Video Laryngoscopes in Simulated Neonatal Intubation: Usability Study
Abstract
1. Introduction
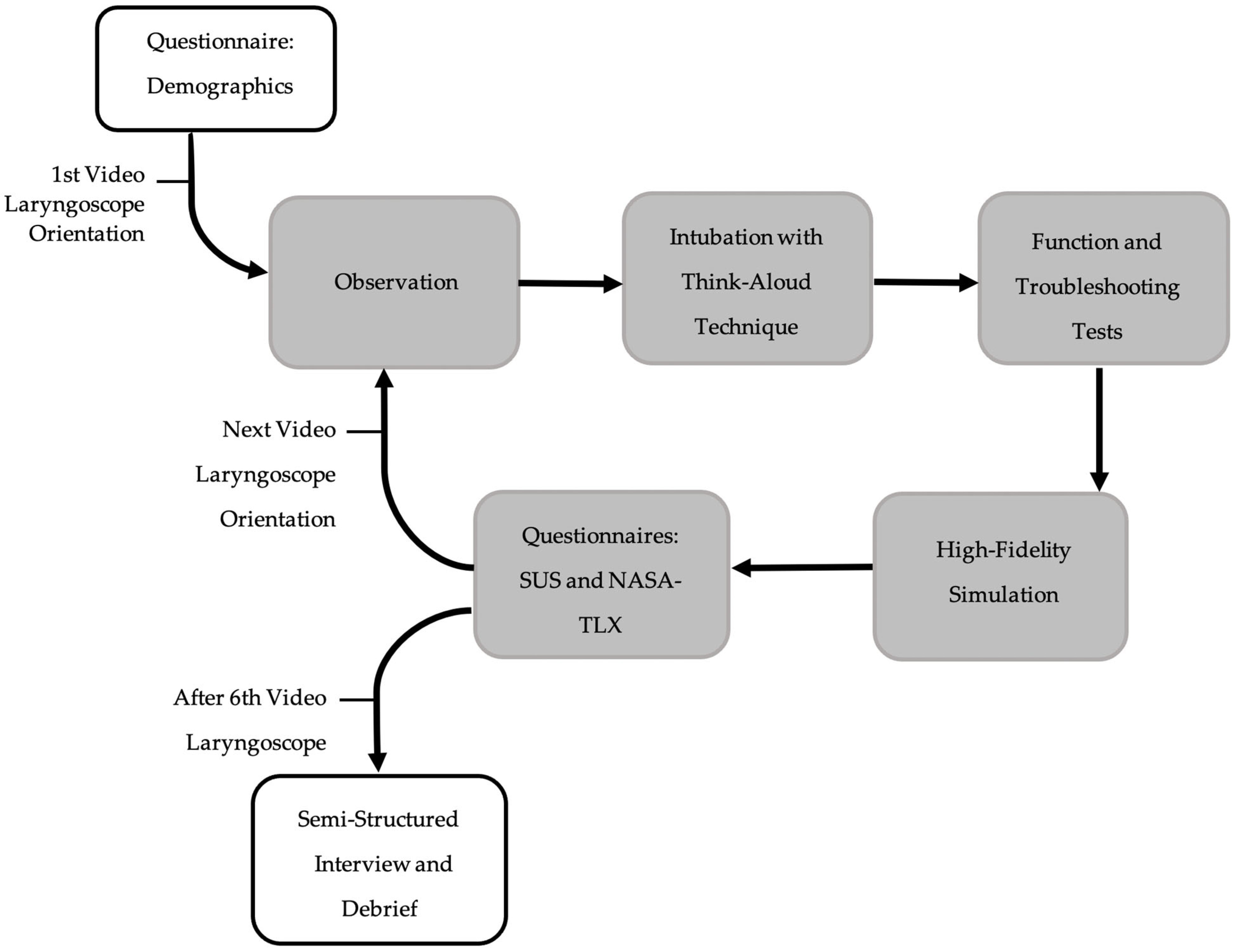
2. Materials and Methods
2.1. Study Design
2.2. Setting and Participants
2.3. Equipment
2.4. Outcome Measures
2.5. Statistics and Analysis
3. Results
3.1. Participant Demographics
3.2. Device Characteristics
3.3. Clinician Satisfaction
3.4. Efficacy
3.5. Efficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SUS | System Usability Scale |
| NASA-TLX | National Aeronautics and Space Administration Task Load Index |
| IQR | Interquartile range |
| SPSS | Statistical Package for the Social Sciences |
| IPPV | Intermittent positive pressure ventilation |
| TT | Tracheal tube |
| ILCOR | International Liaison Committee on Resuscitation |
| P# | Participant number |
References
- Moussa, A.; Sawyer, T.; Puia-Dumitrescu, M.; Foglia, E.E.; Ades, A.; Napolitano, N.; Glass, K.M.; Johnston, L.; Jung, P.; Singh, N.; et al. Does videolaryngoscopy improve tracheal intubation first attempt success in the NICUs? A report from the NEAR4NEOS. J. Perinatol. 2022, 42, 1210–1215. [Google Scholar] [CrossRef]
- Thomas, H.; Lugg, R.; James, B.; Geeroms, C.; Risbridger, A.; Bell, R.; Bartle, D.G. Survey of the use of videolaryngoscopy in neonatal units in the UK. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 89. [Google Scholar] [CrossRef]
- Fawke, J.; Costa-Nobre, D.T.; Antoine, J.; Guinsburg, R.; de Almeida, M.F.; Schmölzer, G.M.; Wyckoff, M.H.; Weiner, G.M.; Liley, H.G. On behalf of the International Liaison Committee on Resuscitation Neonatal Life Support Task Force, Video vs. traditional laryngoscopy for tracheal intubation at birth or in the neonatal unit: A systematic review and meta-analysis. Resusc. Plus 2025, 100965. [Google Scholar] [CrossRef]
- Lingappan, K.; Neveln, N.; Arnold, J.L.; Fernandes, C.J.; Pammi, M. Videolaryngoscopy versus direct laryngoscopy for tracheal intubation in neonates. Cochrane Database Syst. Rev. 2023. [Google Scholar] [CrossRef]
- MacKinnon, J.; McCoy, C. Use of video laryngoscopy versus direct laryngoscopy as a teaching tool for neonatal intubation: A systematic review. Can. J. Respir. Ther. 2023, 59, 111–116. [Google Scholar] [CrossRef]
