Probiotic, Prebiotic, and Synbiotic Supplementation for the Prevention and Treatment of Acute Otitis Media: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Research Question
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
- Randomized and controlled clinical trials, regardless of follow-up duration and specific study design (crossover, parallel, among others).
- Trials presenting multiple study groups will be included when the arm assigned to treatment with probiotics, prebiotics, or synbiotics can be isolated.
- Research published between January 2000 and July 2024.
- Studies published in English, Spanish, and Portuguese.
- Studies in populations under 18 years old, with acute otitis media or at risk of developing the disease, in which the effectiveness of prebiotics, probiotics, or synbiotics—using single or combined strains, regardless of presentation (capsule, food, fermented, powder), duration of administration, and treatment regimen—is evaluated.
- Studies that consider at least one of the following outcomes: development of acute otitis media, time until its onset, recurrence of the disease (3 episodes in 6 months or 4 episodes in 12 months), duration of the clinical episode, requirement for antibiotics, hospitalizations, incidence of upper respiratory tract infections, and adverse events.
2.3.2. Exclusion Criteria
- Articles in preprint format.
- Studies published as conference abstracts.
- Articles in the form of letters to the editor with insufficient information.
- Studies not available in an accessible format.
- Studies employing the same cohort of patients used in previous similar investigations.
- Studies that include patients with acquired or congenital immunodeficiency, craniofacial abnormalities, or chronic diseases.
- Studies that do not specify the strain present in the product supplied to the patients.
- Studies using an animal model of AOM.
- Studies in which it is difficult to evaluate the specific contribution of prebiotics, probiotics, or synbiotics to the outcome due to the simultaneous application of other interventions.
2.4. Data Sources and Search Strategy
2.5. Selection and Data Extraction
2.6. Risk of Bias Assessment
2.7. Assessment of the Quality of Evidence
2.8. Statistical Analysis
3. Results
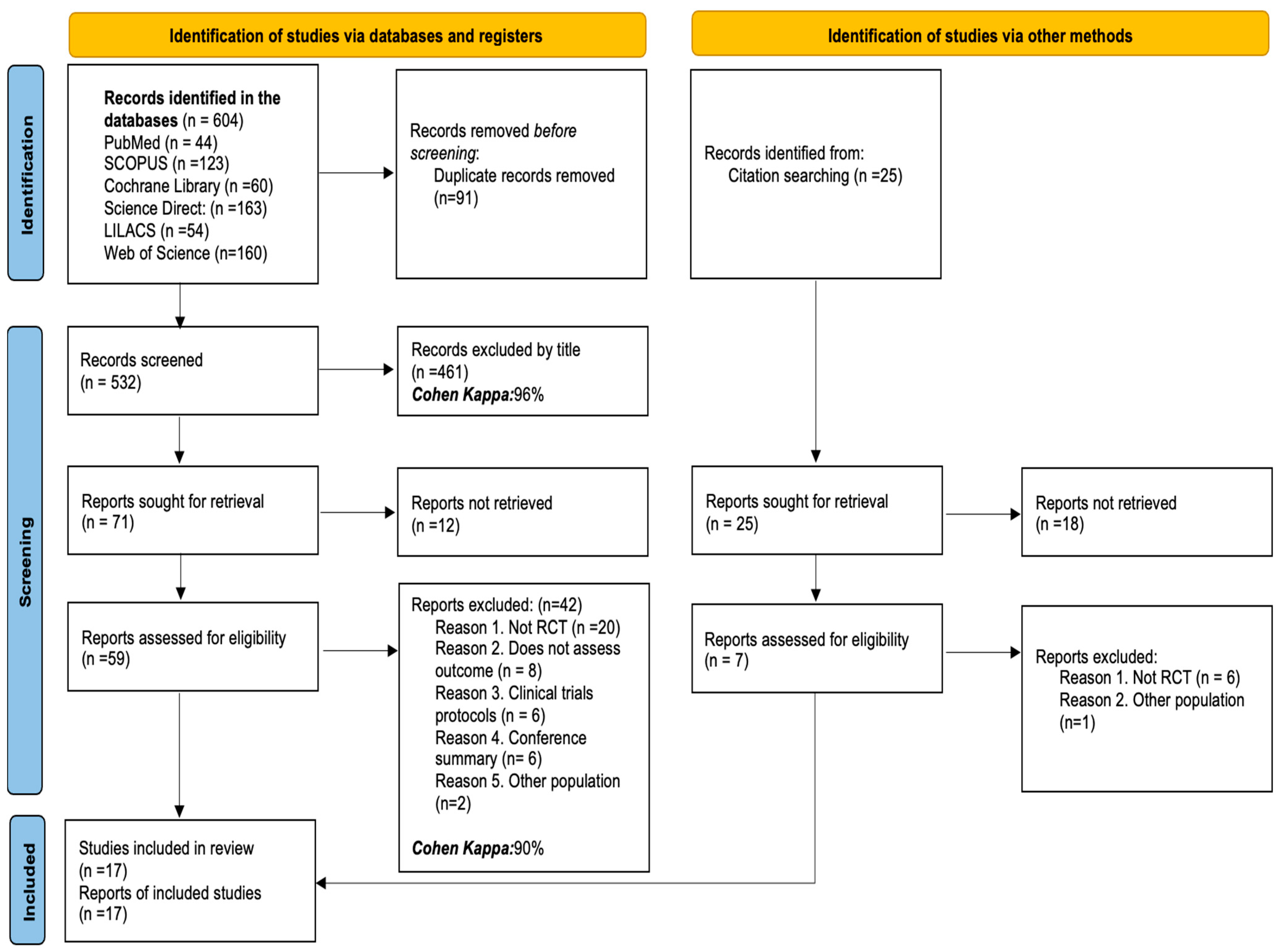
3.1. Studies Identified for the Review
3.2. Characteristics of the Studies Included in the Review
3.3. Strains Administered for the Prevention or Treatment of AOM
3.4. Characteristics of the Population and the Applied Intervention
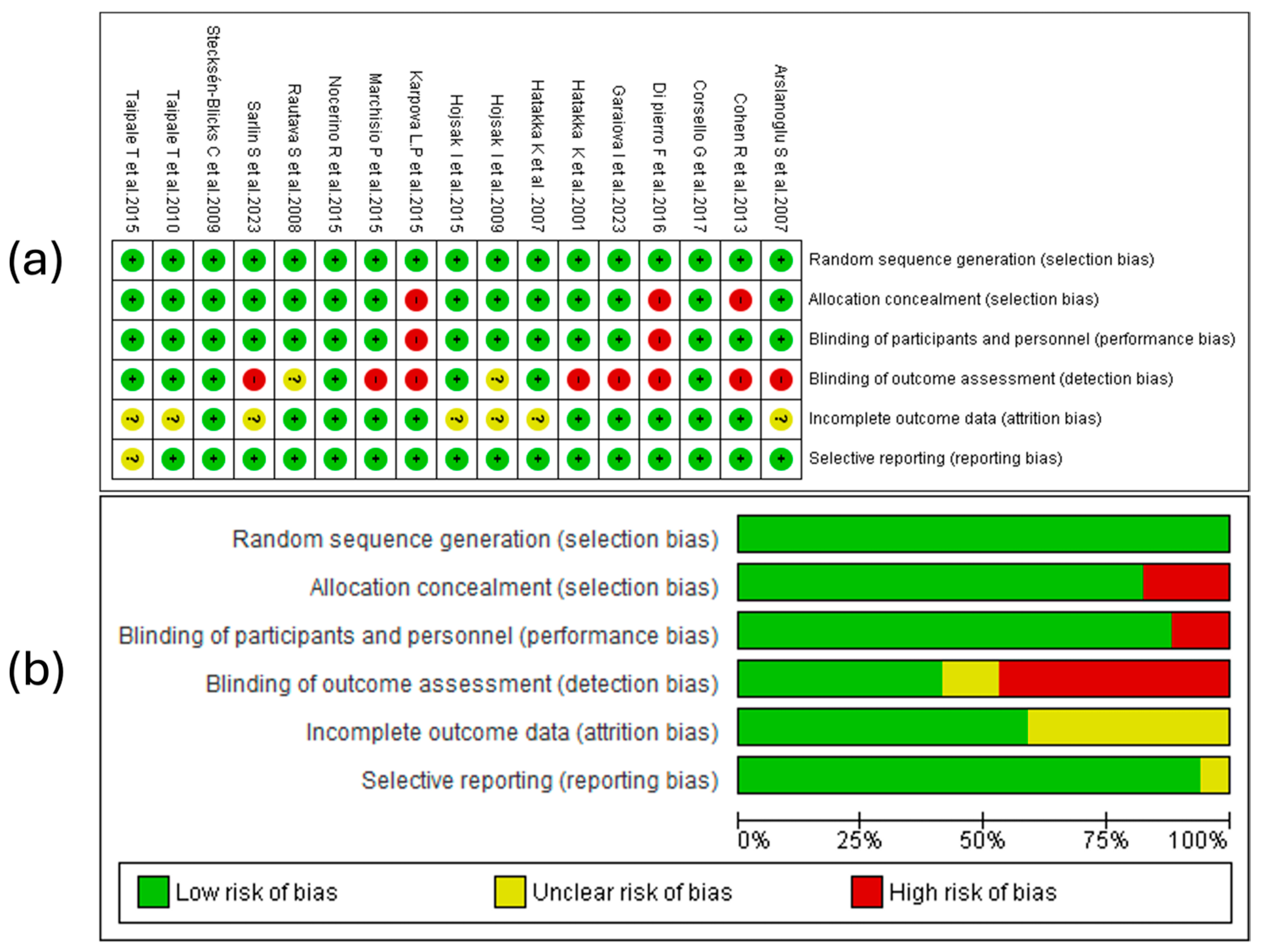
3.5. Result of Risk of Bias Assessment
3.5.1. Random Sequence Generation
3.5.2. Allocation Concealment
3.5.3. Blinding of Participants and Personnel
3.5.4. Blinding of Outcome Assessment
3.5.5. Incomplete Outcome Data
3.5.6. Selective Reporting
3.5.7. Summary of Risk of Bias
3.6. Qualitative Synthesis of the Scientific Evidence
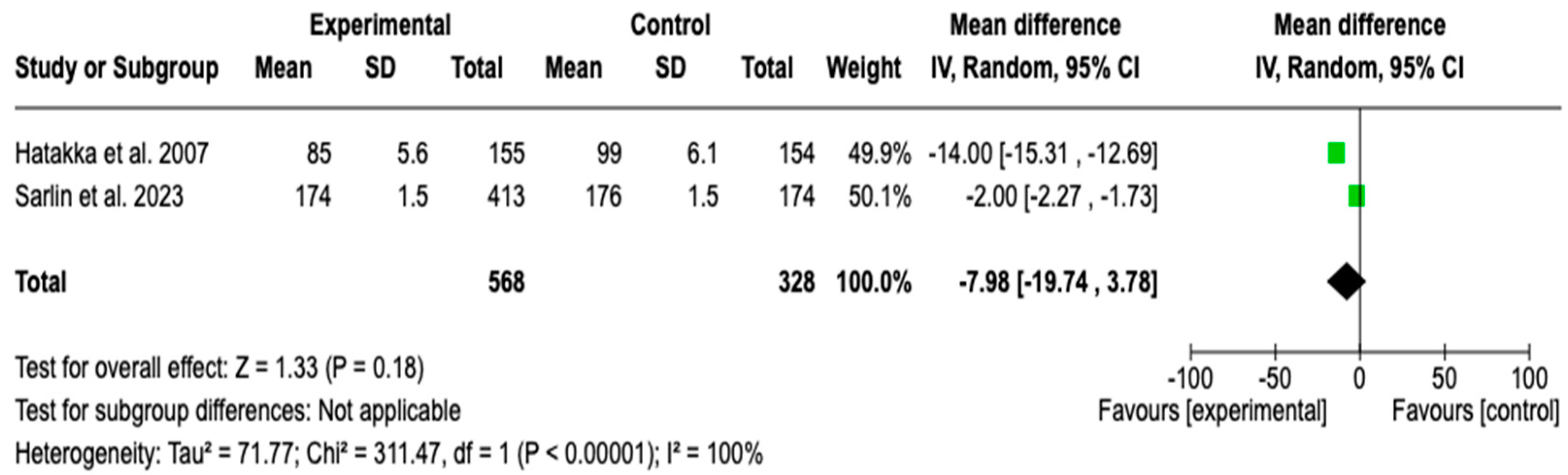
3.6.1. Duration of AOM Episodes
3.6.2. Hospitalizations
3.6.3. Incidence of Upper Respiratory Tract Infections
3.6.4. Adverse Effects
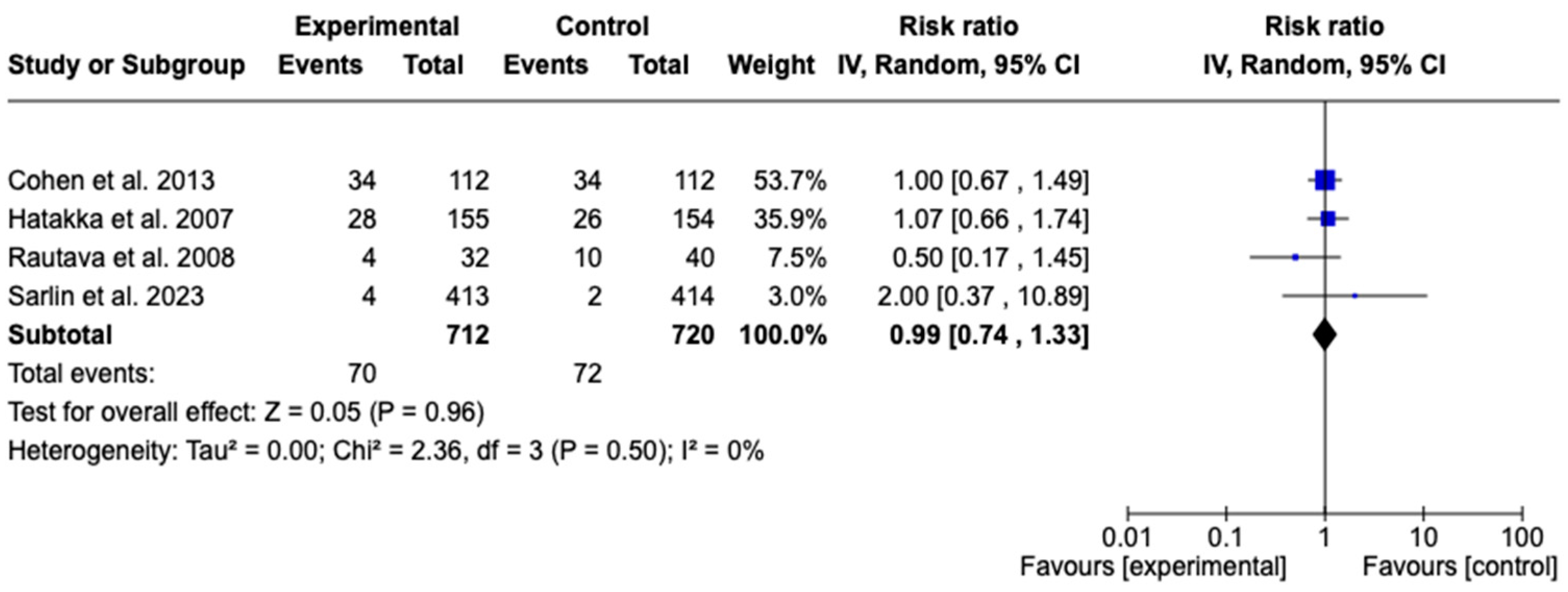
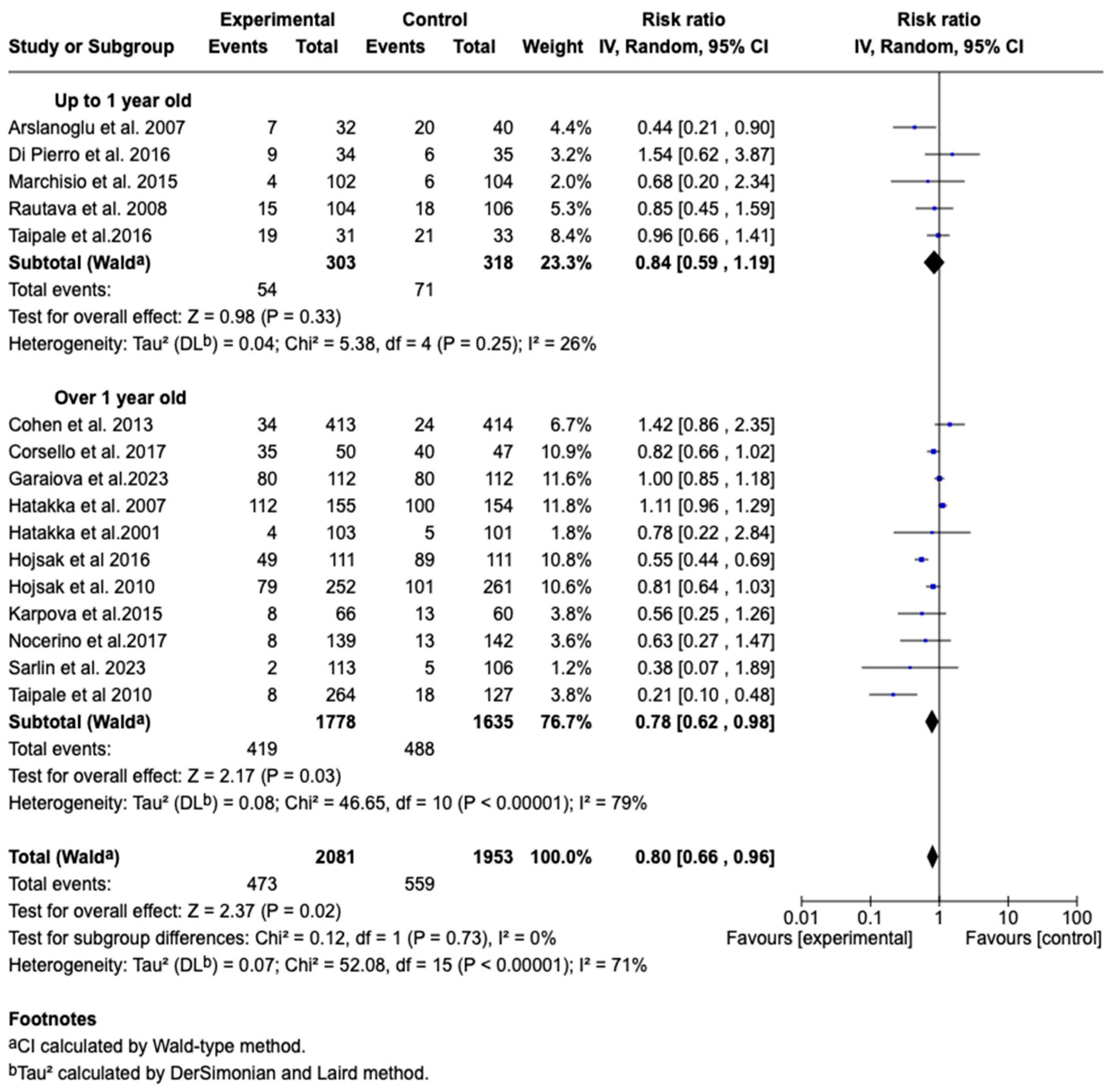
3.7. Meta-Analysis
3.7.1. Result of the Assessment of the Quality of Evidence
3.7.2. Time to AOM Episode
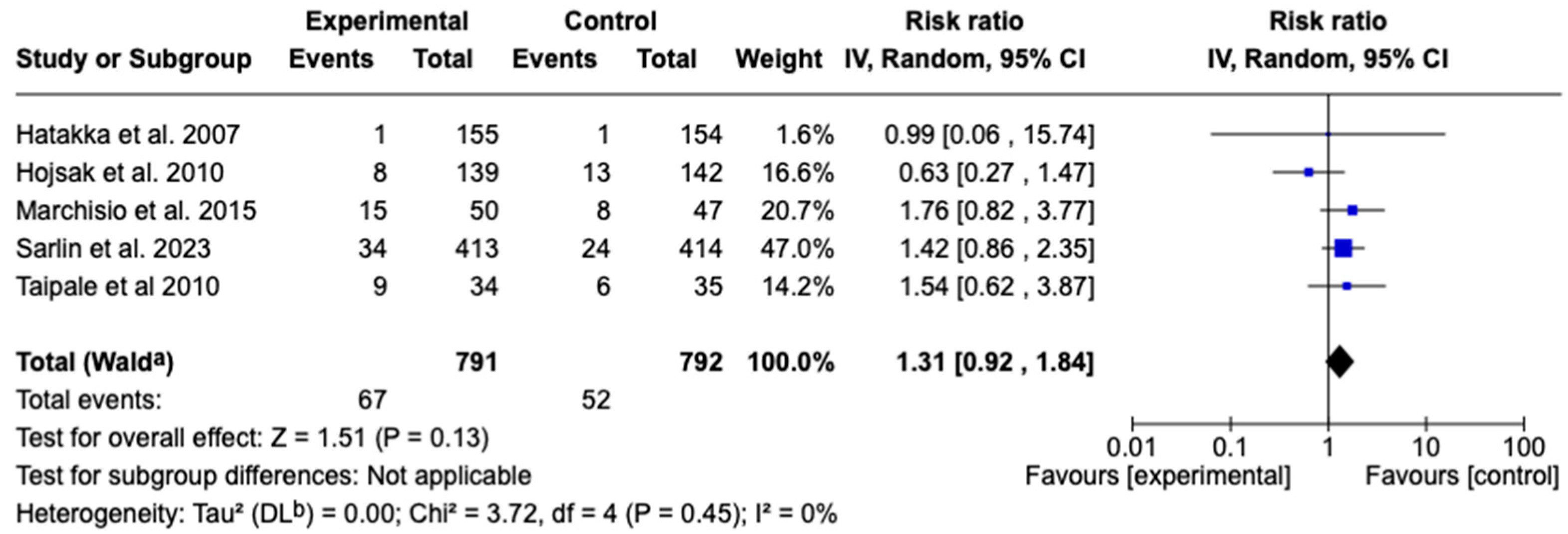
3.7.3. Recurrence of Acute Otitis Media
3.7.4. Antibiotic Requirement for Acute Otitis Media
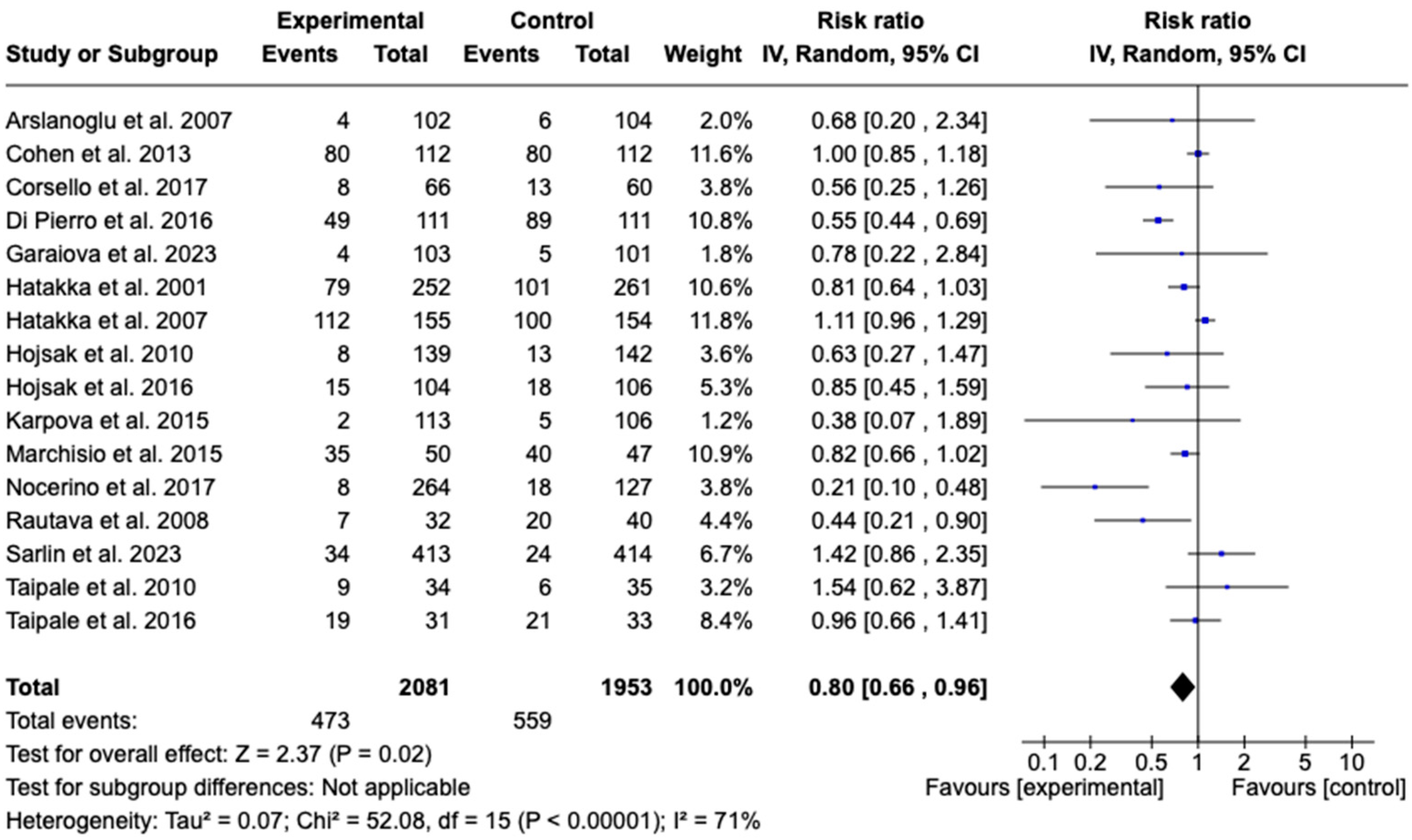
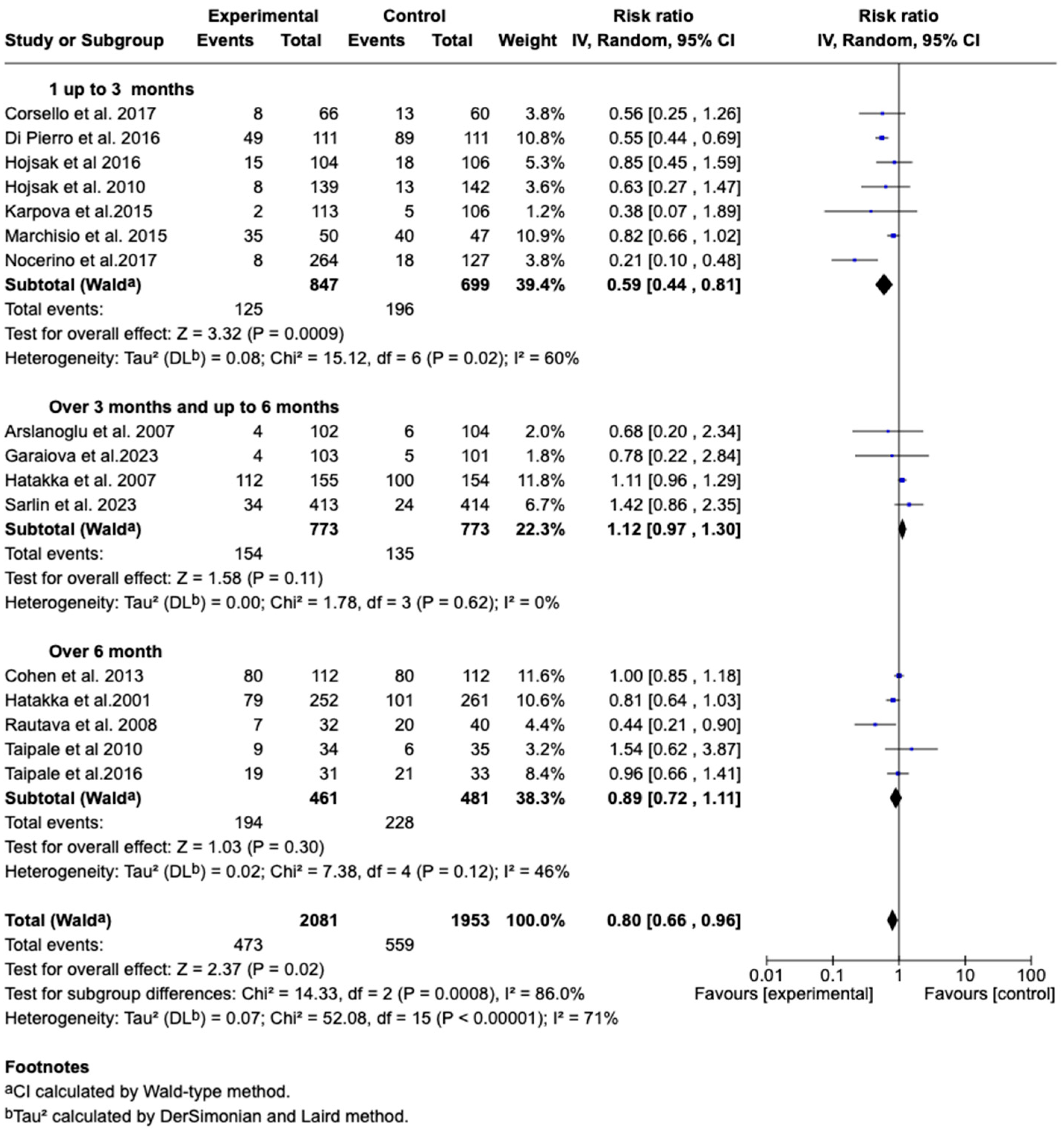
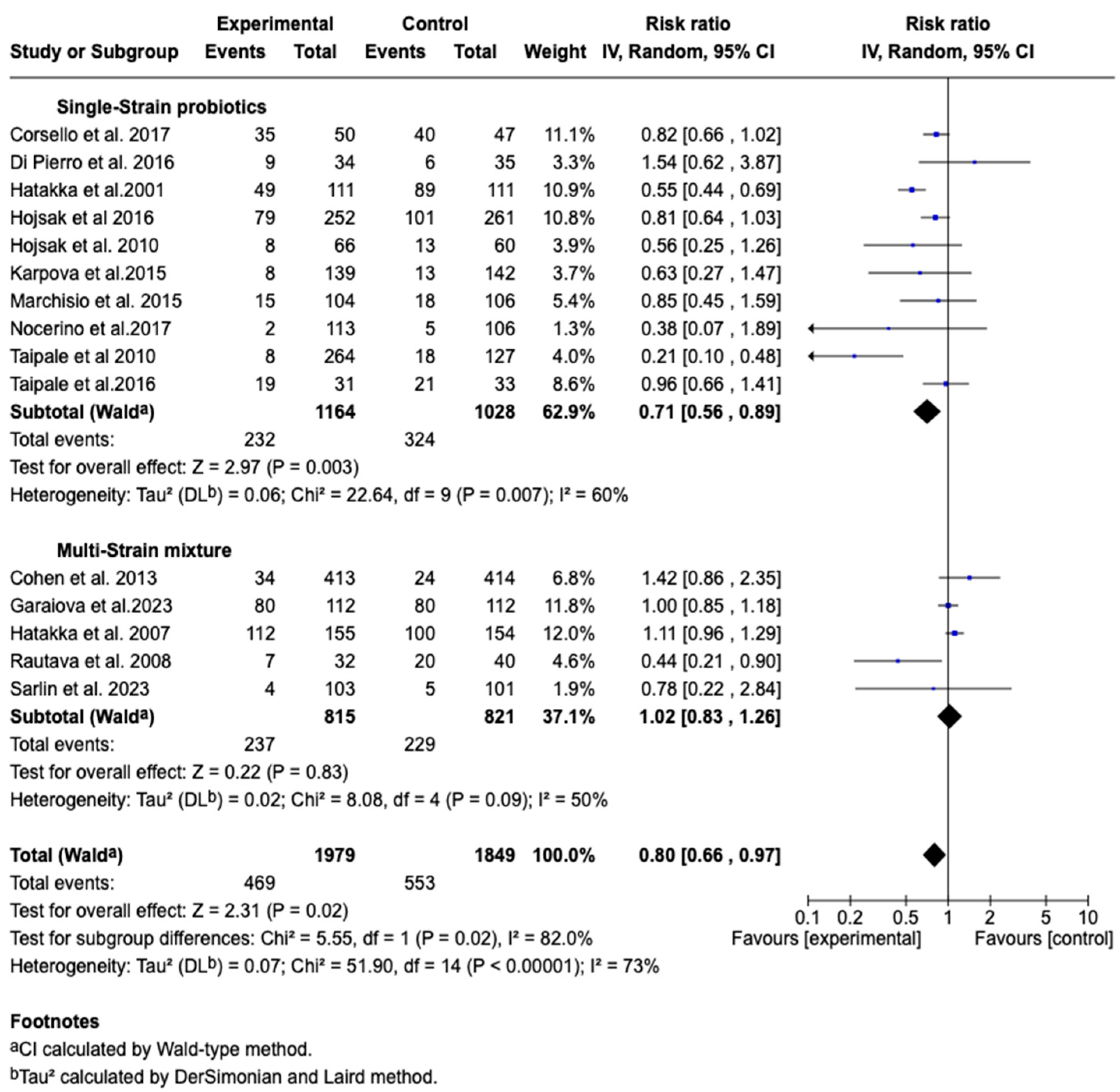
3.7.5. Incidence of Acute Otitis Media
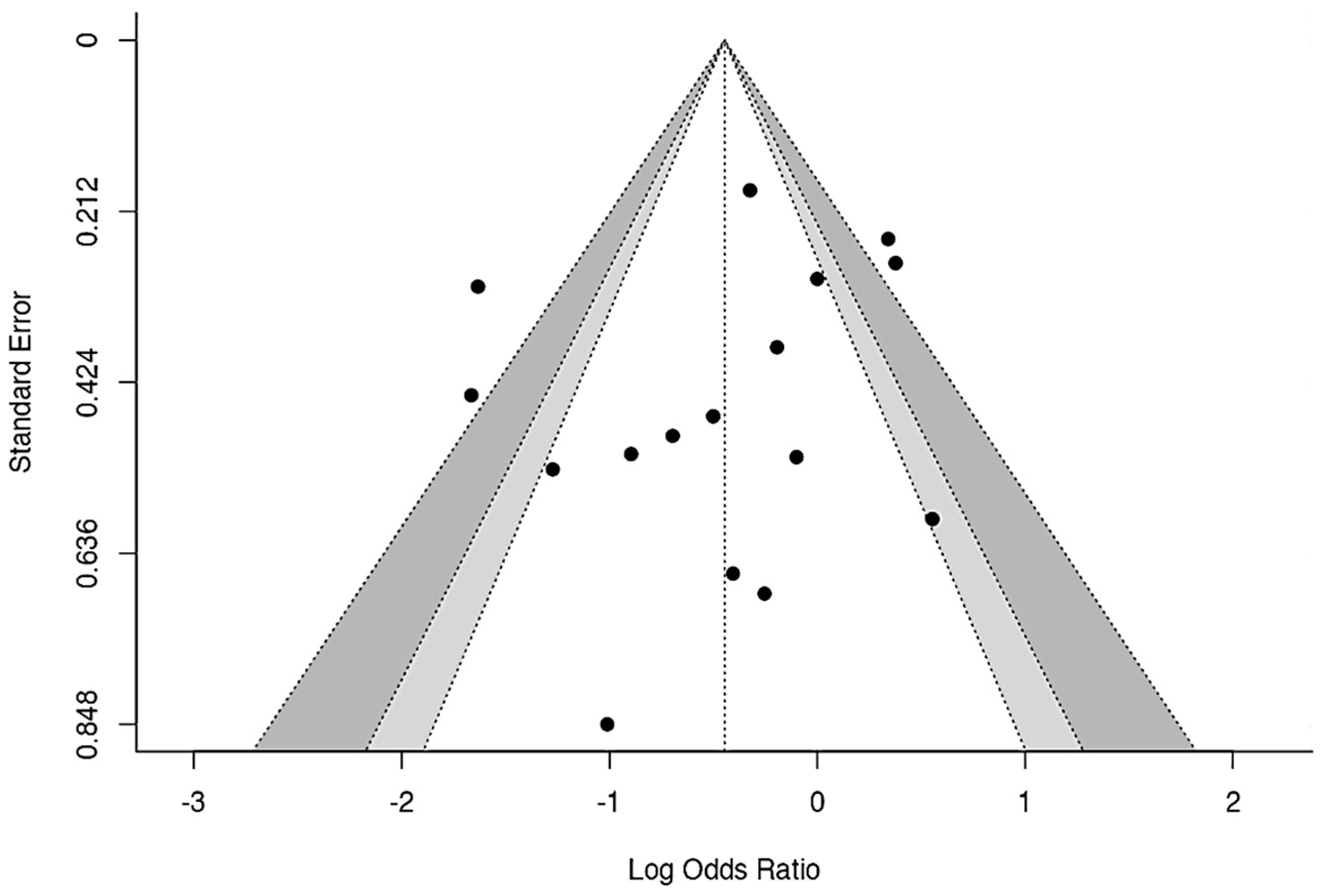
3.7.6. Publication Bias
4. Discussion
4.1. Main Findings
4.2. Comparison with Previous Studies
4.3. Possible Mechanisms of Action
4.4. Limitations of the Included Studies
4.5. Clinical Implications
4.6. Recommendations for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conlon, C.; Zupan, B.; Pirie, E.; Gupta, C. The impact of otitis media on speech production in children: A systematic review. J. Commun. Disord. 2025, 113, 106490. [Google Scholar] [CrossRef]
