1.82%).
Table 2 summarized the treated conditions. Accordingly, the predominant surgical approach was TORS for lesion excision and/or tissue reduction (75/110 cases; 68.2%), followed by cleft repair (21/110; 19.1%), release procedures of aerodigestive tract strictures (6/110; 5.5%), retroauricular excision (6/110; 5.5%), robot-assisted hyoepiglottopexy (1/110; 0.9%), and posterior cordectomy with subtotal arytenoidectomy (1/110; 0.9%).

Table 2.
Summary of conditions treated with robotic-assisted surgery in the included pediatric cases. OSA, obstructive sleep apnea; LTGDC, lingual thyroglossal duct cyst; BOT, base of tongue.
The associated procedures described included neoadjuvant and adjuvant chemotherapy and radiotherapy in malignant cases, with one case also requiring a neck dissection performed via cervicotomy. In another instance, CO2 laser was utilized through a robotic arm. Additionally, two cases required a combined external approach to achieve complete excision of the pathology.
The mean operative time, reported for 84 procedures, was 74.48 ± 72.69 min. For the 35 procedures where docking time was available, the mean docking time was 14.95 ± 10.05 min. Blood loss data were reported in 31 procedures, in which 1 case involved <5 mL, 27 cases between 5 and 10 mL, 2 cases between 10 and 15 mL, and 1 case with 250 mL due to external jugular vein rupture. The most frequently utilized robotic platform was the Da Vinci Si system (91/110 procedures, 82.73%), followed by the Da Vinci Xi system (13/110, 11.82%).
Preoperative tracheostomy was present in 8 out of 104 patients (7.69%); no intraoperative or postoperative tracheostomies were required.
Hospitalization data were available for 72 procedures, with a mean length of stay of 5.11 ± 5.66 days. Two studies reported hospital stays ranging from 1 to 14 days (Table 3).

Table 3.
Summary of study characteristics, patient demographics, procedural data, and complications.
The most frequently cited advantages across studies included high-resolution three-dimensional visualization, tremor filtration, and enhanced instrument maneuverability. Additional reported benefits included high magnification, minimally invasive access, fewer postoperative complications, shorter hospital stays, improved swallowing function, and lower morbidity. Overall, robotic surgery was consistently described as a feasible and safe alternative to other transoral approaches.
Nevertheless, several limitations were noted. Chief among them were the restricted operative field and the fact that current robotic systems are not specifically designed for pediatric anatomy, leading to size mismatches between the robotic arms and the oral cavity. Other drawbacks included high costs, lengthy setup and docking times, and potential robotic arm collisions that can disrupt intraoperative workflow. Limited visualization and challenges in assisting bedside surgeons were also mentioned in some cases.
The mean follow-up duration among the 18 studies with available data was approximately 11.4 months. No recurrences were observed during the follow-up period, although one laryngeal neurofibroma required retreatment for residual disease. One patient remained tracheostomy-dependent at 12 months, and another required a second transoral laser microsurgery (TLM) procedure.
4. Discussion
TORS is a widely accepted approach for the management of both benign and malignant otolaryngologic conditions in adults [27,28]. Due to its several advantages, its use has increasingly extended to pediatric airway pathologies in recent years [14]. This systematic review investigates the indications, safety profile, clinical outcomes, and potential advantages and limitations of robotic-assisted surgical techniques in the management of upper airway and neck pathologies in the pediatric population.
The first work on this topic by Rahbar et al. [4] reports the use of TORS for the repair of laryngeal clefts. According to the authors, robotic-assisted surgery was feasible and effective, though only in highly selected cases. In fact, only 21 patients with laryngeal cleft had been treated using robotic technology up to that point [4,5,14]. Although enhanced visualization and instrument articulation improve maneuvers to manage complex airway anomalies in confined anatomical spaces, limited transoral access requires careful patient selection and technological advancements to enhance applicability [4,11,27]. Over the years, the number of reported cases has increased, while most of the studies included in the review are still case reports. In 2024, Worden et al. [14] presented the largest case series to date, involving 40 patients and demonstrating that TORS outcomes—including operative times, complication rates, and hospital stays—were comparable to traditional surgical methods. In case of type I laryngeal clefts and lymphatic malformations, operative times were generally longer with the robotic approach. However, postoperative swallow outcomes were significantly improved in patients undergoing TORS for type I laryngeal cleft repair. In this regard, robotic-assisted surgery demonstrated promising functional results in management of pediatric palatal clefts, including improvements on middle ear function and hearing. In these patients, shorter hospital stays and a reduced incidence of otitis media with effusion (OME) were noted [29,30,31,32].