- Liley, H.G.; Weiner, G.M.; Wyckoff, M.H.; Rabi, Y.; Schmölzer, G.M.; de Almeida, M.F.; Costa Nobre, D.; Daripa Kawakami, M.; Davis, P.G.; Dawson, J.A.; et al. 2025 International Liaison Committee on Resuscitation Consensus on Science with Treatment Recommendations Neonatal Life Support; ILCOR: Rotterdam, The Netherlands, 2025; Available online: https://ilcor.org/uploads/NLS-2025-COSTR-Full-Chapter.pdf (accessed on 19 March 2025).
- Gupta, A.; Sharma, R.; Gupta, N. Evolution of videolaryngoscopy in pediatric population. J. Anaesthesiol. Clin. Pharmacol. 2021, 37, 14–27. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.E.; Thio, M.; Kamlin, C.O.; McGrory, L.; Wong, C.; John, J.; Roberts, C.; Kuschel, C.; Davis, P.G. Videolaryngoscopy to Teach Neonatal Intubation: A Randomized Trial. Pediatrics 2015, 136, 912–919. [Google Scholar] [CrossRef]
- Roozendaal, B.; Okuda, S.; de Quervain, D.J.; McGaugh, J.L. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience 2006, 138, 901–910. [Google Scholar] [CrossRef]
- Fawke, J.; Stave, C.; Yamada, N. Use of briefing and debriefing in neonatal resuscitation, a scoping review. Resusc. Plus 2021, 5, 100059. [Google Scholar] [CrossRef]
- O’Shea, J.E.; Loganathan, P.; Thio, M.; Kamlin, C.O.F.; Davis, P.G. Analysis of unsuccessful intubations in neonates using videolaryngoscopy recordings. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F408–F412. [Google Scholar] [CrossRef]
- Balaban, O.; Tobias, J.D. Videolaryngoscopy in Neonates, Infants, and Children. Pediatr. Crit. Care Med. 2017, 18, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Kirolos, S.; O’Shea, J.E. Comparison of conventional and videolaryngoscopy blades in neonates. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Liljegren, E. Usability in a medical technology context assessment of methods for usability evaluation of medical equipment. Int. J. Ind. Ergon. 2006, 36, 345–352. [Google Scholar] [CrossRef]
- IEC 62366-1:2015; Medical Devices Part 1: Application of Usability Engineering to Medical Devices. The International Electrotechnical Commission (IEC): Geneva, Switzerland, 2015.