- Folino, F.; Di Pasquale, D.; Marchisio, P.; Pignataro, L.; Capaccio, P.; Gaini, L.; Battilocchi, L.; Bosis, S.; Torretta, S. Topical administration of S. salivarius 24SMB—S. oralis 89a in children with adenoidal disease: A double-blind controlled trial. Eur. J. Pediatr. 2024, 183, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Gisselsson-Solen, M.; Gunasekera, H.; Hall, A.; Homoe, P.; Kong, K.; Sih, T.; Rupa, V.; Morris, P. Panel 1: Epidemiology and global health, including child development, sequelae and complications. Int. J. Pediatr. Otorhinolaryngol. 2024, 178, 111861. [Google Scholar] [CrossRef]
- Tamir, S.O.; Bialasiewicz, S.; Brennan-Jones, C.G.; Der, C.; Kariv, L.; Macharia, I.; Marsh, R.L.; Seguya, A.; Thornton, R. ISOM 2023 research Panel 4—Diagnostics and microbiology of otitis media. Int. J. Pediatr. Otorhinolaryngol. 2023, 174, 111741. [Google Scholar] [CrossRef]
- Schilder, A.G.M.; Chonmaitree, T.; Cripps, A.W.; Rosenfeld, R.M.; Casselbrant, M.L.; Haggard, M.P.; Venekamp, R.P. Otitis media. Nat. Rev. Dis. Primer 2016, 2, 16063. [Google Scholar] [CrossRef]
- Margulis, I.; Cohen-Kerem, R.; Stein, N.; Roitman, A.; Cohen-Vaizer, M.; Fridman, E.; Gordin, A. Xylitol nasal spray for prevention of recurrent acute otitis media in children: A prospective two-center cohort study. Int. J. Pediatr. Otorhinolaryngol. 2024, 176, 111818. [Google Scholar] [CrossRef]
- Fan, Y.; Li, D.; Wang, P.; Ren, L.; Chen, X. Case-control study of relationship of infection by respiratory viruses with acute otitis media in Chinese children. Heliyon 2023, 9, e14422. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, N.; Martín, V.; Arroyo, R.; López, M.; Carrera, M.; Badiola, C.; Jiménez, E.; Rodríguez, J.M. Prevention of Recurrent Acute Otitis Media in Children Through the Use of Lactobacillus salivarius PS7, a Target-Specific Probiotic Strain. Nutrients 2019, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Sethi, R.K.V.; Stankovic, K.M. Acute Otitis Media and Associated Complications in United States Emergency Departments. Otol. Neurotol. 2018, 39, 1005–1011. [Google Scholar] [CrossRef]
- Jamal, A.; Alsabea, A.; Tarakmeh, M.; Safar, A. Etiology, Diagnosis, Complications, and Management of Acute Otitis Media in Children. Cureus 2022, 14, e28019. [Google Scholar] [CrossRef]
- Meherali, S.; Hartling, L.; Campbell, A.; Robin, F.; Scott, S. Parent information needs and experience regarding acute otitis media in children: A systematic review. Patient Educ. Couns. 2021, 104, 554–562. [Google Scholar] [CrossRef] [PubMed]
- El Feghaly, R.E.; Nedved, A.; Katz, S.E.; Frost, H.M. New insights into the treatment of acute otitis media. Expert Rev. Anti Infect. Ther. 2023, 21, 523–534. [Google Scholar] [CrossRef]
- Monasta, L.; Ronfani, L.; Marchetti, F.; Montico, M.; Vecchi Brumatti, L.; Bavcar, A.; Grasso, D.; Barbiero, C.; Tamburlini, G. Burden of Disease Caused by Otitis Media: Systematic Review and Global Estimates. PLoS ONE 2012, 7, e36226. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Morris, M.; Pichichero, M.E. Epidemiology of Acute Otitis Media in the Postpneumococcal Conjugate Vaccine Era. Pediatrics 2017, 140, e20170181. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics 2004, 113, 1451–1465. [Google Scholar]
- Storch-De-Gracia, P.; Antoñanzas-Bernar, V.; Vergara-Muñoz, B.; Lamagrande-Casanova, N.; Di Campli-Zaghlul, M.; Suárez-Bustamante, M.; Añón-Hidalgo, J.; Maiques, M. Comparison of amoxicillin administered twice and three times daily in children with acute otitis media. Eur. J. Pediatr. 2023, 182, 5599–5605. [Google Scholar] [CrossRef]
- Dimo, J.; Poole, N.M. Less is More: The Evidence for Shorter Durations of Antimicrobial Therapy for Acute Otitis Media. Curr. Treat. Options Pediatr. 2024, 10, 265–275. [Google Scholar] [CrossRef]
- Frost, H.M.; Bizune, D.; Gerber, J.S.; Hersh, A.L.; Hicks, L.A.; Tsay, S.V. Amoxicillin Versus Other Antibiotic Agents for the Treatment of Acute Otitis Media in Children. J. Pediatr. 2022, 251, 98–104.e5. [Google Scholar] [CrossRef]
