Abstract
Background/Objectives: Efficient postoperative recovery room care in pediatric patients is crucial for optimizing perioperative safety, patient outcome, and effective pain management. However, this area is frequently underemphasized, leading to higher complication rates compared to the operating room, which in turn increases healthcare costs. Improving pediatric recovery room care offers a significant opportunity to enhance the quality and safety of perioperative pediatric care. From an economic perspective, this is prudent; however, more importantly, every child has the right to the highest attainable standard of health, as outlined by the United Nations. Key aspects of recovery room care include ensuring adequate staffing and equipment, while also prioritizing the child’s privacy and parental presence, both of which are crucial for enhancing patient well-being. A +multimodal approach to postoperative pain management is essential for minimizing fear and stress, alongside strict adherence to established guidelines for the management of postoperative nausea, vomiting, and emergence delirium. Furthermore, addressing risk factors such as hypothermia and airway complications, as well as promoting early intake of clear fluids, plays a crucial role in optimizing pediatric recovery. Organizational strategies such as quality improvement initiatives, structured handovers, standardized care protocols with checklists, continuous staff training, and well-defined discharge criteria are further essential components to reduce translational gaps and to enhance postoperative pediatric safety. Conclusions: Improving pediatric postoperative anesthetic care is a multifaceted challenge for all healthcare providers that can significantly enhance care quality and safety while also reducing costs. Success in this area requires addressing both structural and medical factors.
1. Introduction
Postoperative anesthetic care for pediatric patients requires a highly specialized approach, tailored to their distinct physiological and psychological characteristics. Despite its critical importance, immediate postoperative care for children frequently suffers from insufficient attention by healthcare providers, with poorly defined treatment protocols and responsibilities often compounding the issue. Alarmingly, research by Murat et al. has highlighted a significantly higher complication rate in pediatric patients in recovery rooms (4.3%) compared to operating rooms (3.1%) [1]. As in adults, it must, therefore, be assumed that postoperative complications are common and result in increased healthcare costs [2,3], although the full scope of this challenge is often under-recognized [4].
The principles enshrined in the United Nations Convention on the Rights of the Child affirm that every child has the right to the highest attainable standard of health. This includes access to comprehensive pain management strategies aimed at alleviating pain, fear, and unnecessary stress during recovery. Furthermore, pediatric patients have the right to the presence of their parents during postoperative recovery and the assurance of privacy throughout their care [5].
Given the complexity and multifaceted nature of postoperative anesthetic care for children, there is a substantial opportunity to enhance both the safety and quality of pediatric care while simultaneously achieving better economic outcomes. Achieving these goals requires a wide range of structural, clinical, and psychosocial factors. This article aims to provide an in-depth exploration of the critical considerations necessary for optimizing safety and outcomes in pediatric patients within recovery rooms, offering a framework to advance the standard of care in this vital area of medicine, particularly for non-anesthesia medical providers.
2. Materials and Methods
The objective of this literature review was to summarize current treatment strategies for children in the recovery room, identify gaps in existing knowledge, and explore opportunities to improve both the safety and quality of pediatric post-anesthesia care.
We followed the IMRAD structure and conducted a systematic search of PubMed, Embase, Scopus, Web of Science, CENTRAL, and EudraCT/CTIS for published studies, and PROSPERO for completed systematic reviews, up to 17 April 2025.
Search terms included recovery room, postoperative complications, pain, anxiety, emergence delirium, and postoperative nausea and vomiting.
Inclusion criteria comprised studies published in English or German, focusing on pediatric populations, and including clinical trials, preclinical studies, observational studies, or meta-analyses. Preference was given to recent double-blind or single-center studies from Europe or North America with a low risk of bias. Additional references were identified using snowballing techniques.
Studies that were not published in full or were available only in other languages were excluded.
Both authors independently conducted the search and assessed the relevance of included studies. Pediatric and selected adult anesthesia studies were included following discussion. The extracted data were categorized thematically, as outlined in the subheadings of the Section 3, and are discussed therein.
3. Results
3.1. Post-Anesthetic Recovery Room
Providing postoperative care for pediatric patients requires a dedicated recovery unit equipped to address the unique challenges of this population [6]. By the critical incident technique, it could be shown that evidence-based care and safety are compromised when technology has limitations and is not adapted for pediatric use [7]. The specific resources and capabilities of the recovery unit should be adapted to the severity of the treated conditions and include the following fundamental components:
3.1.1. Essential Infrastructure and Equipment
- Reliable access to oxygen, air, and vacuum systems.