- Gartlehner, G.; Hansen, R.A.; Nissman, D.; Lohr, K.N.; Carey, T.S. Criteria for Distinguishing Effectiveness from Efficacy Trials in Systematic Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2006. [Google Scholar]
- Fuerch, J.H.; Sanderson, P.; Barshi, I.; Liley, H. Developing safe devices for neonatal care. Semin. Perinatol. 2019, 43, 151176. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J. SUS-A quick and dirty usability scale. Usability Eval. Ind. 1996, 189, 4–7. [Google Scholar]
- Hart, S.G. Nasa-Task Load Index (NASA-TLX); 20 Years Later. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2006, 50, 904–908. [Google Scholar] [CrossRef]
- Nielsen, J. Why You Only Need to Test with 5 Users. 2000. Available online: http://www.useit.com/alertbox/20000319.html (accessed on 19 March 2025).
- Virzi, R.A. Refining the Test Phase of Usability Evaluation: How Many Subjects Is Enough? Hum. Factors 1992, 34, 457–468. [Google Scholar] [CrossRef]
- Foglia, E.E.; Ades, A.; Sawyer, T.; Glass, K.M.; Singh, N.; Jung, P.; Bin Huey, Q.; Johnston, L.C.; Barry, J.; Zenge, J.; et al. Neonatal Intubation Practice and Outcomes: An Internationa Registry Study. Pediatrics 2019, 143, e20180902. [Google Scholar] [CrossRef]
- Chow, S.S.W.; Creighton, P.; Chambers, G.M.; Lui, K. Report of Australian and New Zealand Neonatal Network 2022; University of New South Wales: Sydney, Australia, 2022. [Google Scholar]
- Nadler, I.; McLanders, M.; Sanderson, P.; Liley, H. Time without ventilation during intubation in neonates as a patient-centred measure of performance. Resuscitation 2016, 105, 41–44. [Google Scholar] [CrossRef]
- Sauro, J.; Lewis, J.R. Chapter 8—Standardized usability questionnaires. In Quantifying the User Experience (Second Edition); Sauro, J., Lewis, J.R., Eds.; Morgan Kaufmann: Burlington, MA, USA, 2016. [Google Scholar]
- O’Donnell, C.P.; Kamlin, C.O.; Davis, P.G.; Morley, C.J. Endotracheal intubation attempts during neonatal resuscitation: Success rates, duration, and adverse effects. Pediatrics 2006, 117, e16–e21. [Google Scholar] [CrossRef]
- Borsci, S.; Macredie, R.D.; Martin, J.L.; Young, T. How many testers are needed to assure the usability of medical devices? Expert Rev. Med. Devices 2014, 11, 513–525. [Google Scholar] [CrossRef] [PubMed]
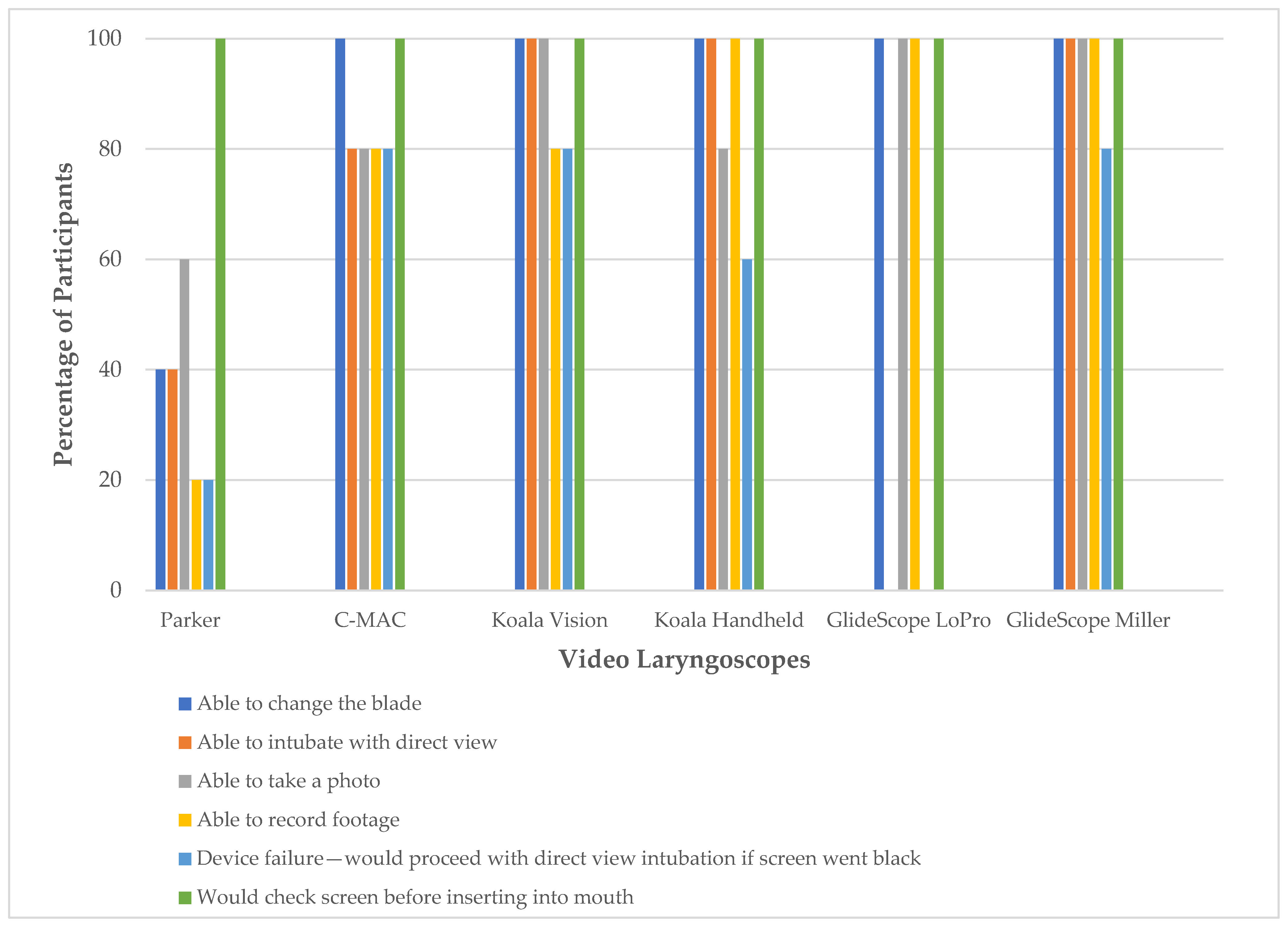
| Participant Demographics | Frequency of Participants N (%) |
|---|---|
| Sex | 2 (40%) Female 3 (60%) Male |