- Smolinski, N.; Antonelli, P.; Winterstein, A. Watchful Waiting for Acute Otitis Media. Pediatrics 2022, 150, e2021055613. [Google Scholar] [CrossRef]
- Gaddey, H.L.; Wright, M.T.; Nelson, T.N. Otitis Media: Rapid Evidence Review. Am. Fam. Physician 2019, 100, 350–356. [Google Scholar]
- Leach, A.; Morris, P. Cochrane review: Antibiotics for the prevention of acute and chronic suppurative otitis media in children. Evid.-Based Child Health 2007, 2, 697–760. [Google Scholar] [CrossRef]
- Hullegie, S.; Venekamp, R.P.; van Dongen, T.M.; Hay, A.D.; Moore, M.V.; Little, P.; Schilder, A.G.; Damoiseaux, R.A. Prevalence and antimicrobial resistance of bacteria in children with acute otitis media and ear discharge: A systematic review. Pediatr. Infect. Dis. J. 2021, 40, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.; Smith, M.E.; George, R.; Dodgson, K.; Lloyd, S.K.W. Changes in antimicrobial resistance in acute otitis media and otitis externa. Clin. Otolaryngol. 2023, 48, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovici, C.; Spoială, E.-L.; Miron, I.-C.; Stârcea, I.M.; Haliţchi, C.O.I.; Zetu, I.N.; Lupu, V.V.; Pânzaru, C. Acute otitis media in children—Challenges of antibiotic resistance in the post-vaccination era. Microorganisms 2022, 10, 1598. [Google Scholar] [CrossRef]
- Coleman, A.; Cervin, A. Probiotics in the treatment of otitis media. The past, the present and the future. Int. J. Pediatr. Otorhinolaryngol. 2019, 116, 135–140. [Google Scholar] [CrossRef]
- Chan, C.K.Y.; Tao, J.; Chan, O.S.; Li, H.-B.; Pang, H. Preventing Respiratory Tract Infections by Synbiotic Interventions: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, A.T.; Edwards, H.A. Review of probiotic use in otolaryngology. Am. J. Otolaryngol. 2021, 42, 102883. [Google Scholar] [CrossRef]
- Rashidi, K.; Darand, M.; Garousi, N.; Dehghani, A.; Alizadeh, S. Effect of infant formula supplemented with prebiotics and probiotics on incidence of respiratory tract infections: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Med. 2021, 63, 102795. [Google Scholar] [CrossRef]
- Scott, A.M.; Clark, J.; Julien, B.; Islam, F.; Roos, K.; Grimwood, K.; Little, P.; Del Mar, C.B. Probiotics for preventing acute otitis media in children. Cochrane Database Syst. Rev. 2019, 6, CD012941. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 96, 102795. [Google Scholar] [CrossRef]
- Knobloch, K.; Yoon, U.; Vogt, P.M. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J. Cranio-Maxillofac. Surg. 2011, 39, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Sarlin, S.; Koskela, U.; Honkila, M.; Taehtinen, P.; Pokka, T.; Renko, M.; Tapiainen, T. Streptococcus salivarius Probiotics to Prevent Acute Otitis Media in Children A Randomized Clinical Trial. JAMA Netw. OPEN 2023, 6, e2340608. [Google Scholar] [CrossRef]
- Garaiova, I.; Paduchová, Z.; Nagyová, Z.; Wang, D.; Michael, D.R.; Plummer, S.F.; Marchesi, J.R.; Ďuračková, Z.; Muchová, J. Probiotics with low dose vitamin C reduce antibiotic prescriptions in children: A secondary analysis of a multicentre randomised double-blind placebo-controlled trial. J. Funct. Foods 2023, 106, 105625. [Google Scholar] [CrossRef]
- Corsello, G.; Carta, M.; Marinello, R.; Picca, M.; De Marco, G.; Micillo, M.; Ferrara, D.; Vigneri, P.; Cecere, G.; Ferri, P.; et al. Preventive Effect of Cow’s Milk Fermented with Lactobacillus paracasei CBA L74 on Common Infectious Diseases in Children: A Multicenter Randomized Controlled Trial. Nutrients 2017, 9, 669. [Google Scholar] [CrossRef]
- Hojsak, I.; Močić Pavić, A.; Kos, T.; Dumančić, J.; Kolaček, S. Bifidobacterium animalis subsp. lactis in prevention of common infections in healthy children attending day care centers—Randomized, double blind, placebo-controlled study. Clin. Nutr. 2016, 35, 587–591. [Google Scholar] [CrossRef]
- Di Pierro, F.; Colombo, M.; Giuliani, M.G.; Danza, M.L.; Basile, I.; Bollani, T.; Conti, A.M.; Zanvit, A.; Rottoli, A.S. Effect of administration of Streptococcus salivarius K12 on the occurrence of streptococcal pharyngo-tonsillitis, scarlet fever and acute otitis media in 3 years old children. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4601–4606. [Google Scholar]