- Tools and expertise for emergency airway management.
- Emergency medications and appropriately scaled dosing tools for pediatric use.
- Immediate access to diagnostic tools, including ultrasound and, where indicated, X-ray facilities.
- Facilities for blood gas analysis and access to clinical laboratories, with a blood bank available depending on the surgical procedures performed.
3.1.2. Trained Personnel and Operational Standards
- Staff thoroughly trained in pediatric care, trained in recognizing the age-specific needs of children [8], and adhering to established standards while utilizing structured care protocols and checklists.
- A clear communication hierarchy with accessible contact information for all relevant personnel [9].
- Implementation strategies for tailoring existing evidence to the target population and effectively applying it in the local clinical setting [10,11,12].
3.1.3. Comprehensive Patient Monitoring
- Pulse oximetry: Mandatory for all patients, with both pre- and post-ductal measurements recommended for infants younger than four weeks.
- Carbon dioxide monitoring: This should be performed via nasal cannula or transcutaneously when an impaired neurological status is present, in order to accurately detect hypoventilation or apnea before desaturation occurs [13].
- Electrocardiographic (ECG) monitoring: Performed based on clinical risk factors. For heart rates exceeding 200 beats per minute, a 12-lead ECG is essential to ensure accurate rhythm differentiation.
- Blood pressure: Measured on admission, before transfer, and at regular intervals based on the patient’s clinical status.
- Temperature: Monitored at a minimum upon admission and before transfer.
- Blood glucose: Regularly assessed in patients at risk for hypo- or hyperglycemia to maintain euglycemia [14] (Table 1).

Table 1.
Risk factors for postoperative hypoglycemia and hyperglycemia in which meticulous monitoring in the recovery room is necessary [15,16,17,18,19].
Table 1.
Risk factors for postoperative hypoglycemia and hyperglycemia in which meticulous monitoring in the recovery room is necessary [15,16,17,18,19].
| Postoperative Risk for | Risk Factors |
|---|---|
| Hypoglycemia in need of therapy for blood glucose values <60 mg/dL (<70 mg/dL in type 1 diabetes) |
○ Age < 12 months/weight for age <5th percentile developmental delay/failure to thrive ○ ASA status ≥ III ○ Long fasting times ○ Abdominal surgery ○ Metabolic disease ○ Low glycogen reserve (parenteral nutrition, liver disease, beta blockers, diabetic mother) |
| Hyperglycemia/ketoacidosis in need of therapy for blood glucose values > 220–250 mg/dL | ○ Type I diabetes |
Primary Objectives of Pediatric Recovery Care
The principal goals in the pediatric recovery room include the following:
- Stabilizing vital parameters.
- Providing effective pain management.
- Timely detection and treatment of complications after anesthesia.
- Minimizing emotional distress.
- Reducing and treating nausea and vomiting.
- Achieving an adequate level of consciousness.
- Ensuring that the level of required monitoring and support is compatible with the capabilities of the ward where further care will be provided.
Additional Influencing Factors
Preoperative anxiety in young children undergoing surgery has been associated with increased postoperative pain, a higher incidence of anxiety, emergence delirium, sleep disturbances, and other problems [22]. A randomized controlled trial by Kain et al. in 2007, involving 408 children, demonstrated that the overall recovery experience can be improved through preoperative interventions, including comprehensive surgical preparation, child-friendly clinical practices, anxiety reduction strategies, parental presence during anesthetic induction, and the administration of premedication (e.g., dexmedetomidine) [23].
3.2. Airway and Respiration
Pediatric patients are uniquely vulnerable to respiratory complications in the postoperative period due to a combination of metabolic and anatomical factors [1]. Infants have a metabolic oxygen demand that is approximately twice that of adults. When faced with sustained hypoxia, they are at an increased risk of hypoxic respiratory depression. Anatomical characteristics, such as a softer and longer epiglottis, a compliant thoracic cage limiting the effective use of accessory respiratory muscles, immature protective reflexes, and a smaller functional residual capacity contribute to rapid desaturation when muscle tone is reduced.
The risk of apnea and bradycardia following surgical procedures is particularly pronounced in neonates born before 36 weeks of gestational age and remains elevated until a post-conceptual age of 56–60 weeks [24]. Monitoring respiratory effort and airway function in these high-risk patients should include clinical parameters such as breathing patterns, respiratory rate, and breath sounds, as well as technical measures like continuous pulse oximetry and electrocardiography, ideally including overnight monitoring.
