Interleukin-2 Receptor as a Marker of Oxidative Stress in Paediatric Patients with Chronic Kidney Disease or Hypertension
Abstract
1. Introduction
2. Materials and Methods
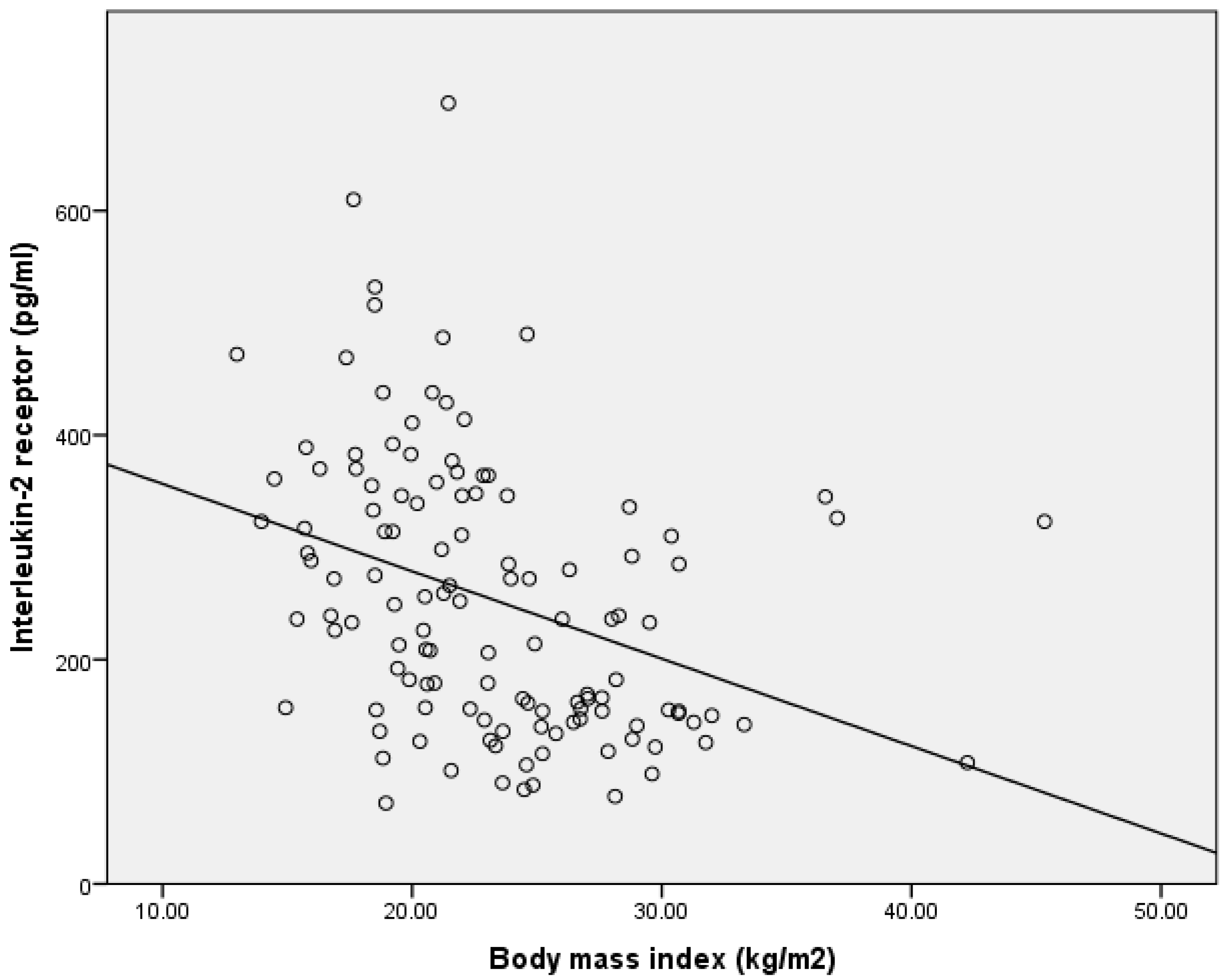
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, W.; Szyguła-Jurkiewicz, B. Oxidative stress and inflammatory markers—The future of heart failure diagnostics? Kardiochir. Torakochirurgia Pol. 2015, 12, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Fayad, Z.A.; Amirbekian, V.; Toussaint, J.F.; Fuster, V. Identification of interleukin-2 for imaging atherosclerotic inflammation. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 111–116. [Google Scholar] [CrossRef]
- Simon, A.D.; Yazdani, S.; Wang, W.; Schwartz, A.; Rabbani, L.E. Elevated plasma levels of interleukin-2 and soluble IL-2 receptor in ischemic heart disease. Clin. Cardiol. 2001, 24, 253–256. [Google Scholar] [CrossRef]
- Hong, Y.M. Atherosclerotic cardiovascular disease beginning in childhood. Korean Circ. J. 2010, 40, 1–9. [Google Scholar] [CrossRef]
- Kotur-Stevuljević, J.; Peco-Antić, A.; Spasić, S.; Stefanović, A.; Paripović, D.; Kostić, M.; Vasić, D.; Vujović, A.; Jelić-Ivanović, Z.; Spasojević-Kalimanovska, V.; et al. Hyperlipidemia, oxidative stress, and intima media thickness in children with chronic kidney disease. Pediatr. Nephrol. 2013, 28, 295–303. [Google Scholar] [CrossRef]
- Ece, A.; Gürkan, F.; Kervancioğlu, M.; Kocamaz, H.; Güneş, A.; Atamer, Y.; Selek, S. Oxidative stress, inflammation and early cardiovascular damage in children with chronic renal failure. Pediatr. Nephrol. 2006, 21, 545–552. [Google Scholar] [CrossRef]