- Taipale, T.J.; Pienihäkkinen, K.; Isolauri, E.; Jokela, J.T.; Söderling, E.M. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in early childhood. Pediatr. Res. 2016, 79, 65–69. [Google Scholar] [CrossRef]
- Marchisio, P.; Santagati, M.; Scillato, M.; Baggi, E.; Fattizzo, M.; Rosazza, C.; Stefani, S.; Esposito, S.; Principi, N. Streptococcus salivarius 24SMB administered by nasal spray for the prevention of acute otitis media in otitis-prone children. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2377–2383. [Google Scholar] [CrossRef]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; De Marco, G.; Micillo, M.; Albano, F.; et al. Cow’s milk and rice fermented with Lactobacillus paracasei CBA L74 prevent infectious diseases in children: A randomized controlled trial. Clin. Nutr. 2017, 36, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Karpova, E.P.; Karpycheva, I.E.; Tulupov, D.A. Prophylaxis of chronic adenoiditis in the children. Vestn. Otorinolaringol. 2015, 80, 43. [Google Scholar] [CrossRef]
- Cohen, R.; Martin, E.; De La Rocque, F.; Thollot, F.; Pecquet, S.; Werner, A.; Boucherat, M.; Varon, E.; Bingen, E.; Levy, C. Probiotics and Prebiotics in Preventing Episodes of Acute Otitis Media in High-risk Children: A Randomized, Double-blind, Placebo-controlled Study. Pediatr. Infect. Dis. J. 2013, 32, 810–814. [Google Scholar] [CrossRef]
- Hojsak, I.; Snovak, N.; Abdović, S.; Szajewska, H.; Mišak, Z.; Kolaček, S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2010, 29, 312–316. [Google Scholar] [CrossRef]
- Taipale, T.; Pienihäkkinen, K.; Isolauri, E.; Larsen, C.; Brockmann, E.; Alanen, P.; Jokela, J.; Söderling, E. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in infancy. Br. J. Nutr. 2011, 105, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Stecksén-Blicks, C.; Sjöström, I.; Twetman, S. Effect of Long-Term Consumption of Milk Supplemented with Probiotic Lactobacilli and Fluoride on Dental Caries and General Health in Preschool Children: A Cluster-Randomized Study. Caries Res. 2009, 43, 374–381. [Google Scholar] [CrossRef]
- Rautava, S.; Salminen, S.; Isolauri, E. Specific probiotics in reducing the risk of acute infections in infancy—A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2008, 101, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Hatakka, K.; Blomgren, K.; Pohjavuori, S.; Kaijalainen, T.; Poussa, T.; Leinonen, M.; Korpela, R.; Pitkäranta, A. Treatment of acute otitis media with probiotics in otitis-prone children—A double-blind, placebo-controlled randomised study. Clin. Nutr. 2007, 26, 314–321. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Moro, G.E.; Boehm, G. Early Supplementation of Prebiotic Oligosaccharides Protects Formula-Fed Infants against Infections during the First 6 Months of Life. J. Nutr. 2007, 137, 2420–2424. [Google Scholar] [CrossRef]
- Hatakka, K.; Savilahti, E.; Ponka, A.; Meurman, J.; Nase, L. Effect of long-term consumption of probiotic milk on infections in children attending day care centres: Double blind, randomised trial. Ambul. Child Health 2001, 7, 334. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Hu, L.; Wang, X. Advancements related to probiotics for preventing and treating recurrent respiratory tract infections in children. Front. Pediatr. 2025, 13, 1508613. [Google Scholar] [CrossRef] [PubMed]
- Laursen, R.P.; Hojsak, I. Probiotics for respiratory tract infections in children attending day care centers—A systematic review. Eur. J. Pediatr. 2018, 177, 979–994. [Google Scholar] [CrossRef]
- Esposito, S.; Rigante, D.; Principi, N. Do children’s upper respiratory tract infections benefit from probiotics? BMC Infect. Dis. 2014, 14, 194. [Google Scholar] [CrossRef]
- Chen, T.Y.; Hendrickx, A.; Stevenson, D.S.; Bird, P.; Walls, T. No evidence from a systematic review for the use of probiotics to prevent otitis media. Acta Paediatr. 2020, 109, 2515–2524. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, B.R.; Hao, Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2022, 8, CD006895. [Google Scholar] [CrossRef] [PubMed]
