Vitamin C in Allergy Mechanisms and for Managing Allergic Diseases: A Narrative Review
Abstract
1. Introduction
2. Methods
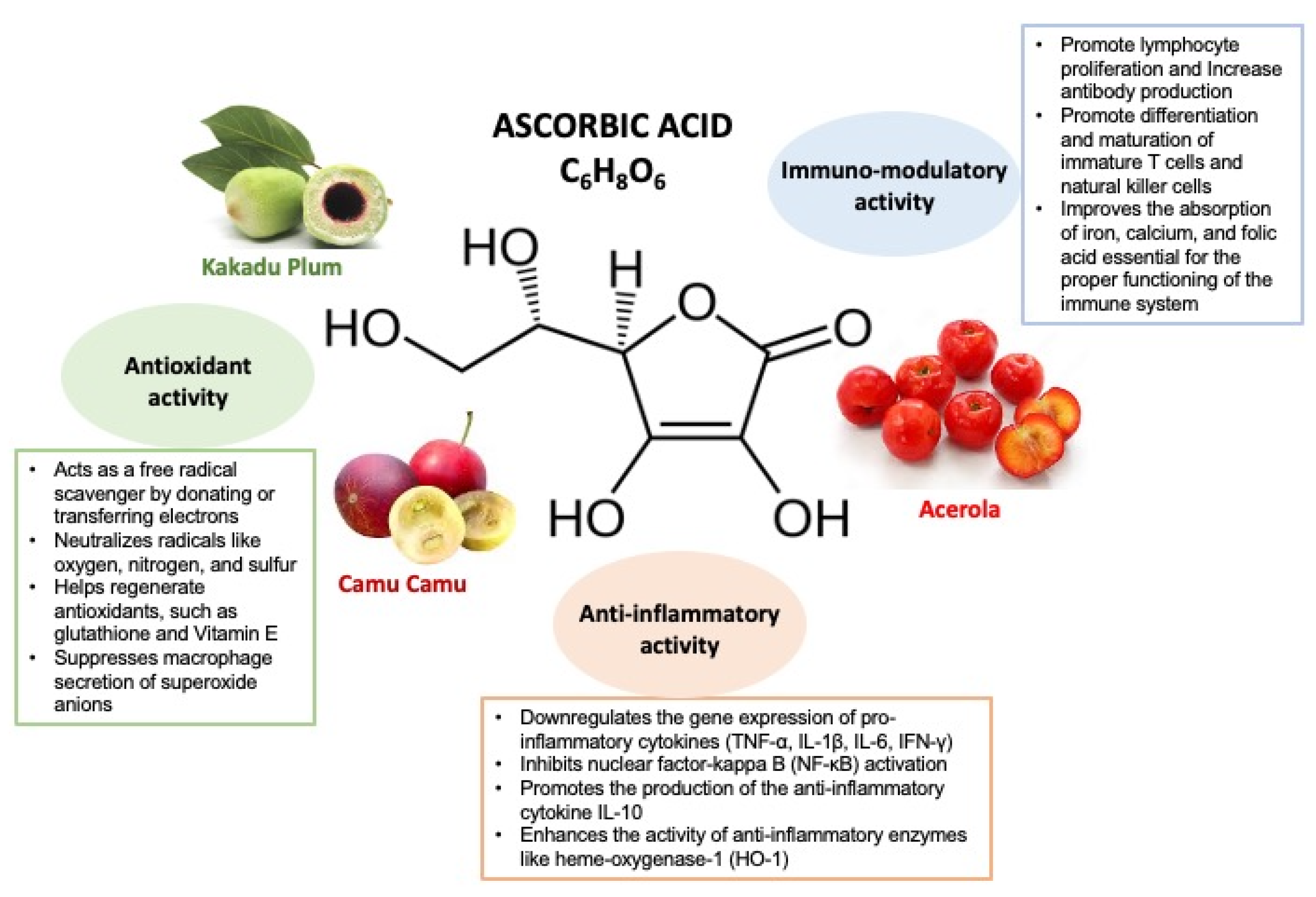
3. Vitamin C Mechanisms of Action
4. The Inflammatory Basis of Allergic Diseases
5. Allergic Rhinitis
6. Asthma and Ascorbic Acid Dietary Intake
7. Asthma and Ascorbic Acid Supplementation
8. Atopic Dermatitis and Ascorbic Acid
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two faces of Vitamin C—Antioxidative and pro-oxidative agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Chan, S.; Xiong, P.; Zhao, M.; Zhang, S.; Zheng, R.; Ye, J.; Chan, K.; Li, C.; Zhong, Z. Anti-inflammatory effects of natural products from vitamin C-rich fruits. Food Front. 2024, 5, 2383–2422. [Google Scholar] [CrossRef]
- Zhou, Y.; Phan, A.D.T.; Akter, S.; Bobasa, E.M.; Seididamyeh, M.; Sivakumar, D.; Sultanbawa, Y. Bioactive Properties of Kakadu Plum-Blended Products. Molecules 2023, 28, 2828. [Google Scholar] [CrossRef]
- Cunha-Santos, E.C.E.; Viganó, J.; Neves, D.A.; Martínez, J.; Godoy, H.T. Vitamin C in camu-camu [Myrciaria dubia (H.B.K.) McVaugh]: Evaluation of extraction and analytical methods. Food Res. Int. 2018, 115, 160–166. [Google Scholar] [CrossRef]
- Cefali, L.C.; de Oliveira Maia, L.; Stahlschimidt, R.; Ataide, J.A.; Tambourgi, E.B.; Rosa, P.C.P.; Mazzola, P.G. Vitamin C in Acerola and Red Plum Extracts: Quantification via HPLC, in Vitro Antioxidant Activity, and Stability of their Gel and Emulsion Formulations. J. AOAC Int. 2018, 101, 1461–1465. [Google Scholar] [CrossRef]
- Tsukaguchi, H.; Tokui, T.; Mackenzie, B.; Berger, U.V.; Chen, X.Z.; Wang, Y.; Brubaker, R.F.; Hediger, M.A. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999, 399, 70–75. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The pharmacokinetics of vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, G.; Negro, M.; Parimbelli, M.; Pecoraro, M.; Perna, S.; Liguori, G.; Rondanelli, M.; Cena, H.; D’Antona, G. The Long History of Vitamin C: From Prevention of the Common Cold to Potential Aid in the Treatment of COVID-19. Front. Immunol. 2020, 11, 574029. [Google Scholar] [CrossRef] [PubMed]
- Martin, A. Nutritional recommendations for the French population: The “apports nutritionnels conseillés” (ANCs). Sci. Aliments 2001, 21, 119–128. [Google Scholar]
- Ghalibaf, M.H.E.; Kianian, F.; Beigoli, S.; Behrouz, S.; Marefati, N.; Boskabady, M.; Boskabady, M.H. The effects of vitamin C on respiratory, allergic and immunological diseases: An experimental and clinical-based review. Inflammopharmacology 2023, 31, 653–672. [Google Scholar] [CrossRef]
- Global Burden of Disease 2016 Disease and Injury Incidenceand Prevalence Collaborators. Global, regional, and national in -cidence, prevalence, and years lived with disability for 328 dis-eases and injuries for 195 countries, 1990–2016: A systematicanalysis for the Global Burden of Disease Study 2016. Lancet 2016, 2017, 1211–1259. [Google Scholar]