According to our findings, the main field of application of robotic surgery in the pediatric population is represented by the treatment of obstructive sleep apnea (OSA) which is often erroneously attributed solely to adenotonsillar hypertrophy. Although adenotonsillectomy represents the first-line treatment for pediatric OSA [33], up to 40% of children may have persistent OSA despite the surgical procedure [18]. Base of tongue and lingual tonsil hypertrophy are well-known contributors to the residual airway obstruction [18,33]. In the literature, many papers have highlighted the efficacy of TORS in reducing the severity of OSA [7,19], improving visualization and minimizing morbidity, when compared to open procedures [5,34,35]. Leonardis et al. [18] reported positive and consistent results with an acceptable length of hospital stay and low rate of postoperative complications. Similarly, Thottam et al. [19] concluded that TORS may be a suitable and safe option for children with residual OSA after adenotonsillectomy and low compliance to other medical therapy. Montevecchi et al. [6] also emphasized the importance of careful patient selection for successful TORS application.
In recent years, the treatment of lingual thyroglossal duct cysts (LTGDC) has become an emerging application of pediatric robotic surgery. Although rare, these cysts may lead to dysphagia, airway obstruction, and OSA [36]. While some authors advocate for simple marsupialization or excision, high recurrence rates have also been reported [37,38]. Johnston et al. [12] highlighted the advantages of TORS in safely accessing and excising cysts located in the post-hyoid space. Similar positive outcomes were observed in studies by Kayhan et al. [7,17] and Turhan et al. [39].
Robotic approaches have also shown value in the excision of cervical masses, offering high surgical precision and improved cosmetic results, particularly when avoiding visible scars is a priority [8,39]. In this review, Lin et al. [8] and Venkatakarthikeyan et al. [9] report cases of cervical mass excision performed through a retroauricolar/transhairline approach highlighting the possibility of complete removal with acceptable aesthetic results, shorter hospital stays, and fewer complications. Moreover, the use of the Da Vinci Xi system in four out of six patients, as reported by the authors, is likely attributable to its slimmer robotic arms, improved maneuverability in confined anatomical spaces, and more efficient docking process—all features frequently emphasized in the literature and particularly advantageous for pediatric head and neck procedures [40,41].
Furthermore, the challenges of accessing and visualizing the narrow spaces of the pediatric airway leads to the increasing adoption of advanced technologies; not only robotic surgery but also the exoscope has been considered. Gaffuri et al. [42] propose that high-definition 3D 4K exoscopy may offer a valuable alternative, helping to overcome the visual limitations of traditional surgical approaches.
Airway management during robotic surgery remains a debated issue, especially in pediatric patients. Although orotracheal or nasotracheal intubation could make the management of small airways challenging, only eight tracheostomies were reported in the included studies, all of which were performed preoperatively [7,15,18,21,23]. As highlighted by Leonardis et al. [18], appropriate tube selection and placement did not interfere with instrument mobility during surgery.
The feasibility of robotic surgery is further supported by an analysis of hospitalization length and complications. According to our findings, the length of stay ranges from a minimum of one day to a maximum of 23 with a mean of 5.11 ± 5.66 days. Given the delicate surgical site and the young patient population, the observed hospitalization duration appears consistent with expectations. Similar results have been described in terms of complications. Minor bleeding has been reported in some cases [7,12,18,19], which was more likely attributable to the high vascularity of the surgical site rather than the robotic technology itself. On the other hand, when considering the need for additional surgery, four cases of revision surgery have been reported by Arnold et al. [23] and Worden et al. [14]. The authors attributed these revisions primarily to the size of the robotic instruments and the limited visualization and accessibility of the surgical field.