- Godur, D.A.; Denton, A.J.; Eshraghi, N.; Mittal, J.; Cooper, J.; Moosa, M.; Mittal, R. Modulation of Gut Microbiome as a Therapeutic Modality for Auditory Disorders. Audiol. Res. 2023, 13, 741–752. [Google Scholar] [CrossRef]
- Peng, X.; Li, Z.; Pei, Y.; Zheng, S.; Liu, J.; Wang, J.; Li, R.; Xu, X. Streptococcus salivarius K12 Alleviates Oral Mucositis in Patients Undergoing Radiotherapy for Malignant Head and Neck Tumors: A Randomized Controlled Trial. J. Clin. Oncol. 2024, 42, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Laws, G.L.; Hale, J.D.F.; Kemp, R.A. Human Systemic Immune Response to Ingestion of the Oral Probiotic Streptococcus salivarius BLIS K12. Probiotics Antimicrob. Proteins 2021, 13, 1521–1529. [Google Scholar] [CrossRef]
- Mosquera, F.E.C.; Lizcano Martinez, S.; Liscano, Y. Effectiveness of Psychobiotics in the Treatment of Psychiatric and Cognitive Disorders: A Systematic Review of Randomized Clinical Trials. Nutrients 2024, 16, 1352. [Google Scholar] [CrossRef]
- Cruz Mosquera, F.E.; Perlaza, C.L.; Naranjo Rojas, A.; Murillo Rios, S.; Carrero Gallego, A.; Fischersworring, S.I.; Rodríguez, J.S.; Liscano, Y. Effectiveness of Probiotics, Prebiotics, and Symbiotic Supplementation in Cystic Fibrosis Patients: A Systematic Review and Meta-Analysis of Clinical Trials. Medicina 2025, 61, 489. [Google Scholar] [CrossRef]
- Martinez Guevara, D.; Vidal Cañas, S.; Palacios, I.; Gómez, A.; Estrada, M.; Gallego, J.; Liscano, Y. Effectiveness of Probiotics, Prebiotics, and Synbiotics in Managing Insulin Resistance and Hormonal Imbalance in Women with Polycystic Ovary Syndrome (PCOS): A Systematic Review of Randomized Clinical Trials. Nutrients 2024, 16, 3916. [Google Scholar] [CrossRef] [PubMed]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. ‘Multi-omics’ data integration: Applications in probiotics studies. npj Sci. Food 2023, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, L.; Butcher, J.; Stintzi, A.; Figeys, D. Advancing functional and translational microbiome research using meta-omics approaches. Microbiome 2019, 7, 154. [Google Scholar] [CrossRef]
| Author and Year | Country | Design | Subjects | Setting | Evaluated Outcomes |
|---|---|---|---|---|---|
| Sarlin S et al., 2024 [34] | Finland | RCT | Healthy children at risk of AOM, between 1 to 6 years old | Daycares and schools | Development of AOM, time to the first AOM episode, disease recurrence, antibiotic requirement, hospitalizations, and incidence of upper respiratory tract infections. |
| Garaiova I et al., 2023 [35] | Slovakia | RCT | Healthy children between 3 and 10 years old | Outpatient and daycares | Development of AOM and incidence of upper respiratory tract infections. |
| Corsello G et al., 2017 [36] | Italy | RCT | Healthy children between 12 and 48 months old | Daycares | Development of AOM and adverse events. |
| Hojsak I et al., 2016 [37] | Croatia | RCT | Healthy children, between 1 and 6 months old | Daycares | Development of AOM and adverse events. |
| Di Pierro F et al., 2016 [38] | Italy | RCT | Healthy children around 3 years old | Daycares | Development of AOM and adverse events. |
| Taipale T et al., 2016 [39] | Finland | RCT | Healthy children from 1 month of age | Outpatient | Development of AOM and adverse events. |
| Marchisio P et al., 2015 [40] | Italy | RCT | Children with recurrent AOM, between 1 and 5 years old | Outpatient, Hospital | Development of AOM, antibiotic requirement, and adverse events. |
| Nocerino R et al., 2015 [41] | Italy | RCT | Healthy children between 12 and 48 months old | Outpatient | Development of AOM and adverse events. |
| Karpova E et al., 2015 [42] | Russia | RCT | Sick children, between 6 and 7 years old | Outpatient | Development of AOM. |
| Cohen R et al., 2013 [43] | France | RCT | Healthy children at risk of AOM, between 7 and 13 months old | Outpatient | Development of AOM, disease recurrence, antibiotic requirement, and adverse events. |
