The Individual and Combined Effects of Prenatal Micronutrient Supplementations on Neurobehavioral Developmental Disorders in Preschool Children
Abstract
1. Introduction
2. Materials and Methods
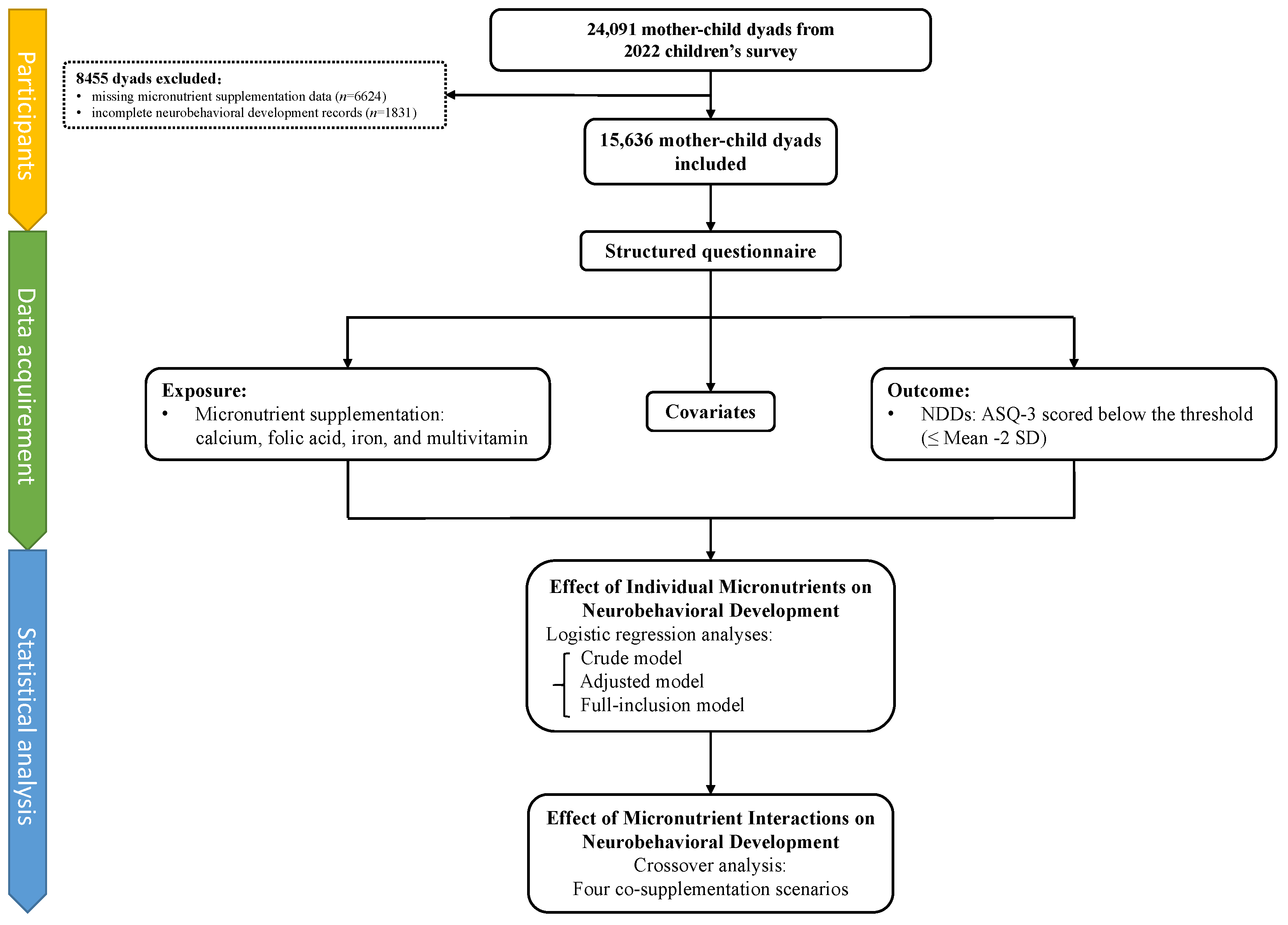
2.1. Participants
2.2. Data Acquisition
2.3. Prenatal Micronutrient Supplementation
2.4. Outcome
2.5. Covariates
2.6. Statistical Analysis
2.6.1. Individual Effects of Micronutrients on Neurobehavioral Development
2.6.2. Combined Effects of Micronutrients on Neurobehavioral Development
3. Results
3.1. Participants’ Characteristics
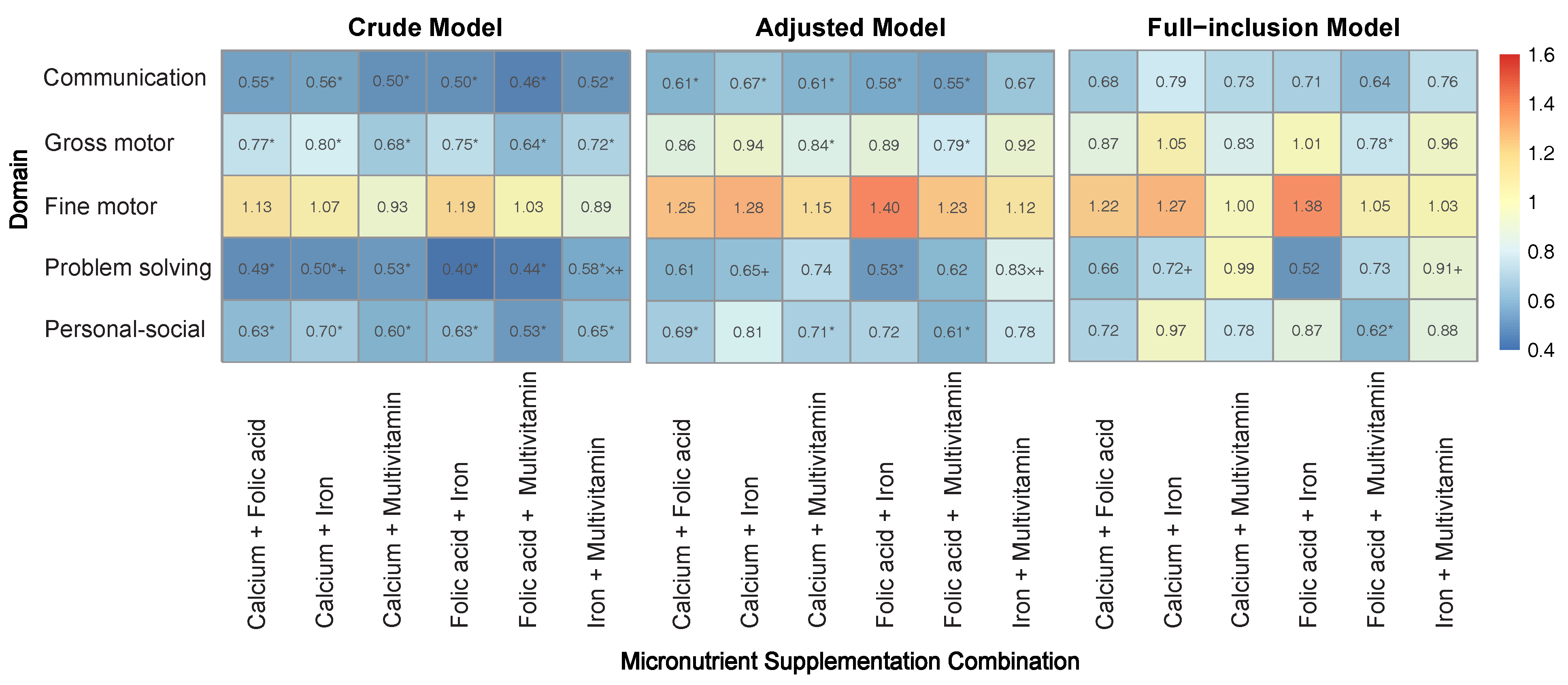
3.2. Individual Effects of Micronutrients on Neurobehavioral Development
3.3. Combined Effects of Micronutrients on Neurobehavioral Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | Attention Deficit Hyperactivity Disorder |
| AP | Attributable Proportion due to Interaction |
| ASQ-3 | Age and Developmental Progress Questionnaire |
| ASD | Autism Spectrum Disorder |
| BMI | Body Mass Index |
| BW | Birth Weight |
| CDE | Controlled Direct Effect |
| DALYs | Disability-Adjusted Life Years |
| IOR | Interaction Odds Ratio |
| IUGR | Intrauterine Growth Restriction |
| NDDs | Neurobehavioral Developmental Disorders |
| NDN | Neurobehavioral Developmental Normality |
| OR | Odds Ratio |
| PMM | Predictive Mean Matching |
| PTB | Preterm Birth |
| RCT | Randomized Controlled Trial |
| RERI | Relative Excess Risk due to Interaction |
| SD | Standard Deviation |
| WHO | World Health Organization |
References
- Shah, P.J.; Boilson, M.; Rutherford, M.; Prior, S.; Johnston, L.; Maciver, D.; Forsyth, K. Neurodevelopmental disorders and neurodiversity: Definition of terms from Scotland’s National Autism Implementation Team. Br. J. Psychiatry 2022, 221, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Grantham-McGregor, S.; Cheung, Y.B.; Cueto, S.; Glewwe, P.; Richter, L.; Strupp, B. Developmental potential in the first 5 years for children in developing countries. Lancet 2007, 369, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Cooper, M.; Rutter, M. Neurodevelopmental disorders. Lancet. Psychiatry 2017, 4, 339–346. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Report on Children with Developmental Disabilities: From the Margins to the Mainstream. Available online: https://www.unicef.org/documents/global-report-children-developmental-disabilities (accessed on 25 August 2024).
- Zhang, J.; Lu, H.; Sheng, Q.; Zang, E.; Zhang, Y.; Yuan, H.; Chen, B.; Tang, W. The Influence of Perinatal Psychological Changes on Infant Neurodevelopment in Shanghai, China: A Longitudinal Group-based Trajectory Analysis. J. Affect. Disord. 2024, 361, 291–298. [Google Scholar] [CrossRef]
- Ma, R.; Wang, P.; Yang, Q.; Zhu, Y.; Zhang, L.; Wang, Y.; Sun, L.; Li, W.; Ge, J.; Zhu, P. Interpregnancy interval and early infant neurodevelopment: The role of maternal-fetal glucose metabolism. BMC Med. 2024, 22, 2. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Shang, X.; He, H. Developmental Screening and Analysis of Influencing Factors in 2,980 Infants Under 3 Months in Beijing. Beijing Med. 2022, 44, 513–517. [Google Scholar] [CrossRef]
- Chen, C.; Lin, Y.; Yan, W.; Zhang, Y. Ages and stages questionnaire in screening developmental levels of infants from 6 to 12 months and risk factors analysis. J. Bio-Educ. 2022, 10, 318–322. [Google Scholar]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. Morb. Mortal. Wkly. Report. Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef]
- Bachmann, C.J.; Scholle, O.; Bliddal, M.; dosReis, S.; Odsbu, I.; Skurtveit, S.; Wesselhoeft, R.; Vivirito, A.; Zhang, C.; Scott, S. Recognition and management of children and adolescents with conduct disorder: A real-world data study from four western countries. Child Adolesc. Psychiatry Ment. Health 2024, 18, 18. [Google Scholar] [CrossRef]