Despite these encouraging findings, several limitations have been acknowledged in the literature.
First, a major constraint is that the Da Vinci Surgical System was not specifically designed for pediatric patients or for the narrow anatomical confines of the upper airway. The large size of the instruments limits their maneuverability, prompting calls for the development of smaller, pediatric-specific robotic tools. Hockstein et al. [41] similarly advocated for the miniaturization of robotic components to better accommodate pediatric applications.
In addition, novice surgeons often experience longer operative times and higher complication rates during their initial cases. This highlights the importance of dedicated training programs tailored to pediatric robotic surgery. Structured curricula incorporating simulation-based learning, mentorship, and supervised operating room experience are strongly recommended [43,44].
Another significant barrier is the high cost of robotic systems. Although reduced hospital stays and fewer complications may help to offset these expenses, comprehensive economic evaluations are needed to determine the true cost-effectiveness of these procedures [45,46].
In conclusion, despite growing interest and expanding clinical experience, high-quality evidence in the field of pediatric head and neck robotic surgery remains limited, underscoring the need for further well-designed studies to validate its safety, efficacy, and long-term outcomes. As reported by Gottman et al. [11], most existing studies are retrospective in nature, with small sample sizes and moderate heterogeneity, which may limit the reliability of their findings. Future research should prioritize prospective, multicenter randomized controlled trials involving larger cohorts. In particular, long-term follow-up data are needed to assess the role of TORS in the management of residual OSA following adenotonsillectomy. The development of specific guidelines for pediatric robotic surgery through future prospective and standardized studies would be desirable. This could promote broader clinical adoption and support the establishment of highly specialized pediatric centers.
5. Conclusions
This review highlights that robotic-assisted surgery may be considered a feasible and safe approach for managing pediatric upper airway procedures, with favorable outcomes in terms of operative times, complication rates, and hospitalization. It offers additional benefits such as improved functional outcomes, low complication rates, and superior cosmetic results. However, long-term prospective studies with larger patient cohorts are strongly needed to objectively assess its efficacy and to enable more robust comparisons with other surgical techniques.
Author Contributions
Conceptualization, I.C.V. and M.G.; methodology, M.R.; validation, E.M.C.T., V.D. and A.M.D.L.; resources, M.R.; writing—original draft preparation, V.D.; writing—review and editing, I.C.V. and M.R.; supervision, M.T.-Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TORS | Transoral Robotic Surgery |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| BOT | Base Of Tongue |
| OSA | Obstructive Sleep Apnea |
| LTGDC | Lingual Thyroglossal Duct Cyst |
| TBR | Tongue Base Reduction |
| TLM | Transoral Laser Microsurgery |
| FAMM | Facial Artery Musculomucosal Flap |
| SD | Standard Deviation |
| n.a. | Not Available |
References
- Kwong, F.N.; Puvanendran, M.; Paleri, V. Transoral robotic surgery in head neck cancer management. B-ENT 2015, 11, 7–13. [Google Scholar]