- Agache, I.; Akdis, C.A.; Akdis, M.; Canonica, G.W.; Casale, T.; Chivato, T.; Corren, J.; Chu, D.K.; Del Giacco, S.; Eiwegger, T.; et al. EAACI Biologicals Guidelines-Recommendations for severe asthma. Allergy 2021, 76, 14–44. [Google Scholar] [CrossRef]
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. 1), S2–S6. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Walker, A.; Pirwani, M.M.; Tahiri, M.; Syed, I. Allergic rhinitis: Diagnosis and management. Br. J. Hosp. Med. 2022, 83, 1–9. [Google Scholar] [CrossRef]
- Wise, S.K.; Damask, C.; Roland, L.T.; Ebert, C.; Levy, J.M.; Lin, S.; Luong, A.; Rodriguez, K.; Sedaghat, A.R.; Toskala, E.; et al. International consensus statement on allergy and rhinology: Allergic rhinitis—2023. Int. Forum Allergy Rhinol. 2023, 13, 293–859. [Google Scholar]
- Mortz, C.G.; Andersen, K.E.; Dellgren, C.; Barington, T.; Bindslev-Jensen, C. Atopic dermatitis from adolescence to adulthood Theme issue: Atopic dermatitis in the TOACS cohort: Prevalence, persistence and comorbidities. Allergy 2015, 70, 836–845. [Google Scholar] [CrossRef]
- Drucker, A.M. Atopic dermatitis: Burden of illness, quality of life, and associated complications. Allergy Asthma Proc. 2017, 38, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Farkas, D.; Brophy, D.F.; Fowler, A.A., 3rd; Natarajan, R. Vitamin C: A novel regulator of neutrophil extracellular trap formation. Nutrients 2013, 5, 3131–3151. [Google Scholar] [CrossRef] [PubMed]
- Conklin, P.L.; Foyer, C.H.; Hancock, R.D.; Ishikawa, T.; Smirnoff, N. Ascorbic acid metabolism and functions. J. Exp. Bot. 2024, 75, 2599–2603. [Google Scholar]
- Lykkesfeldt, J.; Carr, A.C.; Tveden-Nyborg, P. The pharmacology of vitamin C. Pharmacol. Rev. 2025, 77, 100043. [Google Scholar] [CrossRef]
- Gasmi, A.; Shanaida, M.; Oleshchuk, O.; Semenova, Y.; Mujawdiya, P.K.; Ivankiv, Y.; Pokryshko, O.; Noor, S.; Piscopo, S.; Adamiv, S.; et al. Natural Ingredients to Improve Immunity. Pharmaceuticals 2023, 16, 528. [Google Scholar] [CrossRef]
- Sharma, P.; Raghavan, S.A.; Saini, R.; Dikshit, M. Ascorbate-mediated enhancement of reactive oxygen species generation from polymorphonuclear leukocytes: Modulatory effect of nitric oxide. J. Leukoc. Biol. 2004, 75, 1070–1078. [Google Scholar] [CrossRef]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef]
- Micillo, E.; Bianco, A.; D’Auria, D.; Mazzarella, G.; Abbate, G.F. Respiratory infections and asthma. Allergy 2000, 55 (Suppl. 61), 42–45. [Google Scholar]
- Forastiere, F.; Pistelli, R.; Sestini, P.; Fortes, C.; Renzoni, E.; Rusconi, F.; Dell’Orco, V.; Ciccone, G.; Bisanti, L. Consumption of fresh fruit rich in vitamin C and wheezing symptoms in children. Thorax 2000, 55, 283–288. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Li, X.; Yang, F.; Wang, C.; Yu, C. Multivitamin consumption and childhood asthma: A cross-sectional study of the NHANES database. BMC Pediatr. 2024, 24, 84. [Google Scholar] [CrossRef] [PubMed]