- Jensen de López, K.M.; Thirup Møller, H. Prevalence of Autism in Scandinavian Countries (Denmark, Norway, Sweden), and Nordic Countries (Finland, Iceland, the Faroe Islands, and Greenland). Neuropsychiatr. Dis. Treat. 2024, 20, 1597–1612. [Google Scholar] [CrossRef]
- Collaborators, G.N.S.D. Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet. Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef]
- Lavelle, T.A.; Weinstein, M.C.; Newhouse, J.P.; Munir, K.; Kuhlthau, K.A.; Prosser, L.A. Economic burden of childhood autism spectrum disorders. Pediatrics 2014, 133, e520–e529. [Google Scholar] [CrossRef] [PubMed]
- Rogge, N.; Janssen, J. The Economic Costs of Autism Spectrum Disorder: A Literature Review. J. Autism Dev. Disord. 2019, 49, 2873–2900. [Google Scholar] [CrossRef]
- Lewis, A.J.; Galbally, M.; Gannon, T.; Symeonides, C. Early life programming as a target for prevention of child and adolescent mental disorders. BMC Med. 2014, 12, 33. [Google Scholar] [CrossRef]
- McGowan, E.C.; Hofheimer, J.A.; O’Shea, T.M.; Kilbride, H.; Carter, B.S.; Check, J.; Helderman, J.; Neal, C.R.; Pastyrnak, S.; Smith, L.M.; et al. Analysis of Neonatal Neurobehavior and Developmental Outcomes Among Preterm Infants. JAMA Netw. Open 2022, 5, e2222249. [Google Scholar] [CrossRef]
- Cusick, S.E.; Barks, A.; Georgieff, M.K. Nutrition and Brain Development. In Sensitive Periods of Brain Development and Preventive Interventions; Andersen, S.L., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 131–165. [Google Scholar]
- Hu, Y.; Wang, R.; Mao, D.; Chen, J.; Li, M.; Li, W.; Yang, Y.; Zhao, L.; Zhang, J.; Piao, J.; et al. Vitamin D Nutritional Status of Chinese Pregnant Women, Comparing the Chinese National Nutrition Surveillance (CNHS) 2015-2017 with CNHS 2010-2012. Nutrients 2021, 13, 2237. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Han, T.; Dong, J.; Zhang, J.; Zhang, C.; Wang, Y.; Zhang, Z.; Xiang, M. Nutrient supplementation among pregnant women in China: An observational study. Public Health Nutr. 2022, 25, 1537–1542. [Google Scholar] [CrossRef]
- Arija, V.; Hernández-Martínez, C.; Tous, M.; Canals, J.; Guxens, M.; Fernández-Barrés, S.; Ibarluzea, J.; Babarro, I.; Soler-Blasco, R.; Llop, S.; et al. Association of Iron Status and Intake During Pregnancy with Neuropsychological Outcomes in Children Aged 7 Years: The Prospective Birth Cohort Infancia y Medio Ambiente (INMA) Study. Nutrients 2019, 11, 2999. [Google Scholar] [CrossRef]
- Janbek, J.; Sarki, M.; Specht, I.O.; Heitmann, B.L. A systematic literature review of the relation between iron status/anemia in pregnancy and offspring neurodevelopment. Eur. J. Clin. Nutr. 2019, 73, 1561–1578. [Google Scholar] [CrossRef]
- Moumin, N.A.; Shepherd, E.; Liu, K.; Makrides, M.; Gould, J.F.; Green, T.J.; Grzeskowiak, L.E. The Effects of Prenatal Iron Supplementation on Offspring Neurodevelopment in Upper Middle- or High-Income Countries: A Systematic Review. Nutrients 2024, 16, 2499. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, B.; Gallo, S.; Majnemer, A.; Vanstone, C.; Comeau, K.; Jones, G.; L’Abbe, M.; Khamessan, A.; Sharma, A.; Weiler, H.; et al. Impact of Vitamin D Supplementation on Gross Motor Development of Healthy Term Infants: A Randomized Dose-Response Trial. Phys. Occup. Ther. Pediatr. 2016, 36, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Mutua, A.M.; Mogire, R.M.; Elliott, A.M.; Williams, T.N.; Webb, E.L.; Abubakar, A.; Atkinson, S.H. Effects of vitamin D deficiency on neurobehavioural outcomes in children: A systematic review. Wellcome Open Res. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.K.; Murray, D.M.; Malvisi, L.; Kenny, L.C.; J, O.B.H.; Irvine, A.D.; Kiely, M.E. Antenatal Vitamin D Status Is Not Associated with Standard Neurodevelopmental Assessments at Age 5 Years in a Well-Characterized Prospective Maternal-Infant Cohort. J. Nutr. 2018, 148, 1580–1586. [Google Scholar] [CrossRef]