Prophylactic and therapeutic administration of caffeine at doses of 5–10 mg/kg body weight has been shown to reduce the frequency of apnea in a prospective double-blind randomized trial in former premature infants [25]. Caffeine exerts its effects by stimulating the respiratory and cardiovascular systems, increasing responsiveness to arterial carbon dioxide tension, and enhancing diaphragmatic contractility. However, its use is associated with increased oxygen consumption and metabolic rate [26,27].
In early childhood, the enlargement of tonsils and adenoids may predispose children to airway obstruction and obstructive sleep apnea syndrome (OSAS). In non-airway surgery, this necessitates a multimodal approach to pain management, which may include perioperative regional anesthesia and cautious opioid titration, as pediatric patients with OSAS exhibit an increased respiratory sensitivity to opioids [28]. Notably, a recent systematic review found no additional benefit of local or regional anesthesia for pain management in tonsillotomy procedures [29].
A large prospective study on perioperative morbidity in children by Murat et al. in 2004 suggested that children undergoing airway surgeries, such as adenotonsillectomy or tonsillotomy, require vigilant postoperative monitoring, particularly if opioid analgesia is employed [1].
According to a review of high-risk ambulatory pediatric patients from 2023, scheduling surgeries for pediatric OSAS patients early in the day is advantageous, allowing for consistent and extended monitoring in the postoperative recovery unit [30].
The use of a laryngeal mask airway (LMA) has been shown to support airway patency during recovery. As early as 1997, Parry et al. recommend leaving the LMA in place until it is spontaneously expelled by the child, which reduces the need for additional airway maneuvers [31]. This practice has been successfully implemented in numerous hospitals. Spontaneously breathing children should initially be positioned in the lateral decubitus position until fully conscious, as it appears to be associated with less airway obstruction and fewer respiratory adverse events at the time of airway device removal in children [32]. Once stabilized, younger children should transition to the supine position, provided this positioning does not conflict with surgical or anesthetic considerations.
To predict the likelihood of postoperative respiratory complications in infants and children, a validated score, such as the Score for Prediction of Postoperative Respiratory Complications in Pediatric Patients (SPORC) [33], can be used. The eleven predictors, age < 2 yr, ASA physical status > 3, obesity or underweight, thoracic surgery, high dose of neuromuscular blocking agent, expected case duration ≥ 2 h, emergency surgery, upper airway infection within 7 days before surgery, congenital anomalies of nervous or cardiovascular system, congenital anomalies of the respiratory system, and being born as a preterm or small for gestational age infant have been shown to be predictive of an increased incidence of complications such as respiratory infection, aspiration pneumonitis, bronchospasm, laryngospasm, or oxygen desaturation.
Monitoring strategies and the duration of recovery room observation should be tailored accordingly.
Postoperative Residual Neuromuscular Blockade
Postoperative residual neuromuscular blockade remains a significant concern in the recovery room. A recent prospective audit of pediatric patients in 2015 reported an incidence of 28.1%, and that of a severe residual blockade, defined as a TOF ratio < 0.7, of 6.5% [34]. This condition warrants careful postoperative attention, as it may further decrease functional residual capacity and compromise protective pharyngeal and respiratory reflexes, which are essential for maintaining airway patency and respiratory drive [35].
Note: After reversal of steroidal muscle relaxants with sugammadex, children under the age of 2 years are predominantly at risk of experiencing postoperative residual or recurrent weakness [36].
3.3. Hypothermia
Hypothermia in the recovery room poses significant challenges to outcomes in pediatric populations. Heier et al. showed in 2006 that a reduction of 2 °C in body temperature can substantially extend the effects of muscle relaxants by doubling the duration of neuromuscular blockade [37]. Moreover, a decrease of 1 °C elevates postoperative oxygen consumption fourfold, exacerbates acidosis, and prolongs the effects of various drugs due to diminished kidney function and metabolism, which decline by approximately 7% for each 1 °C drop in temperature [38,39].
In term infants, hypothermia induces increases in heart rate, blood pressure, and catecholamine levels, whereas in preterm infants, it may result in bradycardia and reduced cardiac output [40].
A recent retrospective study of 1091 neonates and infants found that these physiological changes contributed to prolonged recovery room stays, increased admissions to intensive care units (ICUs), and extended overall hospitalization durations [41].