- Justin, G.A.; Chang, E.T.; Camacho, M.; Brietzke, S.E. Transoral Robotic Surgery for Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Otolaryngol. Head Neck Surg. 2016, 154, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Raymond, S.L.; Sharafeddin, F.; Sacks, M.A.; Srikureja, D.; Gomez, N.; Moores, D.; Radulescu, A.; Khan, F.A.; Tagge, E.P. Establishment of a successful robotic pediatric general surgery practice. J. Robot. Surg. 2023, 17, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, R.; Ferrari, L.R.; Borer, J.G.; Peters, C.A. Robotic surgery in the pediatric airway: Application and safety. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 46–50. [Google Scholar] [CrossRef]
- Ferrell, J.K.; Roy, S.; Karni, R.J.; Yuksel, S. Applications for transoral robotic surgery in the pediatric airway. Laryngoscope 2014, 124, 2630–2635. [Google Scholar] [CrossRef]
- Montevecchi, F.; Bellini, C.; Meccariello, G.; Hoff, P.T.; Dinelli, E.; Dallan, I.; Corso, R.M.; Vicini, C. Transoral robotic-assisted tongue base resection in pediatric obstructive sleep apnea syndrome: Case presentation, clinical and technical consideration. Eur. Arch. Otorhinolaryngol. 2017, 274, 1161–1166. [Google Scholar] [CrossRef]
- Kayhan, F.T.; Yigider, A.P.; Koc, A.K.; Kaya, K.H.; Erdim, I. Treatment of tongue base masses in children by transoral robotic surgery. Eur. Arch. Otorhinolaryngol. 2017, 274, 3457–3463. [Google Scholar] [CrossRef]
- Lin, H.J.; Lin, F.C.; Yang, T.L.; Chang, C.H.; Kao, C.H.; Tsai, S.C. Cervical lymphatic malformations amenable to transhairline robotic surgical excision in children: A case series. Medicine 2021, 100, e27200. [Google Scholar] [CrossRef]
- Venkatakarthikeyan, C.; Nair, S.; Gowrishankar, M.; Rao, S. Robotic Surgery in Head and Neck in Pediatric Population: Our Experience. Indian J. Otolaryngol. Head Neck Surg. 2020, 72, 98–103. [Google Scholar] [CrossRef]
- Vianini, M.; Fiacchini, G.; Benettini, G.; Dallan, I.; Bruschini, L. Experience in Transoral Robotic Surgery in Pediatric Subjects: A Systematic Literature Review. Front. Surg. 2021, 8, 726739. [Google Scholar] [CrossRef]
- Gottman, D.C.; Corbisiero, M.F.; Saeedi, A.; Bothwell, S.; Svoboda, E.; Ai, A.; Roy, S. Assessing robotic-assisted procedures in pediatric otolaryngology: A systematic review and meta-analysis. Int. J. Pediatr. Otorhinolaryngol. 2024, 187, 112175. [Google Scholar] [CrossRef]
- Johnston, D.R.; Maurrasse, S.E.; Maddalozzo, J.M. Avascular midline oropharyngeal anatomy allows for expanded indications for transoral robotic surgery in pediatric patients. J. Robot. Surg. 2023, 17, 1803–1808. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Worden, C.P.; Prince, A.C.; Kirse, S.N.; Rutter, C.; Shields, B.H.; Hackman, T.G.; Yarbrough, W.G.; Zanation, A.M.; Zdanski, C.J. Transoral robotic surgery for pediatric upper airway pathology: An institutional update. Int. J. Pediatr. Otorhinolaryngol. 2024, 184, 112073. [Google Scholar] [CrossRef]
- Kokot, N.; Mazhar, K.; O’Dell, K.; Huang, N.; Lin, A.; Sinha, U.K. Transoral robotic resection of oropharyngeal synovial sarcoma in a pediatric patient. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1042–1044. [Google Scholar] [CrossRef]
- Wine, T.M.; Duvvuri, U.; Maurer, S.H.; Mehta, D.K. Pediatric transoral robotic surgery for oropharyngeal malignancy: A case report. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1222–1226. [Google Scholar] [CrossRef]
- Kayhan, F.T.; Kaya, K.H.; Koc, A.K.; Altintas, A.; Erdur, O. Transoral surgery for an infant thyroglossal duct cyst. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1620–1623. [Google Scholar] [CrossRef]