- Markhus, M.W.; Dahl, L.; Moe, V.; Abel, M.H.; Brantsæter, A.L.; Øyen, J.; Meltzer, H.M.; Stormark, K.M.; Graff, I.E.; Smith, L.; et al. Maternal Iodine Status is Associated with Offspring Language Skills in Infancy and Toddlerhood. Nutrients 2018, 10, 1270. [Google Scholar] [CrossRef]
- Murcia, M.; Espada, M.; Julvez, J.; Llop, S.; Lopez-Espinosa, M.J.; Vioque, J.; Basterrechea, M.; Riaño, I.; González, L.; Alvarez-Pedrerol, M.; et al. Iodine intake from supplements and diet during pregnancy and child cognitive and motor development: The INMA Mother and Child Cohort Study. J. Epidemiol. Community Health 2018, 72, 216–222. [Google Scholar] [CrossRef]
- Jalali Chimeh, F.; Aghaie, E.; Ghavi, S.; Fatahnia, R. Investigation of the Effects of Maternal Nutrition during Pregnancy on Cognitive Functions of Toddlers: A Systematic Review. Int. J. Prev. Med. 2024, 15, 15. [Google Scholar] [CrossRef]
- Squires, J.; Bricker, D.D.; Twombly, E. Ages & Stages Questionnaires, Third Edition (ASQ-3): A Parent-Completed Child Monitoring System; Paul H. Brookes: Baltimore, MD, USA, 2009. [Google Scholar]
- Devakumar, D.; Fall, C.H.; Sachdev, H.S.; Margetts, B.M.; Osmond, C.; Wells, J.C.; Costello, A.; Osrin, D. Maternal antenatal multiple micronutrient supplementation for long-term health benefits in children: A systematic review and meta-analysis. BMC Med. 2016, 14, 90. [Google Scholar] [CrossRef]
- Bergen, N.E.; Schalekamp-Timmermans, S.; Jaddoe, V.W.; Hofman, A.; Lindemans, J.; Russcher, H.; Tiemeier, H.; Steegers-Theunissen, R.P.; Steegers, E.A. Maternal and Neonatal Markers of the Homocysteine Pathway and Fetal Growth: The Generation R Study. Paediatr. Perinat. Epidemiol. 2016, 30, 386–396. [Google Scholar] [CrossRef]
- Kok, D.E.; Dhonukshe-Rutten, R.A.; Lute, C.; Heil, S.G.; Uitterlinden, A.G.; van der Velde, N.; van Meurs, J.B.; van Schoor, N.M.; Hooiveld, G.J.; de Groot, L.C.; et al. The effects of long-term daily folic acid and vitamin B12 supplementation on genome-wide DNA methylation in elderly subjects. Clin. Epigenetics 2015, 7, 121. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Oestreicher, P.; Cousins, R.J. Copper and zinc absorption in the rat: Mechanism of mutual antagonism. J. Nutr. 1985, 115, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Doets, E.L.; Ueland, P.M.; Tell, G.S.; Vollset, S.E.; Nygård, O.K.; Van’t Veer, P.; de Groot, L.C.; Nurk, E.; Refsum, H.; Smith, A.D.; et al. Interactions between plasma concentrations of folate and markers of vitamin B(12) status with cognitive performance in elderly people not exposed to folic acid fortification: The Hordaland Health Study. Br. J. Nutr. 2014, 111, 1085–1095. [Google Scholar] [CrossRef]
- Li, F.; Pei, L.; Huang, G.; Ye, H. Influence of omega-3 fatty acid and vitamin co-supplementation on metabolic status in gestational diabetes: A meta-analysis of randomized controlled studies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 191–197. [Google Scholar] [CrossRef]
- Jamilian, M.; Mirhosseini, N.; Eslahi, M.; Bahmani, F.; Shokrpour, M.; Chamani, M.; Asemi, Z. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth 2019, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bai, S.; Lin, S.; Du, S.; Zhao, X.; Qin, Y.; Yang, X.; Wang, Z. The association between preterm birth and the supplementation with vitamin D and calcium during pregnancy. Clin. Nutr. ESPEN 2024, 63, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Parvez, S.; Fatima, G.; Singh, S.; Magomedova, A.; Batiha, G.E.; Alexiou, A.; Papadakis, M.; Welson, N.N.; Hadi, N. Micronutrient interactions: Magnesium and its synergies in maternal-fetal health. Food Sci. Nutr. 2024, 12, 6913–6928. [Google Scholar] [CrossRef]
- Lu, Q.; Strodl, E.; Liang, Y.; Huang, L.H.; Hu, B.J.; Chen, W.Q. Joint Effects of Prenatal Folic Acid Supplement with Prenatal Multivitamin and Iron Supplement on Obesity in Preschoolers Born SGA: Sex Specific Difference. Nutrients 2023, 15, 380. [Google Scholar] [CrossRef]