- Drożdż, D.; Kwinta, P.; Sztefko, K.; Kordon, Z.; Drożdż, T.; Łątka, M.; Miklaszewska, M.; Zachwieja, K.; Rudziński, A.; Pietrzyk, J.A. Oxidative stress biomarkers and left ventricular hypertrophy in children with chronic kidney disease. Oxid. Med. Cell Longev. 2016, 2016, 7520231. [Google Scholar] [CrossRef]
- Bujanowicz, A.; Skrzypczyk, P. Immunological mechanisms of arterial damage in pediatric patients with primary hypertension. Cent. Eur. J. Immunol. 2023, 48, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Regalbuto, C.; Porri, D.; Pelizzo, G.; Mazzon, E.; Vinci, F.; Zuccotti, G.; Fabiano, V.; Cena, H. Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules 2020, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Correia-Costa, L.; Sousa, T.; Morato, M.; Cosme, D.; Afonso, J.; Areias, J.C.; Schaefer, F.; Guerra, A.; Afonso, A.C.; Azevedo, A.; et al. Oxidative stress and nitric oxide are increased in obese children and correlate with cardiometabolic risk and renal function. Br. J. Nutr. 2016, 116, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Hertiš Petek, T.; Homšak, E.; Svetej, M.; Marčun Varda, N. Metabolic Syndrome, Inflammation, Oxidative Stress, and Vitamin D Levels in Children and Adolescents with Obesity. Int. J. Mol. Sci. 2024, 25, 10599. [Google Scholar] [CrossRef]
- Codazzi, V.; Frontino, G.; Galimberti, L.; Giustina, A.; Petrelli, A. Mechanisms and risk factors of metabolic syndrome in children and adolescents. Endocrine 2024, 84, 16–28. [Google Scholar] [CrossRef]
- Močnik, M.; Golob Jančič, S.; Marčun Varda, N. Liver and kidney ultrasound elastography in children and young adults with hypertension or chronic kidney disease. Pediatr. Nephrol. 2023, 38, 3379–3387. [Google Scholar] [CrossRef]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef]
- Akern. In Bodygram Plus Software Guide; Akern s.r.l.: Florence, Italy, 2016.