- Leonardis, R.L.; Duvvuri, U.; Mehta, D. Transoral robotic-assisted lingual tonsillectomy in the pediatric population. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1032–1036. [Google Scholar] [CrossRef]
- Thottam, P.J.; Govil, N.; Duvvuri, U.; Mehta, D. Transoral robotic surgery for sleep apnea in children: Is it effective? Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 2234–2237. [Google Scholar] [CrossRef]
- Carroll, D.J.; Byrd, J.K.; Harris, G.F. The feasibility of pediatric TORS for lingual thyroglossal duct cyst. Int. J. Pediatr. Otorhinolaryngol. 2016, 88, 109–112. [Google Scholar] [CrossRef]
- Colaianni, C.A.; Bowe, S.N.; Osborn, H.A.; Lin, D.T.; Richmon, J.D.; Hartnick, C.J. Robotic epiglottopexy for severe epiglottic prolapse limiting decannulation. Int. J. Pediatr. Otorhinolaryngol. 2017, 102, 157–159. [Google Scholar] [CrossRef]
- Canevari, F.R.; Montevecchi, F.; Galla, S.; Sorrentino, R.; Vicini, C.; Sireci, F. Trans-oral robotic surgery for a Ewing’s sarcoma of tongue in a pediatric patient: A case report. Braz. J. Otorhinolaryngol. 2020, 86 (Suppl. S1), 26–29. [Google Scholar] [CrossRef]
- Arnold, M.A.; Mortelliti, A.J.; Marzouk, M.F. Transoral resection of extensive pediatric supraglottic neurofibroma. Laryngoscope 2018, 128, 2525–2528. [Google Scholar] [CrossRef]
- Turhan, M.; Bostanci, A. Robotic resection of lingual thyroglossal duct cyst in an infant. J. Robot. Surg. 2019, 13, 331–334. [Google Scholar] [CrossRef]
- Fanous, A.; Couloigner, V.; Gorphe, P.; Galmiche, L.; Alexandru, M.; Garabedian, E.-N.; Coffinet, L.; Blanc, T.; Leboulanger, N.; Denoyelle, F. Unusual presentation of a first Branchial cleft cyst associated with an abnormal bony canal -a case report. J. Otolaryngol. Head Neck Surg. 2020, 49, 32. [Google Scholar] [CrossRef]
- Das, S.; Sekar, R.; Alexander, A.; Ganesan, S. Transoral Robotic Excision of Paediatric Lingual Thyroglossal Duct Cyst. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 2423–2426. [Google Scholar] [CrossRef]
- Albi, C.; Ciorba, A.; Bianchini, C.; Cammaroto, G.; Pelucchi, S.; Sgarzani, R.; Gessaroli, M.; DE Vito, A.; Vicini, C.; Meccariello, G. Transoral robotic surgery for oropharyngeal cancer: A systematic review on the role of margin status. Minerva Surg. 2024, 79, 346–353. [Google Scholar] [CrossRef]
- Cammaroto, G.; Stringa, L.M.; Zhang, H.; Capaccio, P.; Galletti, F.; Galletti, B.; Meccariello, G.; Iannella, G.; Pelucchi, S.; Baghat, A.; et al. Alternative Applications of Trans-Oral Robotic Surgery (TORS): A Systematic Review. J. Clin. Med. 2020, 9, 201. [Google Scholar] [CrossRef]
- Téblick, S.; Ruymaekers, M.; Van de Casteele, E.; Boudewyns, A.; Nadjmi, N. The effect of soft palate reconstruction with the da Vinci robot on middle ear function in children: An observational study. Int. J. Oral. Maxillofac. Surg. 2023, 52, 931–938. [Google Scholar] [CrossRef]
- Nittrouer, S.; Lowenstein, J.H. Early otitis media puts children at risk for later auditory and language deficits. Int. J. Pediatr. Otorhinolaryngol. 2024, 176, 111801. [Google Scholar] [CrossRef]
- Altamimi, A.A.; Robinson, M.; Alenezi, E.M.; Veselinović, T.; Choi, R.S.; Brennan-Jones, C.G. Recurrent otitis media and behaviour problems in middle childhood: A longitudinal cohort study. J. Paediatr. Child. Health. 2024, 60, 12–17. [Google Scholar] [CrossRef]