- Deng, W.; Wang, H.; Cai, J. Analysis of developmental screening results using ASQ-3 among 2,246 infants aged 3 to 4 months in Shenzhen . J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 45, 454–457. (In Chinese) [Google Scholar]
- Ruan, Z.L.; Liu, L.; Strodl, E.; Fan, L.J.; Yin, X.N.; Wen, G.M.; Sun, D.L.; Xian, D.X.; Jiang, H.; Jing, J.; et al. Antenatal Training with Music and Maternal Talk Concurrently May Reduce Autistic-Like Behaviors at around 3 Years of Age. Front. Psychiatry 2017, 8, 305. [Google Scholar] [CrossRef]
- Fang, X.Y.; Strodl, E.; Liu, B.Q.; Liu, L.; Yin, X.N.; Wen, G.M.; Sun, D.L.; Xian, D.X.; Jiang, H.; Jing, J.; et al. Association between prenatal exposure to household inhalants exposure and ADHD-like behaviors at around 3 years of age: Findings from Shenzhen Longhua Child Cohort Study. Environ. Res. 2019, 177, 108612. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Strodl, E.; Huang, L.H.; Chen, J.Y.; Liu, X.C.; Yang, J.H.; Chen, W.Q. Associations between Prenatal Education, Breastfeeding and Autistic-Like Behaviors in Pre-Schoolers. Children 2021, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, L.; Strodl, E.; Chen, J.; Tong, F.; Chen, W. Impact of Breastfeeding Practices on Autistic Traits in Chinese Children Aged from 3 to 4 Years: Cross-Sectional Study. Nutrients 2025, 17, 836. [Google Scholar] [CrossRef]
- Wei, M.; Bian, X.; Squires, J.; Yao, G.; Wang, X.; Xie, H.; Song, W.; Lu, J.; Zhu, C.; Yue, H.; et al. Studies of the norm and psychometrical properties of the ages and stages questionnaires, third edition, with a Chinese national sample. Chin. J. Pediatr. 2015, 53, 913–918. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Shi, L.; Daniel, L.M.; Yang, P.H.; Khoo, P.C.; Quek, B.H.; Zheng, Q.; Rajadurai, V.S. Prospective evaluation of the Ages and Stages Questionnaire 3rd Edition in very-low-birthweight infants. Dev. Med. Child Neurol. 2017, 59, 484–489. [Google Scholar] [CrossRef]
- Lopes, S.; Graça, P.; Teixeira, S.; Serrano, A.M.; Squires, J. Psychometric properties and validation of Portuguese version of Ages & Stages Questionnaires (3rd edition): 9, 18 and 30 Questionnaires. Early Hum. Dev. 2015, 91, 527–533. [Google Scholar] [CrossRef]
- Wang, P.; Xie, J.; Jiao, X.C.; Ma, S.S.; Liu, Y.; Yin, W.J.; Tao, R.X.; Hu, H.L.; Zhang, Y.; Chen, X.X.; et al. Maternal Glycemia During Pregnancy and Early Offspring Development: A Prospective Birth Cohort Study. J. Clin. Endocrinol. Metab. 2021, 106, 2279–2290. [Google Scholar] [CrossRef]
- Alving-Jessep, E.; Botchway, E.; Wood, A.G.; Hilton, A.C.; Blissett, J.M. The development of the gut microbiome and temperament during infancy and early childhood: A systematic review. Dev. Psychobiol. 2022, 64, e22306. [Google Scholar] [CrossRef]
- Zhang, D.; Lan, Y.; Zhang, J.; Cao, M.; Yang, X.; Wang, X. Effects of early-life gut microbiota on the neurodevelopmental outcomes of preterm infants: A multi-center, longitudinal observational study in China. Eur. J. Pediatr. 2024, 183, 1733–1740. [Google Scholar] [CrossRef]
- Guo, X.; Xu, J.; Tian, Y.; Ouyang, F.; Yu, X.; Liu, J.; Yan, C.; Zhang, J. Interaction of prenatal maternal selenium and manganese levels on child neurodevelopmental trajectories-the Shanghai birth cohort study. Sci. Total Environ. 2024, 915, 170095. [Google Scholar] [CrossRef]
- Allotey, P.A.; Harel, O. Multiple Imputation for Incomplete Data in Environmental Epidemiology Research. Curr. Environ. Health Rep. 2019, 6, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, R.; Westlake, M.; Bragg, M.G.; Rando, J.; Bennett, D.H.; Croen, L.A.; Dunlop, A.L.; Ferrara, A.; Hedderson, M.M.; Kerver, J.M.; et al. Maternal Dietary Patterns During Pregnancy and Child Autism-Related Traits in the Environmental Influences on Child Health Outcomes Consortium. Nutrients 2024, 16, 3802. [Google Scholar] [CrossRef]
