Improving Medication Safety Through Medication Reconciliation in Pediatric Neurology: Clinical Pharmacist Recommendations and Physician Uptake in a 13-Week Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
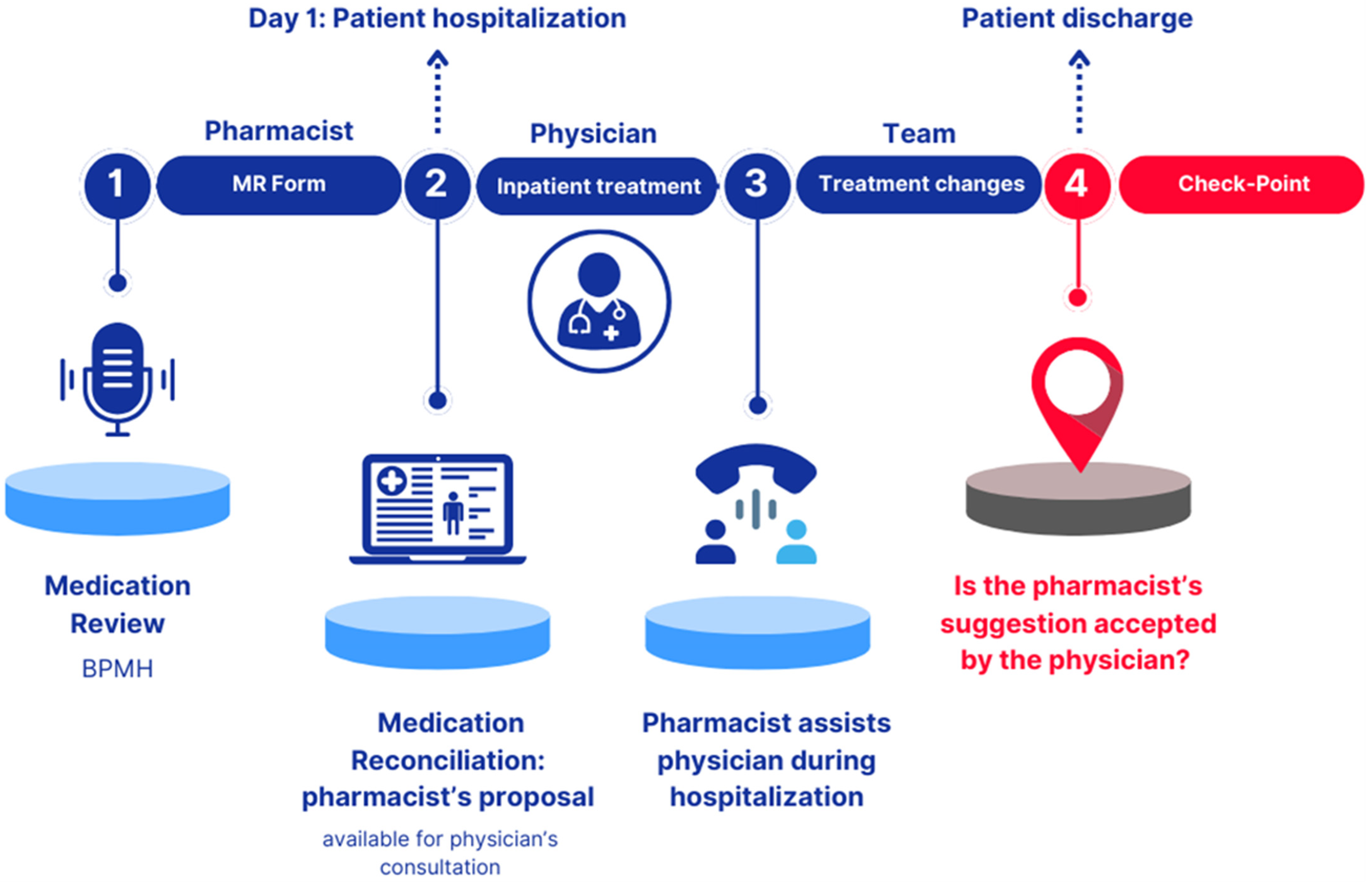
2.1.1. Phase 1 “Pharmacist: Medication Review Form”
- A phone interview with the patient’s parent/caregiver.