- Marčun Varda, N.; Golob Jančič, S.; Močnik, M. Obesity and body composition in relation to liver and kidney ultrasound elastography in paediatric patients with either hypertension or chronic kidney disease. Children 2023, 11, 18. [Google Scholar] [CrossRef]
- Ding, R.; Gao, W.; Ostrodci, D.H.; He, Z.; Song, Y.; Ma, L.; Liang, C.; Wu, Z. Effect of interleukin-2 level and genetic variants on coronary artery disease. Inflammation 2013, 36, 1225–1231. [Google Scholar] [CrossRef]
- Elkind, M.S.; Rundek, T.; Sciacca, R.R.; Ramas, R.; Chen, H.J.; Boden-Albala, B.; Rabbani, L.; Sacco, R.L. Interleukin-2 levels are associated with carotid artery intima-media thickness. Atherosclerosis 2005, 180, 181–187. [Google Scholar] [CrossRef]
- Malek, T.R.; Castro, I. Interleukin-2 receptor signaling: At the interface between tolerance and immunity. Immunity 2010, 33, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Rickert, M.; Garcia, K.C. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science 2005, 310, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Durda, P.; Sabourin, J.; Lange, E.M.; Nalls, M.A.; Mychaleckyj, J.C.; Jenny, N.S.; Li, J.; Walston, J.; Harris, T.B.; Psaty, B.M.; et al. Plasma levels of soluble interleukin-2 receptor α: Associations with clinical cardiovascular events and genome-wide association scan. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2246–2253. [Google Scholar] [CrossRef]
- Teraura, H.; Kotani, K.; Minami, T.; Takeshima, T.; Shimooki, O.; Kajii, E. The serum concentration of soluble interleukin-2 receptor in patients with Kawasaki disease. Ann. Clin. Biochem. 2017, 54, 209–213. [Google Scholar] [CrossRef]
- Bien, E.; Balcerska, A. Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: A review. Biomarkers 2008, 13, 1–26. [Google Scholar] [CrossRef]
- Al-Rawi, K.F.; Ali, H.H.; Guma, M.A.; Mohammed Aldahham, B.J.; Tuleab Alaaraji, S.F.; Al-Ani, O.; Tariq Ali, A. Relationship between IL-2, IL-17 concentrations, and serum creatinine levels in men with chronic kidney diseases. Rep. Biochem. Mol. Biol. 2022, 10, 664–674. [Google Scholar] [CrossRef]
- Silverstein, D.M. Inflammation in chronic kidney disease: Role in the progression of renal and cardiovascular disease. Pediatr. Nephrol. 2009, 24, 1445–1452. [Google Scholar] [CrossRef]
- Zwolińska, D.; Medyńska, A.; Szprynger, K.; Szczepańska, M. Serum concentration of IL-2, IL-6, TNF-alpha and their soluble receptors in children on maintenance hemodialysis. Nephron 2000, 86, 441–446. [Google Scholar] [CrossRef]
- Nairn, J.; Hodge, G.; Henning, P. Intracellular cytokines in peripheral blood leucocytes in children with chronic renal failure. Pediatr. Nephrol. 2006, 21, 251–256. [Google Scholar] [CrossRef]
- Eder, K.; Baffy, N.; Falus, A.; Fulop, A.K. The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res. 2009, 58, 727–736. [Google Scholar] [CrossRef]
- Fain, J.N. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam. Horm. 2006, 74, 443–477. [Google Scholar] [CrossRef] [PubMed]