- Bhatia, R.; Chauhan, A.; Rana, M.; Kaur, K.; Pradhan, P.; Singh, M. Economic Burden of Otitis Media Globally and an Overview of the Current Scenario to Alleviate the Disease Burden: A Systematic Review. Int. Arch. Otorhinolaryngol. 2024, 28, e552–e558. [Google Scholar] [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Ward, S.D.; Sheldon, S.H.; Shiffman, R.N. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, 576–584. [Google Scholar] [CrossRef]
- Meccariello, G.; Cammaroto, G.; Montevecchi, F.; Hoff, P.T.; Spector, M.E.; Negm, H.; Shams, M.; Bellini, C.; Zeccardo, E.; Vicini, C. Transoral robotic surgery for the management of obstructive sleep apnea: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2017, 274, 647–653. [Google Scholar] [CrossRef]
- Meccariello, G.; Cammaroto, G.; Iannella, G.; De Vito, A.; Ciorba, A.; Bianchini, C.; Corazzi, V.; Pelucchi, S.; Vicini, C.; Capaccio, P. Transoral robotic surgery for oropharyngeal cancer in the era of chemoradiation therapy. Auris Nasus Larynx 2022, 49, 535–546. [Google Scholar] [CrossRef]
- Sameer, K.S.; Mohanty, S.; Correa, M.M.; Das, K. Lingual thyroglossal duct cysts--a review. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 165–168. [Google Scholar] [CrossRef]
- Turri-Zanoni, M.; Battaglia, P.; Castelnuovo, P. Thyroglossal Duct Cyst at the Base of Tongue: The Emerging Role of Transoral Endoscopic-Assisted Surgery. J. Craniofacial Surg. 2018, 29, 469–470. [Google Scholar] [CrossRef]
- Burkart, C.M.; Richter, G.T.; Rutter, M.J.; Myer, C.M., 3rd. Update on endoscopic management of lingual thyroglossal duct cysts. Laryngoscope 2009, 119, 2055–2060. [Google Scholar] [CrossRef]
- Zhang, L.C.; Zhang, T.Y.; Sha, Y.; Lin, Y.X.; Chen, Q. Lingual thyroglossal duct cyst with recurrence after cystectomy or marsupialization under endoscopy: Diagnosis and modified Sistrunk surgery. Laryngoscope 2011, 121, 1888–1892. [Google Scholar] [CrossRef]
- Erkul, E.; Duvvuri, U.; Mehta, D.; Aydil, U. Transoral robotic surgery for the pediatric head and neck surgeries. Eur. Arch. Otorhinolaryngol. 2017, 274, 1747–1750. [Google Scholar] [CrossRef]
- Hockstein, N.G.; Nolan, J.P.; O’malley, B.W., Jr.; Woo, Y.J. Robotic microlaryngeal surgery: A technical feasibility study using the daVinci surgical robot and an airway mannequin. Laryngoscope 2005, 115, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Gaffuri, M.; di Lullo, A.M.; Trecca, E.M.C.; Russo, G.; Molinari, G.; Russo, F.Y.; Albera, A.; Mannelli, G.; Ralli, M.; Turri-Zanoni, M. High-Definition 3D Exoscope in Pediatric Otorhinolaryngology: A Systematic Literature Review. J. Clin. Med. 2023, 12, 6528. [Google Scholar] [CrossRef] [PubMed]
- Vinit, N.; Vatta, F.; Broch, A.; Hidalgo, M.; Kohaut, J.; Querciagrossa, S.; Couloigner, V.; Khen-Dunlop, N.; Botto, N.; Capito, C.; et al. Adverse Events and Morbidity in a Multidisciplinary Pediatric Robotic Surgery Program. A prospective, Observational Study. Ann. Surg. 2023, 278, e932–e938. [Google Scholar] [CrossRef]
- Autorino, G.; Mendoza-Sagaon, M.; Scuderi, M.G. Narrative review in learning curve and pediatric robotic training program. Transl. Pediatr. 2024, 13, 343–349. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.R.; Moskowitz, A.J.; Miles, B.A.; Goldstein, D.P.; Teng, M.S.; Sikora, A.G.; Gupta, V.; Posner, M.; Genden, E.M. Cost-effectiveness of transoral robotic surgery versus (chemo)radiotherapy for early T classification oropharyngeal carcinoma: A cost-utility analysis. Head Neck 2016, 38, 589–600. [Google Scholar] [CrossRef]
- Spellman, J.; Coulter, M.; Kawatkar, A.; Calzada, G. Comparative cost of transoral robotic surgery and radiotherapy (IMRT) in early stage tonsil cancer. Am. J. Otolaryngol. 2020, 41, 102409. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).