- A review of clinical documentation, available through the electronic medical record.
- Summary of Product Characteristics (SmPC), Instructions for Use (IFU), or product sheet databases for medicinal products, medical devices, or non-medicinal products, respectively. The completed MR Form was signed by the pharmacist and uploaded into the patient’s electronic medical record. The MR Form collected the following information: patient’s anonymized name, surname, and date of birth (for data analysis); allergies and/or intolerances; recent adverse drug reactions; recently discontinued therapies; meal administration times and feeding route; details of in-use medicinal products, including name, dosage, posology, pharmaceutical form, posology, and route/method of administration; non-medicinal products (e.g., medical devices, homeopathic remedies, supplements); drug–drug, food–drug, and drug–other non-medicinal product contraindications or major interactions; and any other relevant information (e.g., drug handling, supply, or administration issues). The key areas of focus for the reconciliation proposal included pediatric dosages, formulation stability data, and strategies to improve drug administration, either orally or via Percutaneous Endoscopic Gastrostomy (PEG) or Nasogastric Tube (NGT).
2.1.2. Phase 2 “Physician: Inpatient Treatment”
2.1.3. Phase 3 “Team: Treatment Changes”
2.1.4. Checkpoint
2.2. Cluster Model
2.3. Inclusion and Exclusion Criteria
- Patients with a scheduled hospitalization in the Pediatric Neurology and Neurophysiology Unit.