- Tabaeifard, R.; Hashempour, S.; Karim Dehnavi, M.; Mofidi Nejad, M.; Omid, N.; Karimi, M.; Azadbakht, L. Association between oxidative balance score and risk of postpartum depression in Iranian women: A prospective cohort study. Sci. Rep. 2025, 15, 8590. [Google Scholar] [CrossRef]
- Schottenfeld, D. Epidemiology: An introduction. Am. J. Epidemiol. 2002, 156, 188–190. [Google Scholar] [CrossRef]
- Knol, M.J.; VanderWeele, T.J. Recommendations for presenting analyses of effect modification and interaction. Int. J. Epidemiol. 2012, 41, 514–520. [Google Scholar] [CrossRef]
- Correia, K.; Williams, P.L. Estimating the Relative Excess Risk Due to Interaction in Clustered-Data Settings. Am. J. Epidemiol. 2018, 187, 2470–2480. [Google Scholar] [CrossRef]
- Bellocco, R.; Marrone, G.; Ye, W.; Nyrén, O.; Adami, H.O.; Mariosa, D.; Lagerros, Y.T. A prospective cohort study of the combined effects of physical activity and anthropometric measures on the risk of post-menopausal breast cancer. Eur. J. Epidemiol. 2016, 31, 395–404. [Google Scholar] [CrossRef]
- Zhu, W.; Zhu, S.; Sunguya, B.F.; Huang, J. Urban–Rural Disparities in the Magnitude and Determinants of Stunting among Children under Five in Tanzania: Based on Tanzania Demographic and Health Surveys 1991–2016. Int. J. Environ. Res. Public Health 2021, 18, 5184. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, P.; Yan, W.; Hu, Y.; Guo, H.; Chen, F.; Bigambo, F.M.; Wang, X. The Influence of Multivitamins on Neurological and Growth Disorders: A Cross-Sectional Study. Front. Nutr. 2024, 11, 1465875. [Google Scholar] [CrossRef]
- Wei, Q.; Xiao, Y.; Yang, T.; Chen, J.; Chen, L.; Wang, K.; Zhang, J.; Li, L.; Jia, F.; Wu, L.; et al. Predicting Autism Spectrum Disorder Using Maternal Risk Factors: A Multi-Center Machine Learning Study. Psychiatry Res. 2024, 334, 115789. [Google Scholar] [CrossRef]
- Cruz-Rodríguez, J.; Díaz-López, A.; Canals-Sans, J.; Arija, V. Maternal Vitamin B12 Status during Pregnancy and Early Infant Neurodevelopment: The ECLIPSES Study. Nutrients 2023, 15, 1529. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, M.D.; Mead, M.J.; McWhorter, C.A.; Ebeling, M.D.; Shary, J.R.; Newton, D.A.; Baatz, J.E.; Gregoski, M.J.; Hollis, B.W.; Wagner, C.L. Vitamin D and Child Neurodevelopment—A Post Hoc Analysis. Nutrients 2023, 15, 4250. [Google Scholar] [CrossRef]
- Adams, J.B.; Kirby, J.K.; Sorensen, J.C.; Pollard, E.L.; Audhya, T. Evidence Based Recommendations for an Optimal Prenatal Supplement for Women in the US: Vitamins and Related Nutrients. Matern. Health Neonatol. Perinatol. 2022, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, N.; Chegukrishnamurthi, M.; Gadde Venkata, S. Role of Micronutrients in Neurological Development. In Role of Nutrients in Neurological Disorders; Springer: Berlin/Heidelberg, Germany, 2022; pp. 177–199. [Google Scholar]
- Guilarte, T.R. Vitamin B6 and Cognitive Development: Recent Research Findings from Human and Animal Studies. Nutr. Rev. 1993, 51, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Benton, D. Vitamins and Neural and Cognitive Developmental Outcomes in Children. Proc. Nutr. Soc. 2012, 71, 14–26. [Google Scholar] [CrossRef]
- Tafti, M.; Ghyselinck, N.B. Functional Implication of the Vitamin A Signaling Pathway in the Brain. Arch. Neurol. 2007, 64, 1706–1711. [Google Scholar] [CrossRef]
- Chang, J.; Liu, M.; Liu, C.; Zhou, S.; Jiao, Y.; Sun, H.; Ji, Y. Effects of Vitamins and Polyunsaturated Fatty Acids on Cognitive Function in Older Adults with Mild Cognitive Impairment: A Meta-Analysis of Randomized Controlled Trials. Eur. J. Nutr. 2024, 63, 1003–1022. [Google Scholar] [CrossRef]
- Mantovani, E.; Filippini, F.; Bortolus, R.; Franchi, M. Folic Acid Supplementation and Preterm Birth: Results from Observational Studies. BioMed Res. Int. 2014, 2014, 481914. [Google Scholar] [CrossRef]
- WHO. Periconceptional Folic Acid Supplementation to Prevent Neural Tube Defects. Available online: https://www.who.int/tools/elena/interventions/folate-periconceptional (accessed on 25 August 2024).