Notably, thermogenic shivering—a critical mechanism for generating heat—occurs only in children older than 6 years of age [42].
Note: Postoperative hypothermia can lead to the life-threatening triad of hypoxemia, metabolic acidosis, and hypoglycemia in neonates [43]. It is associated with delayed awakening, impaired protective reflexes, increased frequency of apneas, wound infections, coagulation disorders, elevated blood loss, intraventricular cerebral hemorrhage, arrhythmia, prolonged hospitalization, and poorer neurological outcomes [44].
3.4. Postoperative Nausea and/or Vomiting (PO(N)V)
PO(N)V is a common complication in pediatric patients, with an untreated incidence reaching up to 80% [45]. The ability to explicitly report nausea is typically possible only from around 4–5 years of age; thus, in younger children, the assessment predominantly focuses on postoperative vomiting (POV). Its occurrence can lead to dehydration, electrolyte disturbances, increased pain, and compromised surgical outcomes [46].
Preventive strategies for PO(N)V include avoiding volatile anesthetics and nitrous oxide, utilizing total intravenous anesthesia with propofol, minimizing opioid use through regional anesthesia techniques, and ensuring adequate intraoperative hydration. Current guidelines recommend dual prophylaxis for PO(N)V in children over three years old or those presenting with one or two risk factors [47] (Figure 1).
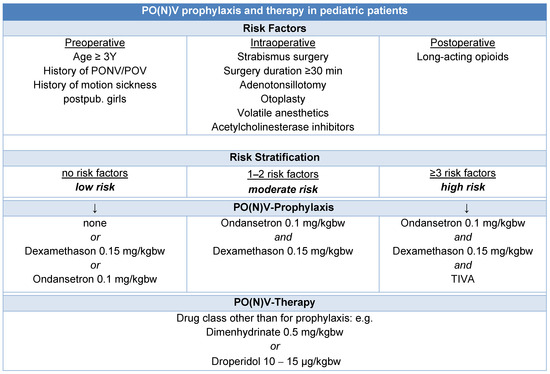
Figure 1.
Algorithm for PO(N)V prophylaxis and therapy in pediatric patients [47,48].
While the effectiveness of alternative therapies such as acupuncture, acupressure, and transcutaneous nerve stimulation for managing PO(N)V in pediatric patients remains inadequately supported by evidence [49,50], a controlled randomized trial has shown that early postoperative intake of clear fluids [51] or ice popsicles can reduce the incidence of PO(N)V and improve overall well-being [52].
3.5. Emergence Delirium (ED)
With an incidence of up to 80%, ED poses a significant challenge in the recovery room, particularly in preschool-aged children and those undergoing head and neck surgeries—both non-modifiable risk factors [53]. ED is characterized by acute disturbances in consciousness and attention, distinct from postoperative agitation [54]. Accurate identification is crucial, as affected children may unintentionally engage in irrational behaviors that increase the risk of self-injury, necessitate additional nursing care, or delay hospital discharge [55].
The Pediatric Anesthesia Emergence Delirium (PAED) scale is a validated diagnostic tool for quantifying the severity of ED in children [56]. It consists of five items [57] and can be divided into a delirium-specific score (ED I) and a nonspecific score (ED II) [58,59] (Figure 2).
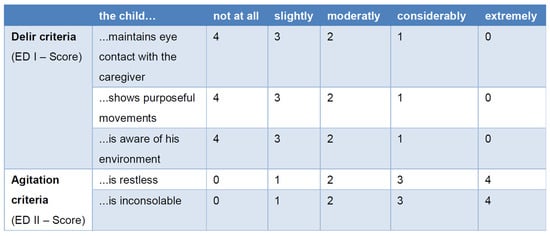
Figure 2.
Pediatric Anesthesia Emergence Delirium (PAED) scale [53,57,60,61].
Currently, a cutoff of ≥6 has been recommended to ensure that no children with ED are missed, despite the increased risk of false positive cases [61]. In contrast, an ED I score ≥ 9 has demonstrated a sensitivity of 0.93 and a specificity of 0.94 [60].
Effective strategies for reducing the incidence of ED include minimizing preoperative anxiety [62] through thorough family preparation and engaging children with digital media or music, both before [22,63] and during surgery [64].