- Patients prescribed at least two different concomitant daily medications, regardless of dosing frequency, were included.
- Hospitalized patients with a high care burden (e.g., requiring multiple daily administrations and/or manipulations of the pharmaceutical form).
- Patients capable of providing informed consent or whose guardians provided consent on their behalf.
2.4. Outcome Measures
2.5. Statistical Analysis
2.6. Ethics Approval
3. Results
3.1. Baseline Characteristics of the Cohort
3.2. Evaluation of Suggestions Shared by the Clinical Pharmacist with Clinicians
3.3. Evaluation of Suggestions Accepted by Clinicians
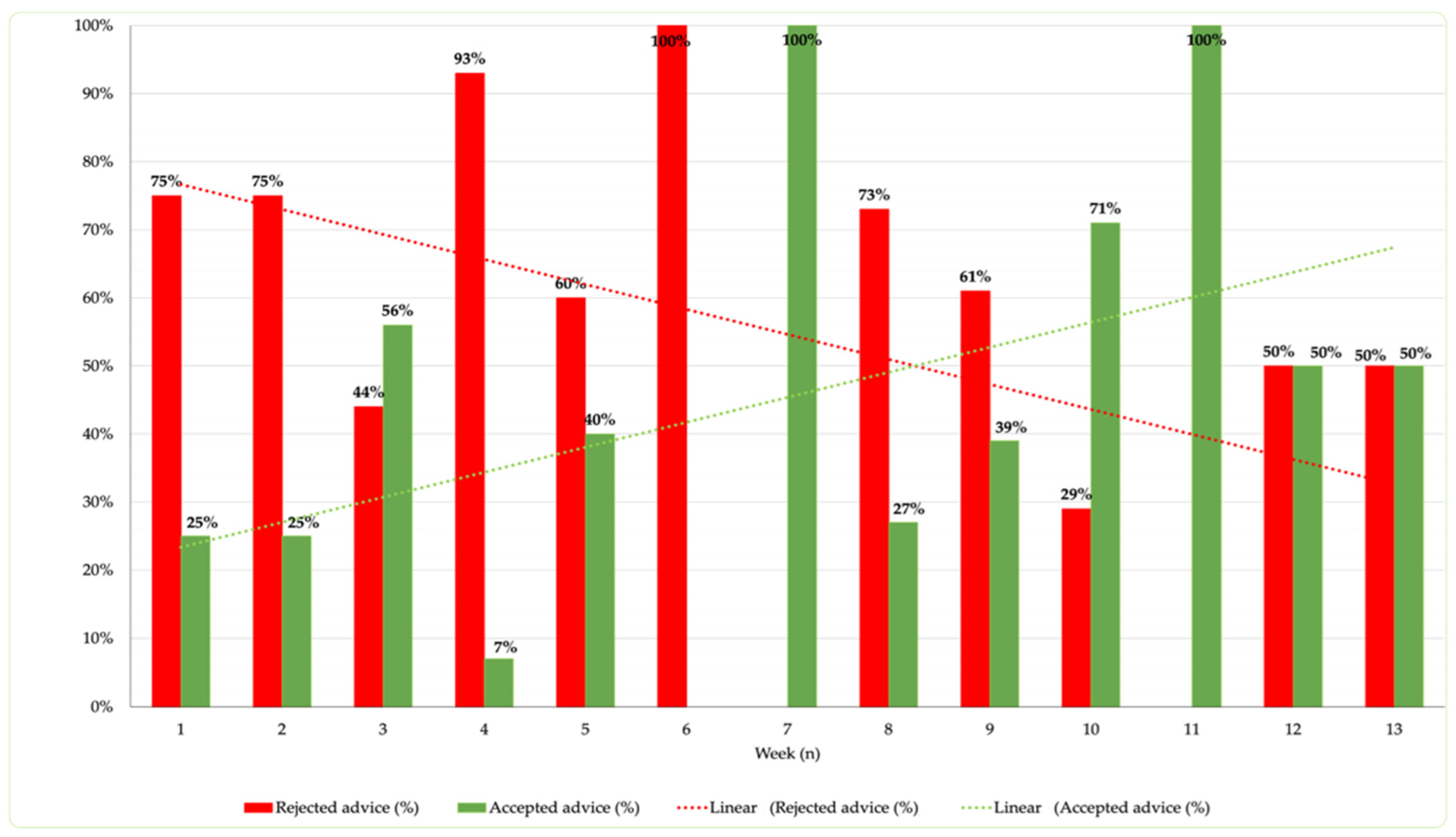
3.4. Analysis of Time to Intervention and Acceptance Rate Correlation
4. Discussion
4.1. Strengths and Weaknesses (Study Limitations)
4.2. Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dreischulte, T.; van den Bemt, B.; Steurbaut, S. European Society of Clinical Pharmacy. European Society of Clinical Pharmacy definition of the term clinical pharmacy and its relationship to pharmaceutical care: A position paper. Int. J. Clin. Pharm. 2022, 44, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Sifact. Available online: https://www.sifact.it/cose-la-farmacia-clinica/ (accessed on 22 November 2024).
- Bernocchi, B.; Casula, C.; Tragni, E. Medication review: An effective tool for appropriate prescribing. GIFF 2016, 8, 21–30. Available online: http://www.sefap.it/web/upload/GIFF2016-3_21_30.pdf (accessed on 22 November 2024).