- Siripornpanich, S.; Chongviriyaphan, N.; Manuyakorn, W.; Matangkasombut, P. Zinc and vitamin C deficiencies associate with poor pulmonary function in children with persistent asthma. Asian Pac. J. Allergy Immunol. 2022, 40, 103–110. [Google Scholar] [PubMed]
- García-García, C.; Kim, M.; Baik, I. Associations of dietary vitamin A and C intake with asthma, allergic rhinitis, and allergic respiratory diseases. Nutr. Res. Pract. 2023, 17, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Larsen, V.; Arthur, R.; Potts, J.F.; Howarth, P.H.; Ahlström, M.; Haahtela, T.; Loureiro, C.; Bom, A.T.; Brożek, G.; Makowska, J. Is fruit and vegetable intake associated with asthma or chronic rhino-sinusitis in European adults? Results from the Global Allergy and Asthma Network of Excellence (GA2LEN) Survey. Clin. Transl. Allergy 2017, 7, 3. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, L.; Luo, H.; Deng, C.; Gong, L.; Chen, Z. Association of serum vitamin C levels with Asthma in adults: Results of NHANES 2003–2006 and mendelian randomization study. BMC Pulm. Med. 2024, 24, 4. [Google Scholar] [CrossRef]
- Misso, N.L.A.; Brooks-Wildhaber, J.; Ray, S.; Vally, H.; Thompson, P.J. Plasma concentrations of dietary and nondietary antioxidants are low in severe asthma. Eur. Respir. J. 2005, 26, 257–264. [Google Scholar] [CrossRef]
- Oh, S.Y.; Chung, J.; Kim, M.K.; Kwon, S.O.; Cho, B.H. Antioxidant nutrient intakes and corresponding biomarkers associated with the risk of atopic dermatitis in young children. Eur. J. Clin. Nutr. 2010, 64, 245–252. [Google Scholar] [CrossRef]
- Fortner, B.R.; Danziger, R.E.; Rabinowitz, P.S.; Nelson, H.S. The effect of ascorbic acid on cutaneous and nasal response to histamine and allergen. J. Allergy Clin. Immunol. 1982, 69, 484–488. [Google Scholar] [CrossRef]
- Tongtako, W.; Klaewsongkram, J.; Mickleborough, T.D.; Suksom, D. Effects of aerobic exercise and vitamin C supplementation on rhinitis symptoms in allergic rhinitis patients. Asian Pac. J. Allergy Immunol. 2018, 36, 222–231. [Google Scholar]
- Vollbracht, C.; Raithel, M.; Krick, B.; Kraft, K.; Hagel, A.F. Intravenous vitamin C in the treatment of allergies: An interim subgroup analysis of a long-term observational study. J. Int. Med. Res. 2018, 46, 3640–3655. [Google Scholar] [CrossRef]
- Tecklenburg, S.L.; Mickleborough, T.D.; Fly, A.D.; Bai, Y.; Stager, J.M. Ascorbic acid supplementation attenuates exercise-induced bronchoconstriction in patients with asthma. Respir. Med. 2007, 101, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, J.; Johnston, S.L. Unravelling synergistic immune interactions between respiratory virus infections and allergic airway inflammation. Clin. Exp. Allergy. 2004, 34, 1153–1155. [Google Scholar] [CrossRef] [PubMed]