| Hojsak I et al., 2010 [44] | Croatia | RCT | Healthy children with an average age of 52 months | Daycares | Development of AOM and antibiotic requirement. |
| Taipale T et al., 2010 [45] | Finland | RCT | Healthy children from 1 month of birth | Outpatient | Development of AOM, antibiotic requirement, and adverse events. |
| Stecksén-Blicks C et al., 2009 [46] | Sweden | RCT | Healthy children between 1 and 5 years old | Daycare | Duration of the AOM episode, antibiotic requirement, and adverse events. |
| Rautava S et al., 2008 [47] | Finland | RCT | Healthy children requiring infant formula before 2 months of age | Outpatient | Development of AOM, disease recurrence, antibiotic requirement, and adverse events. |
| Hatakka K et al., 2007 [48] | Finland | RCT | Healthy children at risk of AOM, between 10 months and 6 years old | Daycares and primary care centers | Development of AOM, time to the first episode of the disease, disease recurrence, duration of the clinical episode, antibiotic requirement, and incidence of upper respiratory tract infections. |
| Arslanoglu S et al., 2007 [49] | Italy | RCT | Healthy children within the first 2 weeks of life | Outpatient | Development of AOM and incidence of upper respiratory tract infections. |
| Hatakka K et al., 2001 [50] | Finland | RCT | Healthy children at risk of AOM, between 1 and 6 years old | Daycares | Development of AOM, antibiotic requirement, and adverse events. |
| Lactobacillus spp. | Bifidobacterium spp. | Streptococcus spp. | Others |
|---|---|---|---|
| L. rhamnosus LPR CGMCC 1.3824 | B. breve 99 | S. salivarius K12 | P. freudenreichii shermanii JS |
| L. rhamnosus GG ATCC 53103 | B. lactis Bb-12 | S. salivarius 24SMB | |
| L. rhamnosus LC 705 | B. bifidum CUL20 | S. salivarius DSM 13084 | |
| L. rhamnosus LB21 | B. animalis subsp. lactis CUL34 | S. thermophilus NCC 2496 | |
| L. acidophilus CUL21 | |||
| L. acidophilus CUL60 | |||
| L. paracasei CBA L74 |
| Study | Patients (I/C) | % Male | Mean Age (Years) | Product (Type, Presentation, Strain, Dose, Route of Administration) | Treatment Duration (Months) | Conclusion |
|---|---|---|---|---|---|---|
| Sarlin S et al., 2024 [34] | I:413 C:414 | 52 | 4.1 | Synbiotic. Soluble powder or chewable tablet. S. salivarius K12 1 × 109 CFU, L. rhamnosus GG 1 × 108 CFU, P. freudenreichii shermanii JS 1 × 108 CFU, fructooligosaccharide 480 mg. Soluble powder or chewable tablet. Oral route. | 6 | The use of a product containing S. salivarius K12 did not reduce the occurrence of acute otitis media in the studied population. |
| Garaiova I et al., 2023 [35] | I:103 C:101 | 50.4 | 6.6 | Probiotic. Chewable tablet. L. acidophilus CUL21 and CUL60, B. bifidum CUL20, B. animalis subsp. lactis CUL34. 1.25 × 1010 CFU. Oral route. | 6 | Supplementation with the probiotic combination used may reduce antibiotic requirements in pediatric patients. |
| Corsello G et al., 2017 [36] | I:66 C:60 | 57 | 2.7 | Probiotic. Powdered cow’s milk. L. paracasei CBA L74. 5.9 × 109 CFU. Oral route. | 3 | The use of fermented cow’s milk with L. paracasei may prevent some childhood infections. |
| Hojsak I et al., 2016 [37] | I:104 C:106 | 43 | 4.4 | Probiotic. Powder. B. animalis subsp. lactis CUL34. 1 × 109 CFU. Oral route. | 3 | The findings suggest that the administered strain does not prevent respiratory infections in the included children. |
| Di Pierro F et al., 2016 [38] | I:111 C:111 | 48 | 3 | Probiotic. Tablets. S. salivarius K12. 1 × 109 CFU. Oral route. | 3 | The administration of S. salivarius K12 was associated with a reduction in AOM episodes. |
| Taipale T et al., 2016 [39] | I:55 C:54 | NS | <1 year | Probiotic. Tablet. B. lactis Bb-12. 1 × 1010 CFU. Oral route. | 12 | The administration of the supplied probiotic may reduce respiratory tract infections. |
| Marchisio P et al., 2015 [40] | I:50 C:47 | NS | 2.9 | Probiotic. Nasal spray. S. salivarius 24SMB. 100 × 109 CFU; 1 dose per nostril. | 3 | Probiotic supplementation with S. salivarius 24SMB via the intranasal route has the capacity to reduce the risk of AOM. |