- Zanin, A.; Baratiri, F.; Roverato, B.; Mengato, D.; Pivato, L.; Avagnina, I.; Maghini, I.; Divisic, A.; Rusalen, F.; Agosto, C.; et al. Polypharmacy in Children with Medical Complexity: A Cross-Sectional Study in a Pediatric Palliative Care Center. Children 2024, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Mengato, D.; Zanin, A.; Baratiri, F.; Pivato, L.; Camuffo, L.; Benini, F.; Venturini, F. Polypharmacy in Pediatric Palliative Care: Exploring Discrepancies Between Physicians and Pharmacists. Children 2025, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Corny, J.; Bailey, B.; Lebel, D.; Bussières, J.-F. Unlicensed and off-label drug use in paediatrics in a mother-child tertiary care hospital. Paediatr. Child. Health 2016, 21, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Cite Eriksson, T.; Melander, A.C. Clinical pharmacists’ services, role and acceptance: A national Swedish survey. Eur. J. Hosp. Pharm. Sci. Pract. 2021, 28, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Sin, C.M.; Huynh, C.; Dahmash, D.; Maidment, I.D. Factors influencing the implementation of clinical pharmacy services on paediatric patient care in hospital settings. Eur. J. Hosp. Pharm. 2022, 29, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, N.; Kaşıkcı, M.; Çelik, H.T.; Allegaert, K.; Demirkan, K.; Yiğit, Ş. Impact of clinical pharmacist-led intervention for drug-related problems in neonatal intensive care unit a randomized controlled trial. Front. Pharmacol. 2023, 14, 1242779. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.; Vest, M.H.; Doligalski, C.; Summerlin, C.M.; Alexander, M.D.; Deyo, Z.M.; Valgus, J.M.; Waldron, K.M. Implementation and results of a standardized process for identifying ambulatory pharmacy clinical outcome measures. Am. J. Health Syst. Pharm. 2023, 80, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Maffre, I.; Leguelinel-Blache, G.; Soulairol, I. A systematic review of clinical pharmacy services in pediatric inpatients. Drugs Ther. Perspect. 2021, 37, 363–375. [Google Scholar] [CrossRef]
- UptoDate. Available online: https://wkhealthce.my.site.com/customers/s/?language=it (accessed on 2 April 2024).
- Meritive Micromedex. Available online: https://www.micromedexsolutions.com/micromedex2/librarian/ (accessed on 2 April 2024).
- PCNE Classification for Drug-Related Problems 2020, Volume 9.1. 2003-2020 Pharmaceutical Care Network Europe Association. Available online: https://www.pcne.org/upload/files/555_09_PCNE_classification_V9-1_final.pdf (accessed on 12 December 2024).