- Jonas, M.I.; Kurylowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Wierzbicki, Z.; Chmura, A.; Pruszczyk, P.; Puzianowska-Kuznicka, M. Interleukins 6 and 15 levels are higher in subcutaneous adipose tissue, but obesity is associated with their increased content in visceral fat depots. Int. J. Mol. Sci. 2015, 16, 25817–25830. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, K.; Jousilahti, P.; Havulinna, A.S.; Borodulin, K.; Männistö, S.; Salomaa, V. Role of inflammation markers in the prediction of weight gain and development of obesity in adults—A prospective study. Metabol. Open 2019, 3, 100016. [Google Scholar] [CrossRef] [PubMed]
- Kochumon, S.; Al Madhoun, A.; Al-Rashed, F.; Thomas, R.; Sindhu, S.; Al-Ozairi, E.; Al-Mulla, F.; Ahmad, R. Elevated adipose tissue associated IL-2 expression in obesity correlates with metabolic inflammation and insulin resistance. Sci. Rep. 2020, 10, 16364. [Google Scholar] [CrossRef]
- van der Zalm, I.J.B.; van der Valk, E.S.; Wester, V.L.; Nagtzaam, N.M.A.; van Rossum, E.F.C.; Leenen, P.J.M.; Dik, W.A. Obesity-associated T-cell and macrophage activation improve partly after a lifestyle intervention. Int. J. Obes. 2020, 44, 1838–1850. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef]
- Vargas, R.; Ryder, E.; Diez-Ewald, M.; Mosquera, J.; Durán, A.; Valero, N.; Pedreañez, A.; Peña, C.; Fernández, E. Increased C-reactive protein and decreased Interleukin-2 content in serum from obese individuals with or without insulin resistance: Associations with leukocyte count and insulin and adiponectin content. Diabetes Metab. Syndr. 2016, 10, S34–S41. [Google Scholar] [CrossRef]
- Meijer, K.; de Vries, M.; Al-Lahham, S.; Bruinenberg, M.; Weening, D.; Dijkstra, M.; Kloosterhuis, N.; van der Leij, R.J.; van der Want, H.; Kroesen, B.J.; et al. Human primary adipocytes exhibit immune cell function: Adipocytes prime inflammation independent of macrophages. PLoS ONE 2011, 6, e17154. [Google Scholar] [CrossRef]
- Aygun, A.D.; Gungor, S.; Ustundag, B.; Gurgoze, M.K.; Sen, Y. Proinflammatory cytokines and leptin are increased in serum of prepubertal obese children. Mediators Inflamm. 2005, 2005, 180–183. [Google Scholar] [CrossRef]
- Dogan, Y.; Akarsu, S.; Ustundag, B.; Yilmaz, E.; Gurgoze, M.K. Serum IL-1beta, IL-2, and IL-6 in insulin-dependent diabetic children. Mediators Inflamm. 2006, 2006, 59206. [Google Scholar] [CrossRef]
- Utsal, L.; Tillmann, V.; Zilmer, M.; Mäestu, J.; Purge, P.; Jürimäe, J.; Saar, M.; Lätt, E.; Maasalu, K.; Jürimäe, T. Elevated serum IL-6, IL-8, MCP-1, CRP, and IFN-γ levels in 10- to 11-year-old boys with increased BMI. Horm. Res. Paediatr. 2012, 78, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.S.; Heydari, S.; Oliver, S.R.; Flores, R.L.; Pontello, A.M.; Ibardolaza, M.; Galassetti, P.R. Inflammatory cytokine profiles during exercise in obese, diabetic, and healthy children. J. Clin. Res. Pediatr. Endocrinol. 2011, 3, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Mihuta, M.S.; Paul, C.; Borlea, A.; Roi, C.M.; Velea-Barta, O.A.; Mozos, I.; Stoian, D. Unveiling the Silent Danger of Childhood Obesity: Non-Invasive Biomarkers Such as Carotid Intima-Media Thickness, Arterial Stiffness Surrogate Markers, and Blood Pressure Are Useful in Detecting Early Vascular Alterations in Obese Children. Biomedicines 2023, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Savant, J.D.; Furth, S.L.; Meyers, K.E. Arterial stiffness in children: Pediatric measurement and considerations. Pulse 2014, 2, 69–80. [Google Scholar] [CrossRef]