| Nocerino R et al., 2015 [41] | I:264 C:127 | 51 | 2.6 | Probiotic. Fermented milk. L. paracasei CBA L74. 5.9 × 109 CFU. Oral route. | 3 | Fermented cow or rice milk with L. paracasei CBA L74 can prevent some common childhood infections. |
| Karpova E et al., 2015 [42] | I:113 C:106 | NS | NS | Probiotic. Tablet. S. salivarius K12. Oral route. | 1 | The administered probiotic complex was associated with a lower frequency of otitis media in the studied patients. |
| Cohen R et al., 2013 [43] | I:112 C:112 | NS | <1 year | Synbiotic. Milk. S. salivarius 2.5 × 107 CFU, S. thermophilus 1 × 107 CFU, L. rhamnosus 1 × 107 CFU + Raftiline. Oral route. | 12 | The synbiotic administered in children did not reduce the risk of AOM, recurrent AOM, antibiotic use, or lower respiratory tract infections within 1 year. |
| Hojsak I et al., 2010 [44] | I:139 C:142 | 56 | 4.3 | Probiotic. Fermented milk. L. rhamnosus GG 1 × 109 CFU in 100 mL. Oral route. | 3 | The probiotic used in the study is recommended as a valid measure to reduce the risk of upper respiratory tract infections in children attending daycare. |
| Taipale T et al., 2010 [45] | I:34 C:35 | NS | NS | Probiotic. Tablets. Bifidobacterium animalis subsp. lactis BB-12. 5 × 109 CFU. Administered orally via a pacifier. | > 6 | The administration of Bifidobacterium animalis subsp. lactis BB-12 in childhood may reduce respiratory infections. However, it was not associated with a lower frequency of AOM. |
| Stecksén-Blicks C et al., 2009 [46] | I:133 C:115 | NS | 3.5 | Probiotic. Milk. L. rhamnosus LB21. 1 × 107 CFU in 150 mL of milk. Oral route. | 21 | Probiotic supplementation did not significantly reduce the days of AOM. |
| Rautava S et al., 2008 [47] | I:32 C:40 | 49 | <1 year | Probiotic. Infant formula. L. rhamnosus GG and B. lactis Bb-12. 1 × 1010 CFU. Oral route. | 10 | The results suggest that the probiotics L. rhamnosus GG and B. lactis Bb-12 are a safe alternative to reduce the risk of early acute otitis media and antibiotic use. In addition, they may decrease the risk of recurrent respiratory infections during the first year of life. |
| Hatakka K et al., 2007 [48] | I:155 C:154 | 58 | 2.4 | Probiotic. Capsule. L. rhamnosus GG ATCC 53103, L. rhamnosus LC 705, B. breve 99; P. freudenreichii ssp. shermanii JS. 8–9 × 109 CFU. Oral route. | 6 | The probiotics did not prevent otitis or the presence of pathogens in the nasopharynx, although a trend toward a reduction in recurrent respiratory infections was observed, which should be confirmed in future studies. |
| Arslanoglu S et al., 2007 [49] | I:102 C:104 | NS | NS | Prebiotic. Infant formula. Galactooligosaccharides/fructooligosaccharides. 8 g/L. Oral route. | 6 | The administration of a prebiotic oligosaccharide mixture in early life appears to modulate immunity in the correct direction. However, it was not associated with a lower frequency of acute otitis media episodes. |
| Hatakka K et al., 2001 [50] | I:252 C:261 | 51 | 4.5 | Probiotic. Milk. L. rhamnosus GG. 5–10 × 105 in 200 mL of milk. Oral route. | 7 | Lactobacillus GG may reduce respiratory infections and their severity among children in daycare. The effects of the probiotic Lactobacillus GG were modest. No lower frequency of AOM was observed in the intervention group. |
| Author, Year | Number of Patients Treated with the Product | Number of Adverse Events (Intervention) | Number of Adverse Events (Control) | Reported Adverse Events | Conclusion on Product Safety |
|---|---|---|---|---|---|
| Corsello G et al., 2017 [36] | 66 | 0 | 0 | None | No adverse events related to the consumption of the active products or placebo were recorded. |
| Di Pierro F et al., 2016 [38] | 111 | 0 | 0 | None | No side effects associated with the treatment were evidenced. |
| Taipale T et al., 2016 [39] | 55 | 2 | 1 | Gastrointestinal discomfort | No serious adverse events related to the probiotic were reported during the follow-up period. |