- The Jamovi Project, Version 2.6.22; Jamovi Desktop. Available online: https://www.jamovi.org (accessed on 2 December 2024).
- European Medicines Agency, Guideline for Good Clinical Practice E6(R2). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-good-clinical-practice-e6r2-step-5_en.pdf (accessed on 15 May 2024).
- EMA Updated Version of EnprEMA “Informed Consent for Paediatric Clinical Trials in Europe”. Available online: https://www.ema.europa.eu/en/events/annual-workshop-european-network-paediatric-research-ema-enpr-ema (accessed on 2 May 2023).
- Clyne, W.; Blenkinsopp, A.; Seal, R. A Guide Medicat. Review 2008. Available online: https://www.cff.org.br/userfiles/52%20-%20CLYNE%20W%20A%20guide%20to%20medication%20review%202008.pdf (accessed on 2 May 2024).
- Zhang, L.; Hu, Y.; Pan, P.; Hong, C.; Fang, L. Estimated Manipulation of Tablets and Capsules to Meet Dose Requirements for Chinese Children: A Cross-Sectional Study. Front. Pediatr. 2021, 9, 747499. [Google Scholar] [CrossRef] [PubMed]
- Naseef, H.; Amria, A.; Asrawi, A.; Al-Shami, N.; Dreidi, M. The acceptance and awareness of healthcare providers towards doctor of pharmacy (Phram D) in the Palestinian health care system. Saudi Pharm. J. 2020, 28, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
| Variables | |
|---|---|
| Gender, n (%) | Total (N = 57) |
| Female | 27 (47%) |
| Male | 30 (53%) |
| Age, n (%) | |
| <1 year | 11 (19%) |
| 1–5 years | 19 (33%) |
| 6–12 years | 17 (30%) |
| 13–18 years | 10 (18%) |
| Age, median years (IQR) | 3.0 (1.00–10.25) |
| Drug Therapy, median (IQR) | 3.2 (1.25–4.00) |
| Polypharmacy, n (%) | |
| drugs taken > 5 | 9 (16%) |
| drugs taken > 10 | 2 (4%) |
| Prescribed drugs by ATC * code, n (%) | Total (N = 160) |
| N—nervous system | 111 (69%) |
| A—alimentary tract and metabolism | 17 (11%) |
| R—respiratory system | 8 (5%) |
| Other ATC codes | 16 (10%) |
| Supplements | 8 (5%) |
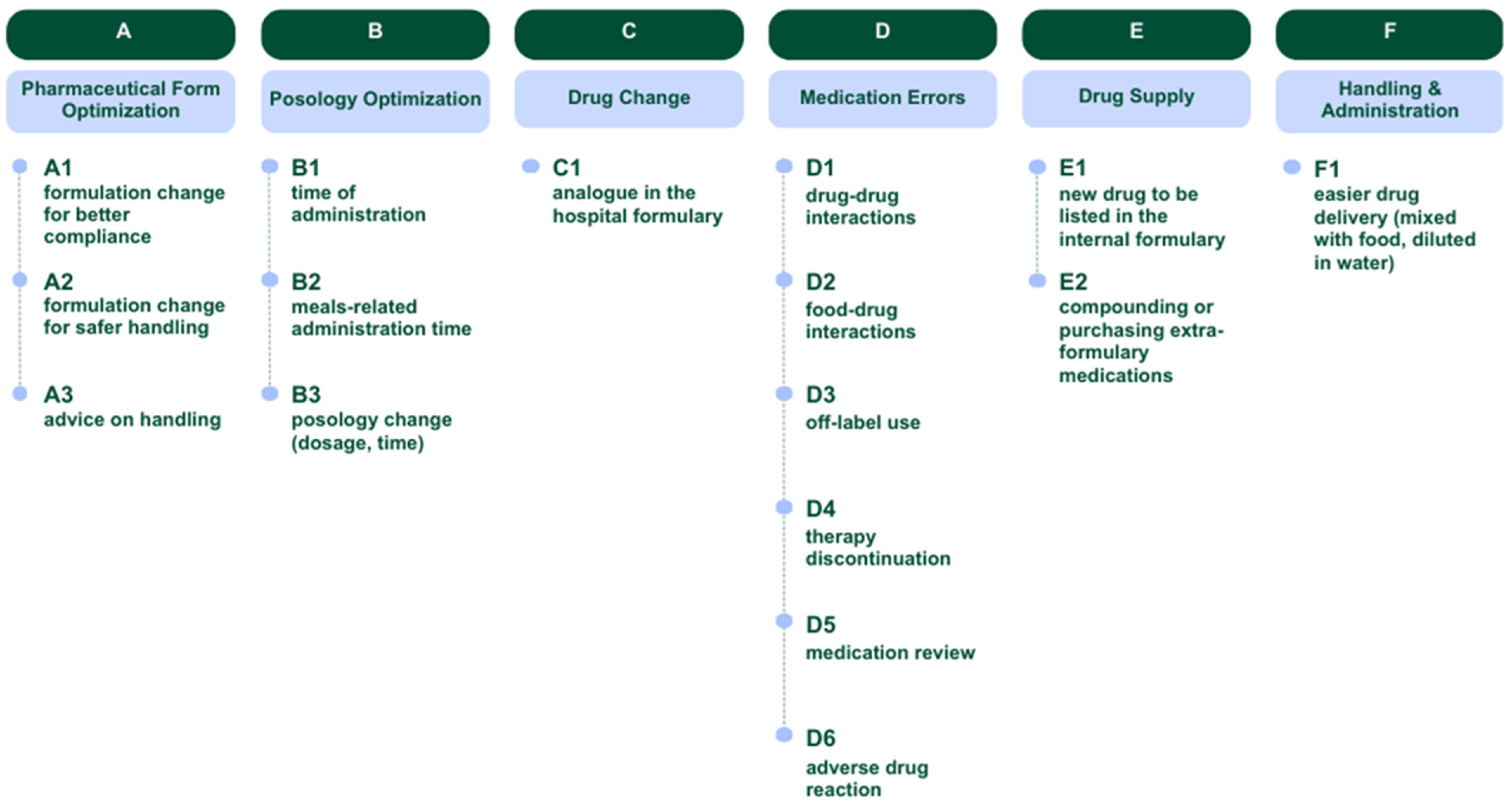
| Not Accepted, n (%) c | Accepted, n (%) b | Total, n (%) a | Cluster of Suggestions |
|---|---|---|---|
| 9 (36%) | 16 (64%) | 25 (18%) | A—Pharmaceutical Form Optimization |
| 1 (100%) | - | 1 (1%) | A1—formulation change for better compliance |
| 6 (55%) | 5 (45%) | 11 (8%) | A2—formulation change for safer handling |
| 2 (15%) | 11 (85%) | 13 (9%) | A3—advice on handling |
| 5 (71%) | 2 (29%) | 7 (5%) | B—Posology Optimization |
| 2 (100%) | - | 2 (1%) | B1—time of administration |
| - | 2 (100%) | 2 (1%) | B2—meals-related administration time |
| 3 (100%) | - | 3 (2%) | B3—posology change (dosage, time) |