| Intubations with conventional laryngoscope | 5 (100%) had completed > 100 conventional laryngoscope intubations |
| Intubations with video laryngoscope | 1 (20%) no intubations with video laryngoscope 2 (40%) < 5 intubations with video laryngoscope 2 (40%) > 10 intubations with video laryngoscope |
| Previous experience with C-MAC | 3 (60%) none 2 (40%) yes |
| Previous experience with GlideScope Miller | 5 (100%) none |
| Previous experience with GlideScope LoPro | 5 (100%) none |
| Previous experience with Koala Vision | 2 (40%) none 3 (60%) yes |
| Previous experience with Koala Handheld | 2 (40%) none 3 (60%) yes |
| Previous experience with Parker | 2 (40%) none 3 (60%) yes |
| Self-rated competence to intubate with video laryngoscope | 1 (20%) not competent 4 (80%) competent |
| Self-rated competence to supervise video laryngoscope intubation | 3 (60%) not competent 2 (40%) competent |
| Video Laryngoscope | Blade | Screen and Mount | Image | Thematic Exemplar of Participant Comments (Participant Number) |
| C-MAC® (KARL STORZ, Brisbane, Australia) | Reusable Miller blades size 0, 1 | 8” screen unmounted |  | Ergonomics of Handle and Blade: “Thick handle doesn’t feel comfortable” (P4) “Size 1 is enormous. Blade is slim in the baby’s mouth” (P5) “Cord from screen to handle too long” (P2) Screen and Mount: “Screen can mount it on something. Would be better mounted” (P4) “Screen is very heavy, designed to be mounted. Would be worried without it mounted” (P2) Screen placed at the end of the bed near feet. Comparison to Conventional Laryngoscope: “Mental load of having to look down, would prefer just to look into the baby’s mouth to put the tube in” (P4) |
| GlideScope® CoreTM system (GlideScope Miller) (Verathon, Brisbane, Australia) | Single-use Miller blades size 0, 1 | 10” screen mounted on stand with articulated arm |  | Ergonomics of Handle and Blade: “Weight of blade different” (P2) “Changing blade is easy” (P1) Screen and Mount: “Magnified picture” (P4) “Screen is fantastic, moves up and down” (P4) Stand is “bit bulky and heavy” (P1) Comparison to Conventional Laryngoscope: “Forgot to turn on prior to putting blade in mouth, because its open, looks like a regular laryngoscope jumped to that assumption” (P5) |