Anesthesia management plays a pivotal role in prevention. Total intravenous anesthesia, particularly with a propofol bolus or a brief infusion of up to 3 mg/kg shortly before discharge to the recovery room, has shown efficacy in a meta-analysis of RCTs [65]. Additionally, a calm recovery environment, effective analgesia [66,67,68], and early intake of clear fluids contribute significantly to the child’s well-being and the reduction in ED episodes.
According to Davies et al., initial evidence suggests that intraoperative electroencephalographic (EEG) monitoring can enhance the prediction and prevention of ED. Anesthesiologists may reduce ED by timing patient emergence from anesthesia to coincide with appropriate EEG patterns indicative of natural sleep transitions [69].
Pharmacological interventions with propofol, 0.5–1 mg/kgbw, or, alternatively, ketamine S, 1 mg/kgbw iv, have demonstrated clinical success. Clonidine or dexmedetomidine can be administered in a dosage of 1–2 µg/kgbw for both prophylaxis and therapy [70].
A systematic review and meta-analysis of randomized controlled trials in 2022 found that administering magnesium sulfate also reduced the occurrence and severity of emergence agitation or delirium in children following general anesthesia [71].
3.6. Pain Assessment and Pain Management
Recent results from a study of registry data from 11 European hospitals continue to highlight substantial deficits in the concept, application, and dosing of analgesics in pediatric patients following surgery [72].
Effective pain management in the recovery room requires the timely administration of adequate analgesic doses, and the use of standardized pain assessment scales tailored to all age groups. For children under four years of age, external assessment tools are essential, as verbalization of discomfort is often ineffective in this age group. Reliable and validated tools such as the revised Face, Legs, Activity, Cry, and Consolability (r-FLACC) scale and the Children’s and Infants’ Postoperative Pain Scale (CHIPPS) are particularly suited for this purpose [73,74,75,76] (Figure 3).
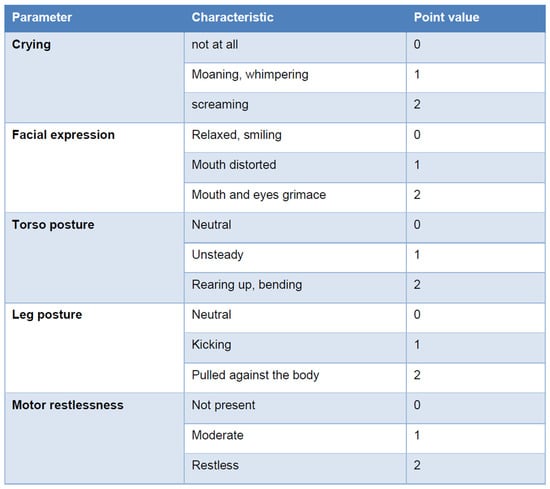
Figure 3.
Children’s and Infants’ Postoperative Pain Scale (CHIPPS). The score value is calculated by adding the individual parameter point values after observing the child for 15 s. The need for analgesic therapy starts at four points [73].
3.6.1. Prevention of Chronic Post-Surgical Pain (CPSP)
According to Furuta et al., CPSP presents a significant challenge in pediatric care, occurring in 6–30% of cases within 3 to 12 months postoperatively [77]. CPSP is associated with considerable pain-related disabilities and increased healthcare costs. Preventative strategies emphasize comprehensive perioperative and postoperative pain management during the recovery phase, including techniques such as regional anesthesia.
Preoperative factors, including preexisting pain, anxiety, depressive symptoms, and sleep disturbances [77,78], have been considered potential risk factors for CPSP.
A recent systematic review and meta-analysis identified pre-surgical pain as the only preoperative risk factor that significantly predicted pediatric CPSP, emphasizing the critical need for effective pain management throughout the perioperative period, beginning before surgery [79].
3.6.2. Non-Opioid Analgesics (NOPAs)
Nonsteroidal anti-inflammatory drugs (NSAIDs) are a cornerstone of pediatric pain management and are commonly used in infants, children, and adolescents [80]. In many RCTs, they demonstrate superior efficacy compared to acetaminophen and, when combined with opioids, reduce required opioid dosages while enhancing analgesic efficacy and minimizing adverse effects such as vomiting and sedation [81,82] (Figure 4).
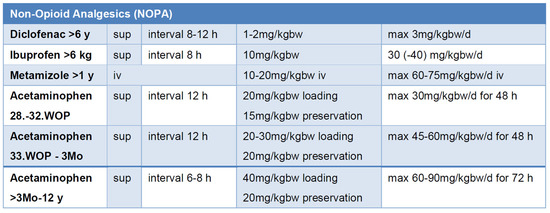
Figure 4.