| 1 (50%) | 1 (50%) | 2 (1%) | C—Drug Change |
| 1 (50%) | 1 (50%) | 2 (1%) | C1—analog in the hospital formulary |
| 42 (68%) | 20 (32%) | 62 (45%) | D—Medication Errors |
| 28 (88%) | 4 (12%) | 32 (23%) | D1—drug–drug interactions |
| 4 (50%) | 4 (50%) | 8 (6%) | D2—food–drug interactions |
| - | 1 (100%) | 1 (1%) | D3—off-label use |
| 1 (100%) | - | 1 (1%) | D4—therapy discontinuation |
| 8 (57%) | 6 (43%) | 14 (10%) | D5—medication review |
| 1 (17%) | 5 (83%) | 6 (4%) | D6—adverse drug reaction |
| 21 (58%) | 15 (42%) | 36 (26%) | E—Drug Supply |
| 4 (33%) | 8 (67%) | 12 (9%) | E1—new drug to be listed in the internal formulary |
| 17 (71%) | 7 (29%) | 24 (17%) | E2—compounding or purchasing extra-formulary medications |
| 2 (33%) | 4 (67%) | 6 (4%) | F—handling and administration |
| 2 (33%) | 4 (67%) | 6 (4%) | F1—easier drug delivery (mixed with food, diluted in water) |
| 80 (58%) | 58 (42%) | 138 (100%) | Total |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zennaro, M.; Trotter, A.; Mengato, D.; Camuffo, L.; Ancona, C.; Toldo, I.; Giron, M.C.; Pelizza, M.F.; Nosadini, M.; Perilongo, G.; et al. Improving Medication Safety Through Medication Reconciliation in Pediatric Neurology: Clinical Pharmacist Recommendations and Physician Uptake in a 13-Week Study. Children 2025, 12, 625. https://doi.org/10.3390/children12050625
Zennaro M, Trotter A, Mengato D, Camuffo L, Ancona C, Toldo I, Giron MC, Pelizza MF, Nosadini M, Perilongo G, et al. Improving Medication Safety Through Medication Reconciliation in Pediatric Neurology: Clinical Pharmacist Recommendations and Physician Uptake in a 13-Week Study. Children. 2025; 12(5):625. https://doi.org/10.3390/children12050625
Chicago/Turabian StyleZennaro, Margherita, Anna Trotter, Daniele Mengato, Laura Camuffo, Claudio Ancona, Irene Toldo, Maria Cecilia Giron, Maria Federica Pelizza, Margherita Nosadini, Giorgio Perilongo, and et al. 2025. "Improving Medication Safety Through Medication Reconciliation in Pediatric Neurology: Clinical Pharmacist Recommendations and Physician Uptake in a 13-Week Study" Children 12, no. 5: 625. https://doi.org/10.3390/children12050625
APA StyleZennaro, M., Trotter, A., Mengato, D., Camuffo, L., Ancona, C., Toldo, I., Giron, M. C., Pelizza, M. F., Nosadini, M., Perilongo, G., Sartori, S., & Venturini, F. (2025). Improving Medication Safety Through Medication Reconciliation in Pediatric Neurology: Clinical Pharmacist Recommendations and Physician Uptake in a 13-Week Study. Children, 12(5), 625. https://doi.org/10.3390/children12050625