| GlideScope® CoreTM system (GlideScope LoPro) (Verathon, Brisbane, Australia) | Single-use hyperangle LoPro blades size S1, S2 | 10” screen mounted on stand with articulated arm |  | Ergonomics of Handle and Blade: “Never seen blade like this” (P4) “Just need to get angle right. Very angle dependent. If have right angle easy to do. Otherwise ETT goes into oesophagus” (P2) Screen and Mount: “Display is good. High quality. High resolution” (P3) “Screen allows movement up and down which is handy” (P4) Stand is “quite bulky” (P3) “Problem is positioning it during resuscitation” (P2) “Like video laryngoscope because magnifies view, makes easier for me” (P2) |
| Koala® Vision Ultra (Koala Vision) (Koala Medical, Sydney, Australia) | Reusable Miller blades 00, 0, 1 | 8” screen mounted on stand |  | Ergonomics of Handle and Blade: “Quite good with 00 Miller” (P3) Screen and Mount: “Good resolution” (P3) “Can’t change the height…would like it lower” (P5) Stand is “quite easy to move” (P4) “Need to think about where to place with stand…Easier to move around than the GlideScope with stand” (P2) Comparison to Conventional Laryngoscope: “Similar to direct laryngoscope for changing blades” (P1) |
| Koala® Handheld (Koala Medical, Sydney, Australia) | Reusable Miller blades 00, 0, 1 | 3.5” screen mounted on handle |  | Ergonomics of Handle and Blade: “Feels like blade is really big in the mouth, big and wide” (P5) “Blade is similar to direct laryngoscope” (P1) Screen and Mount: “Like it because its compact” (P1) “I like that screen in field of vision” (P2) “Advantage not twisting to look at screen, everyone can see it.” (P3) “Top of handle can hit top of the cot” (P1) Teaching: Good for teaching because “instructor can see with magnification, can intubate directly” (P3) “Still use as teaching tool, get trainee close to look at screen” (P4) Comparison to Conventional Laryngoscope: “Similar to direct laryngoscope for changing blades” (P1) “Feels more intuitive because connected to blade and baby” (P5) |
| Parker Neonatal (Parker, Healthcare, Melbourne, Australia) | Reusable Miller blades sizes 000, 00, 0, 1 | 5” screen unmounted |  Parker Neonatal with size 1 blade without sleeve  Size 00 blade with sleeve | Ergonomics of Handle and Blade: “Blade is small. Wouldn’t give good control of the tongue for difficult intubation” (P1) “They have really small blades 000, if 300-400g helpful as profile of blade is very small” (P2) “Handle not very ergonomical” (P4) “Change the blade matter of plugging in another blade… sleeve not intuitive… size once out of packaging not labelled, need to ensure correct sleeve attached” (P2) Screen and Mount: “Getting a lot of glare onto screen” (P5) “Screen fell over, would need assistant” (P3) |
| Video Laryngoscope | SUS Median (IQR) |