- WHO. WHO Model List of Essential Medicines 19th Edition. Available online: https://publichealthupdate.com/who-model-list-of-essential-medicines-april-2015-19th-edition/ (accessed on 25 August 2024).
- Chmielewska, A.; Dziechciarz, P.; Gieruszczak-Białek, D.; Horvath, A.; Pieścik-Lech, M.; Ruszczyński, M.; Skórka, A.; Szajewska, H. Effects of Prenatal and/or Postnatal Supplementation with Iron, PUFA or Folic Acid on Neurodevelopment: Update. Br. J. Nutr. 2019, 122, S10–S15. [Google Scholar] [CrossRef]
- Caffrey, A.; McNulty, H.; Rollins, M.; Prasad, G.; Gaur, P.; Talcott, J.B.; Witton, C.; Cassidy, T.; Marshall, B.; Dornan, J.; et al. Effects of Maternal Folic Acid Supplementation during the Second and Third Trimesters of Pregnancy on Neurocognitive Development in the Child: An 11-Year Follow-Up from a Randomised Controlled Trial. BMC Med. 2021, 19, 73. [Google Scholar] [CrossRef]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 100–111. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.C.; Hong, X.; Selhub, J.; Paul, L.; Wood, R.A.; Matsui, E.C.; Keet, C.A.; Wang, X. Association Between Folate Metabolites and the Development of Food Allergy in Children. J. Allergy Clin. Immunol. Pract. 2020, 8, 132–140.e135. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Piña, D.A.; Rivera-Ramírez, N.; García-López, G.; Díaz, N.F.; Molina-Hernández, A. Calcium and Neural Stem Cell Proliferation. Int. J. Mol. Sci. 2024, 25, 4073. [Google Scholar] [CrossRef] [PubMed]
- Prado, E.L.; Dewey, K.G. Nutrition and Brain Development in Early Life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef]
- Kiely, M.E.; McCarthy, E.K.; Hennessy, Á. Iron, Iodine and Vitamin D Deficiencies during Pregnancy: Epidemiology, Risk Factors and Developmental Impacts. Proc. Nutr. Soc. 2021, 80, 290–302. [Google Scholar] [CrossRef]
- Rodionov, D.A.; Arzamasov, A.A.; Khoroshkin, M.S.; Iablokov, S.N.; Leyn, S.A.; Peterson, S.N.; Novichkov, P.S.; Osterman, A.L. Micronutrient Requirements and Sharing Capabilities of the Human Gut Microbiome. Front. Microbiol. 2019, 10, 1316. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D Deficiency: Infertility and Neurodevelopmental Diseases (Attention Deficit Hyperactivity Disorder, Autism, and Schizophrenia). Am. J. Physiol. Cell Physiol. 2018, 314, C135–C151. [Google Scholar] [CrossRef]
- Gusso, D.; Prauchner, G.R.K.; Rieder, A.S.; Wyse, A.T.S. Biological Pathways Associated with Vitamins in Autism Spectrum Disorder. Neurotox. Res. 2023, 41, 730–740. [Google Scholar] [CrossRef]
- Hill, T.R.; Verlaan, S.; Biesheuvel, E.; Eastell, R.; Bauer, J.M.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; Mets, T.; et al. A Vitamin D, Calcium and Leucine-Enriched Whey Protein Nutritional Supplement Improves Measures of Bone Health in Sarcopenic Non-Malnourished Older Adults: The PROVIDE Study. Calcif. Tissue Int. 2019, 105, 383–391. [Google Scholar] [CrossRef]
- Gariballa, S.; Yasin, J.; Alessa, A. A Randomized, Double-Blind, Placebo-Controlled Trial of Vitamin D Supplementation with or without Calcium in Community-Dwelling Vitamin D Deficient Subjects. BMC Musculoskelet. Disord. 2022, 23, 415. [Google Scholar] [CrossRef]
- Liu, S.H.; Bobb, J.F.; Claus Henn, B.; Gennings, C.; Schnaas, L.; Tellez-Rojo, M.; Bellinger, D.; Arora, M.; Wright, R.O.; Coull, B.A. Bayesian Varying Coefficient Kernel Machine Regression to Assess Neurodevelopmental Trajectories Associated with Exposure to Complex Mixtures. Stat. Med. 2018, 37, 4680–4694. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Tessing, J.; Lee, B.K.; Lyall, K. Maternal Dietary Factors and the Risk of Autism Spectrum Disorders: A Systematic Review of Existing Evidence. Autism Res. Off. J. Int. Soc. Autism Res. 2020, 13, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, G.; Wu, W.; Zhang, M.; Liu, W.; Chen, Q.; Wang, X. The Efficacy and Safety of Vitamin C for Iron Supplementation in Adult Patients with Iron Deficiency Anemia: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2023644. [Google Scholar] [CrossRef] [PubMed]