| Hojsak I et al., 2016 [37] | 104 | 0 | 0 | None | No adverse effects were found in the studied population. |
| Nocerino R et al., 2015 [41] | 264 | 0 | 0 | None | No adverse effects associated with the use of the active products or placebo were recorded throughout the study. |
| Marchisio P et al., 2015 [40] | 50 | 35 | 13 | Sneezing, itching, cough, nasal congestion, epistaxis, and rhinorrhea | The percentage of children who experienced at least one adverse event was 42% in the intervention group versus 15% in the control group. No systemic or severe adverse events were reported. |
| Cohen R et al., 2013 [43] | 112 | 4 | 1 | Lack of appetite for milk, regurgitation, dry skin, diarrhea, constipation, and abdominal pain | Most adverse events reported in the participants were not attributed to the treatment. No severe adverse events were reported. |
| Taipale T et al., 2010 [45] | 55 | 2 | 1 | Gastrointestinal discomfort and dermatological reactions | No serious adverse effects were identified during the probiotic supplementation period. |
| Stecksén-Blicks C et al., 2009 [46] | 133 | 0 | 0 | None | None of the participants in the study groups reported any intervention-related side effects. |
| Rautava S et al., 2008 [47] | 38 | 1 | 3 | Vomiting and flatulence | No serious adverse events related to probiotic supplementation were reported during the study. |
| Hatakka K et al., 2001 [50] | 282 | 0 | 0 | None | No adverse effects associated with the administration of the probiotic product were found in the participants. |
| Author | The Study Is Randomized | The Intervention Is Double-Blind | Study Withdrawals Are Accounted for and Described | The Randomization Procedure Is Adequately Performed | Selection Criteria | Score |
|---|---|---|---|---|---|---|
| Sarlin S et al., 2023 [34] | 1 | 1 | 0 | 1 | 1 | 4 |
| Marchisio P et al., 2015 [40] | 1 | 1 | 1 | 1 | 1 | 5 |
| Cohen R et al., 2013 [43] | 1 | 1 | 1 | 1 | 1 | 5 |
| Hatakka K et al., 2007 [48] | 1 | 1 | 0 | 1 | 1 | 4 |
| Rautava S et al., 2008 [47] | 1 | 1 | 0 | 1 | 1 | 4 |
| Taipale T et al., 2010 [45] | 1 | 1 | 0 | 1 | 1 | 4 |
| Arslanoglu S et al., 2007 [49] | 1 | 1 | 0 | 1 | 1 | 4 |
| Garaiova I et al., 2023 [35] | 1 | 1 | 1 | 1 | 1 | 5 |
| Di Pierro F et al., 2016 [38] | 1 | 0 | 1 | 0 | 1 | 3 |
| Hatakka K et al. 2001 [50] | 1 | 1 | 1 | 1 | 1 | 5 |
| Stecksén-Blicks C et al., 2009 [46] | 1 | 1 | 1 | 0 | 1 | 4 |
| Corsello G et al., 2017 [36] | 1 | 1 | 1 | 1 | 1 | 5 |
| Hojsak I et al., 2009 [44] | 1 | 1 | 0 | 1 | 1 | 4 |
| Hojsak I et al., 2016 [37] | 1 | 1 | 0 | 1 | 1 | 4 |
| Karpova L et al., 2015 [42] | 1 | 0 | 1 | 0 | 1 | 3 |
| Nocerino R et al., 2015 [41] | 1 | 1 | 1 | 1 | 1 | 5 |
| Taipale T et al., 2016 [39] | 1 | 1 | 0 | 1 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosquera, F.E.C.; de la Rosa Caldas, M.; Naranjo Rojas, A.; Perlaza, C.L.; Liscano, Y. Probiotic, Prebiotic, and Synbiotic Supplementation for the Prevention and Treatment of Acute Otitis Media: A Systematic Review and Meta-Analysis. Children 2025, 12, 591. https://doi.org/10.3390/children12050591
Mosquera FEC, de la Rosa Caldas M, Naranjo Rojas A, Perlaza CL, Liscano Y. Probiotic, Prebiotic, and Synbiotic Supplementation for the Prevention and Treatment of Acute Otitis Media: A Systematic Review and Meta-Analysis. Children. 2025; 12(5):591. https://doi.org/10.3390/children12050591
Chicago/Turabian StyleMosquera, Freiser Eceomo Cruz, Mayerli de la Rosa Caldas, Anisbed Naranjo Rojas, Claudia Lorena Perlaza, and Yamil Liscano. 2025. "Probiotic, Prebiotic, and Synbiotic Supplementation for the Prevention and Treatment of Acute Otitis Media: A Systematic Review and Meta-Analysis" Children 12, no. 5: 591. https://doi.org/10.3390/children12050591
APA StyleMosquera, F. E. C., de la Rosa Caldas, M., Naranjo Rojas, A., Perlaza, C. L., & Liscano, Y. (2025). Probiotic, Prebiotic, and Synbiotic Supplementation for the Prevention and Treatment of Acute Otitis Media: A Systematic Review and Meta-Analysis. Children, 12(5), 591. https://doi.org/10.3390/children12050591