- Bhoot, H.R.; Zamwar, U.M.; Chakole, S.; Anjankar, A. Dietary Sources, Bioavailability, and Functions of Ascorbic Acid (Vitamin C) and Its Role in the Common Cold, Tissue Healing, and Iron Metabolism. Cureus 2023, 15, e49308. [Google Scholar] [CrossRef]
- Miller, R.L.; Grayson, M.H.; Strothman, K. Advances in asthma: New understandings of asthma’s natural history, risk factors, underlying mechanisms, and clinical management. J. Allergy Clin. Immunol. 2021, 148, 1430–1441. [Google Scholar] [CrossRef]
- Doss, A.M.A.; Stokes, J.R. Viral Infections and Wheezing in Preschool Children. Immunol. Allergy Clin. 2022, 42, 727–741. [Google Scholar] [CrossRef]
- Bove, P.F.; van der Vliet, A. Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Radic. Biol. Med. 2006, 41, 515–527. [Google Scholar] [CrossRef]
- Van Muylem, A.; Malinovschi, A.; Haccuria, A.; Michils, A. Exhaled nitric oxide and its predictive power related to lung function and bronchial inflammation. Biochem. Pharmacol. 2020, 179, 114101. [Google Scholar] [CrossRef]
- Biedrzycki, G.; Wolszczak-Biedrzycka, B.; Dorf, J.; Maciejczyk, M. The antioxidant barrier, oxidative/nitrosative stress, and protein glycation in allergy: From basic research to clinical practice. Front. Immunol. 2024, 15, 1440313. [Google Scholar] [CrossRef]
- Licari, A.; Castagnoli, R.; Brambilla, I.; Marseglia, A.; Tosca, M.A.; Marseglia, G.L.; Ciprandi, G. Asthma endotyping and biomarkers in childhood asthma. Pediatr. Allergy Immunol. Pulmonol. 2018, 31, 44–55. [Google Scholar] [CrossRef]
- McKeever, T.M.; Lewis, S.A.; Smit, H.; Burney, P.; Britton, J.; Cassano, P.A. Serum nutrient markers and skin prick testing using data from the Third National Health and Nutrition Examination Survey. J. Allergy Clin. Immunol. 2004, 114, 1398–1402. [Google Scholar] [CrossRef]
- Kompauer, I.; Heinrich, J.; Wolfram, G.; Linseisen, J. Association of carotenoids, tocopherols and vitamin C in plasma with AGTOPIand allergic sensitization in adults. Allergo J. 2006, 15, 133–135. [Google Scholar]
- Nakamura, K.; Wada, K.; Sahashi, Y.; Tamai, Y.; Tsuji, M.; Watanabe, K.; Ohtsuchi, S.; Ando, K.; Nagata, C. Associations of intake of antioxidant vitamins and fatty acids with asthma in pre-school children. Public Health Nutr. 2013, 16, 2040–2045. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sackesen, C.; Ercan, H.; Dizdar, E.; Soyer, O.; Gumus, P.; Tosun, B.N.; Büyüktuncer, Z.; Karabulut, E.; Besler, T.; Kalayci, O. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J. Allergy Clin. Immunol. 2008, 122, 78–85. [Google Scholar] [CrossRef]
- Shanklin, D.R.; O’Dell, T.E. Ascorbic acid and the lung. Nature 1966, 210, 1329–1331. [Google Scholar] [CrossRef]