- Rocha Dda, S.; Capanema, F.D.; Netto, M.P.; de Almeida, C.A.; Franceschini Sdo, C.; Lamounier, J.A. Effectiveness of Fortification of Drinking Water with Iron and Vitamin C in the Reduction of Anemia and Improvement of Nutritional Status in Children Attending Day-Care Centers in Belo Horizonte, Brazil. Food Nutr. Bull. 2011, 32, 340–346. [Google Scholar] [CrossRef]
- da Cunha, M.S.; Siqueira, E.M.; Trindade, L.S.; Arruda, S.F. Vitamin A Deficiency Modulates Iron Metabolism via Ineffective Erythropoiesis. J. Nutr. Biochem. 2014, 25, 1035–1044. [Google Scholar] [CrossRef]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Ahangari, R.; Mohammadbeigi, A.; Miraj, S.; Rajabi, N.; Mohammadpour, R. Monitoring the Utilization and Effectiveness of Iron and Vitamin D Supplementations Program and Its Predictive Factors in High Schools’ Girls in Qom, Iran. J. Prev. Med. Hyg. 2024, 65, E36–E42. [Google Scholar] [CrossRef]
- Mukherjee, R.; Gupta Bansal, P.; Lyngdoh, T.; Medhi, B.; Sharma, K.A.; Prashanth, T.; Pullakhandam, R.; Chowdhury, R.; Taneja, S.; Yadav, K.; et al. Recommendations for India-Specific Multiple Micronutrient Supplement Through Expert Consultation. Indian J. Med Res. 2024, 159, 547–556. [Google Scholar] [CrossRef]
- Bourre, J.M. Effects of Nutrients (in Food) on the Structure and Function of the Nervous System: Update on Dietary Requirements for Brain. Part 1: Micronutrients. J. Nutr. Health Aging 2006, 10, 377–385. [Google Scholar]
- Moerkerke, M.; Daniels, N.; Van der Donck, S.; Tang, T.; Prinsen, J.; Yargholi, E.; Steyaert, J.; Alaerts, K.; Boets, B. Impact of Chronic Intranasal Oxytocin Administration on Face Expression Processing in Autistic Children: A Randomized Controlled Trial Using fMRI. Mol. Autism 2024, 15, 53. [Google Scholar] [CrossRef]

| Characteristic | Overall (n = 15,636) 1 | NDDs (n = 13,804) 1 | NDN (n = 1832) 1 | p-Value 2 |
|---|---|---|---|---|
| Child’s age | 4.6 ± 0.6 | 4.6 ± 0.6 | 4.6 ± 0.5 | <0.001 |
| Child’s sex | <0.001 | |||
| Male | 8346 (53.4%) | 7219 (52.3%) | 1127 (61.5%) | |
| Female | 7290 (46.6%) | 6585 (47.7%) | 705 (38.5%) | |
| Birth season | <0.001 | |||
| Spring | 4225 (27.0%) | 3746 (27.1%) | 479 (26.1%) | |
| Summer | 4482 (28.7%) | 3845 (27.9%) | 637 (34.8%) | |
| Autumn | 2945 (18.8%) | 2618 (19.0%) | 327 (17.8%) | |
| Winter | 3984 (25.5%) | 3595 (26.0%) | 389 (21.2%) | |
| Residence type | <0.001 | |||
| Shenzhen residents | 9415 (60.2%) | 8507 (61.6%) | 908 (49.6%) | |
| Non-Shenzhen residents | 6221 (39.8%) | 5297 (38.4%) | 924 (50.4%) | |
| Maternal education | <0.001 | |||
| Less than high school | 1614 (10.3%) | 1262 (9.14%) | 352 (19.2%) | |
| High school and higher | 14,022 (89.7%) | 12,542 (90.9%) | 1480 (80.8%) | |
| Household income | <0.001 | |||
| <RMB 20,000 | 7314 (46.8%) | 6262 (45.4%) | 1052 (57.4%) | |
| ≥RMB 20,000 | 8322 (53.2%) | 7542 (54.6%) | 780 (42.6%) | |
| Maternal conception age | 34.0 ± 5.5 | 34.0 ± 5.5 | 33.7 ± 5.7 | 0.028 |
| Pre-pregnancy BMI | <0.001 | |||
| BMI < 18.5 | 2890 (18.5%) | 2550 (18.5%) | 340 (18.6%) | |
| 18.5 ≤ BMI < 24 | 10,571 (67.6%) | 9392 (68.0%) | 1179 (64.4%) | |
| BMI ≥ 24 | 2175 (13.9%) | 1862 (13.5%) | 313 (17.1%) | |
| Intrauterine growth retardation | <0.001 | |||
| No | 15,497 (99.1%) | 13,697 (99.2%) | 1800 (98.3%) | |
| Yes | 139 (0.89%) | 107 (0.78%) | 32 (1.75%) | |
| Parity | 0.21 | |||
| No | 8810 (56.3%) | 7752 (56.2%) | 1058 (57.8%) | |
| Yes | 6826 (43.7%) | 6052 (43.8%) | 774 (42.2%) | |
| Preterm birth | <0.001 | |||
| No | 14,517 (92.8%) | 12,851 (93.1%) | 1666 (90.9%) | |
| Yes | 1119 (7.2%) | 953 (7.0%) | 166 (9.1%) | |
| Child’s birth weight | 3.1 ± 0.6 | 3.1 ± 0.6 | 3.0 ± 0.7 | <0.001 |
| Parental depression | <0.001 | |||
| No | 13,652 (87.3%) | 12,140 (87.9%) | 1512 (82.5%) | |
| Yes | 1984 (12.7%) | 1664 (12.1%) | 320 (17.5%) | |
| Family function | <0.001 | |||
| Normal | 9697 (62.0%) | 8772 (63.5%) | 925 (50.5%) | |
| Dysfunction | 5939 (38.0%) | 5032 (36.5%) | 907 (49.5%) | |
| Feeding pattern | <0.001 | |||
| Breastfeeding | 8803 (56.3%) | 7815 (56.6%) | 988 (53.9%) | |
| Formula feeding | 1665 (10.6%) | 1415 (10.3%) | 250 (13.6%) | |
| Mixed feeding | 5168 (33.1%) | 4574 (33.1%) | 594 (32.4%) | |
| Calcium supplementation | 0.003 | |||
| No | 3781 (24.2%) | 3286 (23.8%) | 495 (27.0%) | |