|---|---|
| Parker | 32.5 (23.8) |
| GlideScope LoPro | 62.5 (23.8) |
| C-MAC | 65.0 (10.0) |
| Koala Vision | 72.5 (10.0) |
| GlideScope Miller | 75.0 (22.5) |
| Koala Handheld | 87.5 (25) |
| Video Laryngoscope | Overall NASA-TLX Median (IQR) | Mental Demand Median (IQR) | Physical Demand Median (IQR) | Temporal Demand Median (IQR) | Performance Median (IQR) | Effort Median (IQR) | Frustration Median (IQR) |
|---|---|---|---|---|---|---|---|
| Parker | 50.8 (22.9) | 60 (40) | 60 (28) | 30 (33) | 25 (23) | 60 (25) | 55 (33) |
| GlideScope LoPro | 45.0 (52.5) | 65 (58) | 50 (60) | 35 (43) | 40 (58) | 40 (68) | 20 (45) |
| Koala Vision | 39.2 (33.8) | 50 (58) | 45 (35) | 40 (50) | 35 (23) | 45 (48) | 25 (33) |
| Koala Handheld | 39.2 (30.4) | 30 (48) | 30 (38) | 45 (35) | 10 (33) | 55 (55) | 20 (33) |
| GlideScope Miller | 32.5 (48.3) | 35 (53) | 50 (58) | 30 (43) | 20 (35) | 35 (60) | 20 (60) |
| C-MAC | 26.7 (18.8) | 20 (35) | 35 (43) | 30 (18) | 15 (20) | 25 (45) | 20 (30) |
| Video Laryngoscope | First-Pass Success N (%) | Number of Attempts Median (IQR) | Overall Success N (%) | Time to Successful Intubation Median (IQR) |
|---|---|---|---|---|
| GlideScope LoPro | 4 (80%) * | 1 (2.0) * | 4 (80%) * | 35.5 s (9.8) * |
| Koala Vision | 4 (80%) | 1 (1.0) | 5 (100%) | 31.0 s (14.0) |
| GlideScope Miller | 4 (80%) | 1 (1.0) | 5 (100%) | 25.0 s (10.5) |
| Parker | 5 (100%) | 1 (0.0) | 5 (100%) | 31.0 s (19.0) |
| Koala Handheld | 5 (100%) | 1 (0.0) | 5 (100%) | 26.0 s (12.5) |
| C-MAC | 5 (100%) | 1 (0.0) | 5 (100%) | 23.0 s (10.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoine, J.; McLeod, K.; Jardine, L.; Liley, H.G.; McLanders, M. Video Laryngoscopes in Simulated Neonatal Intubation: Usability Study. Children 2025, 12, 723. https://doi.org/10.3390/children12060723
Antoine J, McLeod K, Jardine L, Liley HG, McLanders M. Video Laryngoscopes in Simulated Neonatal Intubation: Usability Study. Children. 2025; 12(6):723. https://doi.org/10.3390/children12060723
Chicago/Turabian StyleAntoine, Jasmine, Kirsty McLeod, Luke Jardine, Helen G. Liley, and Mia McLanders. 2025. "Video Laryngoscopes in Simulated Neonatal Intubation: Usability Study" Children 12, no. 6: 723. https://doi.org/10.3390/children12060723
APA StyleAntoine, J., McLeod, K., Jardine, L., Liley, H. G., & McLanders, M. (2025). Video Laryngoscopes in Simulated Neonatal Intubation: Usability Study. Children, 12(6), 723. https://doi.org/10.3390/children12060723