- Larsson, N.; Rankin, G.D.; Bicer, E.M.; Roos-Engstrand, E.; Pourazar, J.; Blomberg, A.; Mudway, I.S.; Behndig, A.F. Identification of vitamin C transporters in the human airways: A cross-sectional in vivo study. BMJ Open 2015, 5, e006979. [Google Scholar] [CrossRef]
- Hemilä, H. The effect of vitamin C on bronchoconstriction and respiratory symptoms caused by exercise: A review and statistical analysis. Allergy Asthma Clin. Immunol. 2014, 10, 58. [Google Scholar] [CrossRef]
- Schock, B.C.; Koostra, J.; Kwack, S.; Hackman, R.M.; Van Der Vliet, A.; Cross, C.E. Ascorbic acid in nasal and tracheobronchial airway lining fluids. Free Radic. Biol. Med. 2004, 37, 1393–1401. [Google Scholar] [CrossRef]
- Behndig, A.F.; Blomberg, A.; Helleday, R.; Kelly, F.J.; Mudway, I.S. Augmentation of respiratory tract lining fluid ascorbate concentrations through supplementation with vitamin C. Inhal. Toxicol. 2009, 21, 250–258. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Kim, J.H.; Kang, J.S.; Lee, W.J.; Hwang, Y.I. Mega-dose vitamin C attenuated lung inflammation in mouse asthma model. Anat. Cell Biol. 2010, 43, 294. [Google Scholar] [CrossRef]
- Sipahi, E.; Ercan, Z.S. The mechanism of the relaxing effect of ascorbic acid in guinea pig isolated tracheal muscle. Gen. Pharmacol. 1997, 28, 757–760. [Google Scholar] [CrossRef]
- Kitahata, K.; Matsuo, K.; Sato, M.; Susami, Y.; Hara, Y.; Morikawa, T.; Oiso, N.; Kawada, A.; Otsuka, A.; Nakayama, T. Anti-allergic effect of ascorbic acid derivative DDH-1 in a mouse model of atopic dermatitis. Exp. Dermatol. 2022, 31, 1234. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Wu, Y.W.; Hung, J.I.; Chen, M.C. Epigallocatechin gallate/L-ascorbic acid-loaded poly-γ-glutamate microneedles with antioxidant, anti-inflammatory, and immunomodulatory effects for the treatment of atopic dermatitis. Acta Biomater. 2021, 130, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of vitamin C in skin diseases. Front. Physiol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yun, H.; Cho, Y. Analysis of ceramide metabolites in differentiating epidermal keratinocytes treated with calcium or vitamin C. Nutr. Res. Pract. 2011, 5, 396–403. [Google Scholar] [CrossRef]
- Martindale, S.; McNeill, G.; Devereux, G.; Campbell, D.; Russell, G.; Seaton, A. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am. J. Respir. Crit. Care Med. 2005, 171, 121–128. [Google Scholar] [CrossRef]
| Reference | Authors, Years | Type of Study | Number of Patients and Characteristics | Type of Intervention | Effects Described |
|---|---|---|---|---|---|
| [30] | Forastiere et al., 2000 | O | 18,737 Italian children aged 6–7 years | Observation of the effects of fresh fruit consumption on respiratory symptoms using standardized respiratory questionnaires filled in by parents. | Higher fruit consumption (5–7 times/week) reduces the risk of wheezing. Even 1–2 times/week offered some protective effects. This finding was especially noticeable in children with a history of asthma. |