| Yes | 11,855 (75.8%) | 10,518 (76.2%) | 1337 (73.0%) | |
| Folic acid supplementation | 0.013 | |||
| No | 1846 (11.8%) | 1597 (11.6%) | 249 (13.6%) | |
| Yes | 13,790 (88.2%) | 12,207 (88.4%) | 1583 (86.4%) | |
| Iron supplementation | 0.11 | |||
| No | 8451 (54.0%) | 7428 (53.8%) | 1023 (55.8%) | |
| Yes | 7185 (46.0%) | 6376 (46.2%) | 809 (44.2%) | |
| Multivitamin supplementation | <0.001 | |||
| No | 8690 (55.6%) | 7549 (54.7%) | 1141 (62.3%) | |
| Yes | 6946 (44.4%) | 6255 (45.3%) | 691 (37.7%) | |
| Probiotic intake | 0.010 | |||
| No | 3098 (19.8%) | 2777 (20.1%) | 321 (17.5%) | |
| Yes | 12,538 (80.2%) | 11,027 (79.9%) | 1511 (82.5%) |
| Domains | Score, Mean ± SD | Prevalence, N (%) |
|---|---|---|
| Communication | 58 ± 5.4 | |
| Normal | 15,457 (98.9%) | |
| Delay | 179 (1.14%) | |
| Gross motor | 54 ± 8.6 | |
| Normal | 14,248 (91.1%) | |
| Delay | 1388 (8.88%) | |
| Fine motor | 52 ± 10.2 | |
| Normal | 15,214 (97.3%) | |
| Delay | 422 (2.70%) | |
| Problem-solving | 57 ± 5.7 | |
| Normal | 15,526 (99.3%) | |
| Delay | 110 (0.70%) | |
| Personal-social | 57 ± 5.6 | |
| Normal | 15,281 (97.7%) | |
| Delay | 355 (2.27%) | |
| Total | 277 ± 26.4 | |
| Normal | 13,804 (88.3%) | |
| Delay | 1832 (11.7%) |
| Micronutrients | N (%) | OR | IOR | RERI | AP | |
|---|---|---|---|---|---|---|
| Calcium | Folic acid | |||||
| No | No | 210 (13.5%) | 1.00 (ref) | — | — | — |
| No | Yes | 39 (13.3%) | 0.98 (0.67, 1.40) | — | — | — |
| Yes | No | 285 (12.8%) | 0.94 (0.77, 1.14) | — | — | — |
| Yes | Yes | 1298 (11.2%) | 0.81 (0.69, 0.95) | 0.88 (0.60, 1.32) | (, 0.28) | (, 0.34) |
| Calcium | Iron | |||||
| No | No | 457 (13.2%) | 1.00 (ref) | — | — | — |
| No | Yes | 38 (12.3%) | 0.93 (0.64, 1.30) | — | — | — |
| Yes | No | 566 (11.4%) | 0.85 (0.74, 0.97) | — | — | — |
| Yes | Yes | 771 (11.2%) | 0.83 (0.74, 0.94) | 1.06 (0.74, 1.56) | 0.06 (, 0.40) | 0.07 (, 0.49) |
| Calcium | Multivitamin | |||||
| No | No | 423 (13.3%) | 1.00 (ref) | — | — | — |
| No | Yes | 718 (13.0%) | 0.98 (0.86, 1.12) | — | — | — |
| Yes | No | 72 (12.1%) | 0.90 (0.68, 1.17) | — | — | — |
| Yes | Yes | 619 (9.7%) | 0.71 (0.62, 0.80) | 0.80 (0.60, 1.08) | (, 0.10) | (, 0.13) |
| Folic acid | Iron | |||||
| No | No | 233 (13.8%) | 1.00 (ref) | — | — | — |
| No | Yes | 16 (10.5%) | 0.74 (0.42, 1.22) | — | — | — |
| Yes | No | 790 (11.7%) | 0.83 (0.71, 0.97) | — | — | — |
| Yes | Yes | 793 (11.3%) | 0.80 (0.68, 0.93) | 1.30 (0.78, 2.33) | 0.23 (, 0.63) | 0.29 (, 0.81) |
| Folic acid | Multivitamin | |||||
| No | No | 235 (14.1%) | 1.00 (ref) | — | — | — |
| No | Yes | 906 (12.9%) | 0.91 (0.78, 1.06) | — | — | — |
| Yes | No | 14 (8.0%) | 0.53 (0.29, 0.90) | — | — | — |
| Yes | Yes | 677 (10.0%) | 0.68 (0.58, 0.80) | 1.41 (0.82, 2.61) | 0.24 (, 0.56) | 0.36 (, 0.84) |
| Iron | Multivitamin | |||||
| No | No | 795 (13.4%) | 1.00 (ref) | — | — | — |
| No | Yes | 346 (12.6%) | 0.93 (0.81, 1.06) | — | — | — |
| Yes | No | 228 (9.1%) | 0.64 (0.55, 0.75) | — | — | — |
| Yes | Yes | 463 (10.5%) | 0.75 (0.67, 0.85) | 1.26 (1.02, 1.57) | 0.18 (0.02, 0.35) | 0.24 (0.02, 0.46) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Strodl, E.; Zhang, M.; Chen, W. The Individual and Combined Effects of Prenatal Micronutrient Supplementations on Neurobehavioral Developmental Disorders in Preschool Children. Children 2025, 12, 602. https://doi.org/10.3390/children12050602
Ding L, Strodl E, Zhang M, Chen W. The Individual and Combined Effects of Prenatal Micronutrient Supplementations on Neurobehavioral Developmental Disorders in Preschool Children. Children. 2025; 12(5):602. https://doi.org/10.3390/children12050602
Chicago/Turabian StyleDing, Liwen, Esben Strodl, Maolin Zhang, and Weiqing Chen. 2025. "The Individual and Combined Effects of Prenatal Micronutrient Supplementations on Neurobehavioral Developmental Disorders in Preschool Children" Children 12, no. 5: 602. https://doi.org/10.3390/children12050602
APA StyleDing, L., Strodl, E., Zhang, M., & Chen, W. (2025). The Individual and Combined Effects of Prenatal Micronutrient Supplementations on Neurobehavioral Developmental Disorders in Preschool Children. Children, 12(5), 602. https://doi.org/10.3390/children12050602