| Variable | CKD Group (N = 46) MW | HTN Group (N = 50) MW | Control Group (N = 33) | KW |
|---|---|---|---|---|
| IL-2Rα (pg/mL) | 232 (118) p = 0.160 | 147 (35) p < 0.001 | 295 (172) | p< 0.001 |
| Overweight/Obesity (N = 51) | Normal Weight (N = 78) | Comparison (MW) | ||
| IL-2Rα (pg/mL) | 162 (119) | 286.5 (186) | p< 0.001 | |
| Anthropometric Measurements, Body Composition, Blood Pressure, Elastography Measurements | Laboratory Measurements | ||
|---|---|---|---|
| Variable | IL-2Rα | Variable | IL-2Rα |
| Age | r = −0.188 p = 0.038 | AST | r = 0.047 p = 0.606 |
| Height | r = −0.256 p = 0.004 | ALT | r = −0.178 p = 0.049 |
| Weight | r = −0.447 p < 0.001 | GGT | r = −0.265 p = 0.003 |
| BMI | r = −0.443 p < 0.001 | Urea | r = 0.182 p = 0.044 |
| Waist circumference | r = −0.477 p < 0.001 | Creatinine | r = 0.045 p = 0.623 |
| Hip circumference | r = −0.441 p < 0.001 | Cystatin C | r = 0.288 p = 0.002 |
| FFM | r = −0.335 p < 0.001 | Urate | r = −0.199 p = 0.039 |
| TBW | r = −0.316 p = 0.001 | Total cholesterol | r = −0.013 p = 0.889 |
| ECW | r = −0.284 p = 0.003 | LDL | r = −0.060 p = 0.515 |
| BCM | r = −0.350 p < 0.001 | HDL | r = 0.203 p = 0.027 |
| FM | r = −0.484 p < 0.001 | Triglycerides | r = −0.220 p = 0.016 |
| PA | r = −0.307 p = 0.001 | Vitamin D | r = 0.116 p = 0.210 |
| Systolic pressure | r = −0.442 p < 0.001 | Homocysteine | r = −0.086 p = 0.367 |
| Diastolic pressure | r = −0.142 p = 0.117 | Urinary albumin/creatinine | r = 0.318 p = 0.001 |
| Pulse wave velocity | r = −0.047 p = 0.661 | ||
| Liver elastography | r = −0.184 p = 0.041 | ||
| Left kidney elastography | r = −0.355 p < 0.001 | ||
| Right kidney elastography | r = −0.292 p = 0.001 | ||
| Dependent Variable | Beta Coefficient | Significance |
|---|---|---|
| Systolic pressure | −2.748 | 0.011 |
| Diastolic pressure | +0.050 | 0.973 |
| Creatinine | +0.468 | 0.488 |
| Cystatin C | +204.676 | 0.021 |
| Urinary albumin/creatinine | +0.077 | 0.894 |
| Body mass index | −4.946 | 0.040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marčun Varda, N.; Močnik, M.; Filipič, M.; Homšak, E.; Svetej, M.; Golob Jančič, S. Interleukin-2 Receptor as a Marker of Oxidative Stress in Paediatric Patients with Chronic Kidney Disease or Hypertension. Children 2025, 12, 569. https://doi.org/10.3390/children12050569
Marčun Varda N, Močnik M, Filipič M, Homšak E, Svetej M, Golob Jančič S. Interleukin-2 Receptor as a Marker of Oxidative Stress in Paediatric Patients with Chronic Kidney Disease or Hypertension. Children. 2025; 12(5):569. https://doi.org/10.3390/children12050569
Chicago/Turabian StyleMarčun Varda, Nataša, Mirjam Močnik, Martina Filipič, Evgenija Homšak, Mateja Svetej, and Sonja Golob Jančič. 2025. "Interleukin-2 Receptor as a Marker of Oxidative Stress in Paediatric Patients with Chronic Kidney Disease or Hypertension" Children 12, no. 5: 569. https://doi.org/10.3390/children12050569
APA StyleMarčun Varda, N., Močnik, M., Filipič, M., Homšak, E., Svetej, M., & Golob Jančič, S. (2025). Interleukin-2 Receptor as a Marker of Oxidative Stress in Paediatric Patients with Chronic Kidney Disease or Hypertension. Children, 12(5), 569. https://doi.org/10.3390/children12050569