| [31] | Zhang et al., 2024 | O | 4715 children and adolescents aged 2–17 years | Observation of the relationship between dietary intake of various vitamins and their supplements (including vitamins A, C, D, E, B1, B2, B6, B12, K, niacin, folic acid, and choline) and childhood asthma. The data were extracted from the NHANES database. | The odds of childhood asthma decreased with elevated vitamin C intake. |
| [32] | Siripornpanich et al., 2022 | O | 76 asthmatic children aged 7–17 years | Measured PGF2α concentrations, a marker of oxidative stress, measured plasma levels of zinc, vitamin C, and vitamin E, and correlation with altered pulmonary function. | A total of 72 participants with high oxidative stress. All patients had zinc deficiency, and 40% of them were deficient in vitamin C, a condition linked to more severe asthma and airway obstruction. |
| [33] | García-García et al., 2023 | O | 6293 adults aged 20–49 year | Investigate the associations of vitamin A and C intake with asthma and AR, using information from KNHANES. | Higher vitamin C intake (≥75 mg) was significantly associated with a lower prevalence of asthma in participants with high hs-CRP. Vitamin C intake was not associated with AR. |
| [34] | Garcia-Larsen et al., 2017 | O | 3206 European adults, 22.8% with asthma symptoms and 19.5% with CRS | Observation of the association between asthma and CRS with intake of fruits and vegetables using information from the GA2LEN screening survey and FFQ. | No consistent evidence was found linking fruit and vegetable intake with asthma or CRS. |
| [35] | Wang et al., 2024 | O | 8504 adults, including 639 with asthma and 7865 without asthma | Examine the connection between adult asthma and serum vitamin C levels using a multivariate logistic regression model. | After sample weighting, serum vitamin C was not associated with adult asthma risk (OR = 0.829, 95% CI: 0.660~1.042, p 0.104). |
| [36] | Misso et al., 2005 | O | 53 mild-to-moderate and 28 severe asthmatic patients and 43 non-asthmatic subjects | Determine whether lower antioxidant intake and plasma antioxidant concentrations are associated with more severe asthma. | Plasma AA was lower in severe (31.9 ± 3.6 microM) compared with mild-to-moderate asthmatic (52.3 ± 2.6) or control subjects (52.7 ± 2.9). |
| [37] | Oh et al., 2010 | O | 180 children with AD and 242 without AD | To investigate the association of antioxidant nutritional status with the risk of AD. Diet was assessed using a validated semi-quantitative FFQ. Fasting blood samples were used to analyze fat-soluble vitamins (retinol, alpha-tocopherol, and beta-carotene) and vitamin C. | Vitamin C intake and micronutrient supplementation (mean 50 mg/day) showed no significant association with AD risk. |
| Reference | Authors, Years | Type of Study | Number of Patients and Characteristics | Type of Intervention | Effects Described |
|---|---|---|---|---|---|
| [38] | Fortner et al., 1982 | RCDB | 8 adults with AR | A total of 2 g/day of ascorbic acid or placebo for 4 days. | No difference in the nasal response to the instillation of allergen. |
| [39] | Tongtako et al., 2018 | RCT | 27 adults with AR | Three groups: a control group, an exercise group (walking or running for 30 min per session, three times per week for 8 weeks), and a group combining exercise with vitamin C supplementation (2 g/day). | Aerobic exercise significantly improves AR symptoms, with no change with vitamin C supplementation. |
| [40] | Vollbracht et al., 2018 | O | 71 patients with allergy-related diseases (30 with AR and 10 with asthma) with a diagnosed vitamin C deficiency | The patients received IV treatment with 7.5 g of vitamin C diluted in a 0.9% NaCl solution for 2–3 weeks for acute deficiency and 11–12 weeks for chronic deficiency. | In total, 97.1% of patients registered symptom improvement, with the mean disease-specific symptom (pruritus, rhinitis, or restlessness) score significantly decreasing at the final visit (p < 0.0001). |
| [41] | Tecklenburg et al., 2007 | RCBD | 8 asthmatic adults with documented EIB | The patients received either 2 weeks of ascorbic acid supplementation (1.5 g/day) or placebo, followed by a 1-week washout period before switching to the alternative diet. Pre- and post-exercise pulmonary function, asthma symptom scores, and FENO were assessed at the beginning of the trial (usual diet) and at the end of each treatment period. | The ascorbic acid diet significantly reduced (p < 0.05) the maximum fall in post-exercise FEV1 compared to the usual and placebo diet. Asthma symptom scores significantly improved, and Post-exercise FENO was significantly lower (p < 0.05) on the ascorbic acid diet compared to the placebo and usual diet. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trincianti, C.; Naso, M.; Tosca, M.A.; Ciprandi, G. Vitamin C in Allergy Mechanisms and for Managing Allergic Diseases: A Narrative Review. Children 2025, 12, 718. https://doi.org/10.3390/children12060718
Trincianti C, Naso M, Tosca MA, Ciprandi G. Vitamin C in Allergy Mechanisms and for Managing Allergic Diseases: A Narrative Review. Children. 2025; 12(6):718. https://doi.org/10.3390/children12060718
Chicago/Turabian StyleTrincianti, Chiara, Matteo Naso, Maria Angela Tosca, and Giorgio Ciprandi. 2025. "Vitamin C in Allergy Mechanisms and for Managing Allergic Diseases: A Narrative Review" Children 12, no. 6: 718. https://doi.org/10.3390/children12060718
APA StyleTrincianti, C., Naso, M., Tosca, M. A., & Ciprandi, G. (2025). Vitamin C in Allergy Mechanisms and for Managing Allergic Diseases: A Narrative Review. Children, 12(6), 718. https://doi.org/10.3390/children12060718