Dosing of non-opioid analgesics (NOPAs) to achieve effective drug levels [82,83]; bw: body weight, iv: intravenous, sup: suppositorium, WOP: week of pregnancy.
As NSAIDs impair platelet and kidney function in children, they can lead to ductus arteriosus occlusion and increase the risk of necrotizing enterocolitis or intraventricular hemorrhage [84,85].
Acetaminophen is widely used, including in neonates, though caution is required due to potential hepatotoxicity. To maximize analgesic potential and ensure safe dosing, age, body weight, therapy duration, maximum daily dose, and dosing intervals must be considered [82,86,87] (Figure 4).
Metamizole, despite its controversial status, offers minimal respiratory depression and is effective in managing visceral pain [88,89].
Consistent documentation of medication timing and dosage is particularly critical during the transfer to a peripheral ward to prevent both overdosing and prolonged treatment intervals.
3.6.3. Opioids
Piritramide and morphine are effective postoperative analgesics, with piritramide preferred due to its stable hemodynamic profile and lower incidence of pruritus. Patient-controlled analgesia (PCA) by proxy remains a safe and efficient method of pain administration for pediatric patients, provided that limitations like developmental and neurological disabilities are respected [90].
Opioids in newborns and premature infants pose unique challenges as they act more strongly in the brainstem than in the midbrain, where the modulation of emotional pain perception and cortical pain transmission is mediated. Therefore, they lead to significant respiratory depression at levels that are insufficient for analgesia.
Note: Newborns and premature babies can exhibit pronounced respiratory depression at opioid levels that do not provide sufficient analgesia. Opioid titration to analgesia without respiratory depression is, therefore, not possible [91].
Nalbuphine, a functional µ-antagonist and κ-agonist, is suitable for the management of mild to moderate pain. It has a pronounced sedative effect without causing respiratory depression. At a dosage of 0.3–0.4 mg/kgbw iv, nalbuphine demonstrates a ceiling effect, preventing further analgesic enhancement [92].
For tonsillectomy, systematic reviews have identified the combination of acetaminophen, NSAIDs, and dexamethasone as the most effective approach, whereas regional or local anesthesia provides no additional benefit in this context [29,93].
3.6.4. Co-Analgesics
Dexamethasone, clonidine, and dexmedetomidine are well-established co-analgesics that reduce opioid requirements while effectively managing PO(N)V and pain [94].
Gabapentinoids such as gabapentin and pregabalin have shown promise in reducing pain, nausea, and emergence delirium in specific pediatric populations (e.g., after adenotonsillectomy or posterior spinal fusion). While side effects like sedation and respiratory depression are common in adults, they appear to be less frequent in children [95].
Non-pharmacological interventions can further alleviate pain and reduce reliance on analgesics, thereby minimizing their adverse effects. Techniques such as tablet use, mobile phones, pacifiers, and caregiver presence are beneficial across various pediatric age groups. In preterm neonates, interventions like non-nutritive sucking, facilitated tucking, and swaddling help reduce pain behaviors. In full-term neonates, non-nutritive sucking is particularly effective. However, evidence indicates that none of these interventions is promising in reducing pain in older infants [96].
For inpatient settings, the ESPA Pain Management Ladder Initiative offers guidance to encourage optimal pain management practice and to support institutions in developing individual pain management concepts according to their financial and human resources [97,98].
3.7. Early Postoperative Clear Fluid Intake
When clinically appropriate, permitting early oral intake after surgery has been shown to enhance patient satisfaction, reduce opioid consumption, and decrease the incidence of nausea and vomiting [51]. The administration of small portions of water ice, typically around 40 mL, is particularly effective and well tolerated, even in very young children, with virtually no contraindications.
For school-aged children and older, chewing gum can serve as a valuable adjunct by providing distraction, improving well-being, and alleviating nausea. However, a systematic review and meta-analysis demonstrated that gum chewing is not associated with earlier postoperative gastrointestinal recovery in children [99].
Note: A multimodal analgesia approach—integrating regional anesthesia, non-opioid analgesics, opioids, and co-analgesics—remains a cornerstone to effective postoperative anesthetic care [100].
3.8. Discharge Criteria from Postoperative Recovery Room Care
Readiness for discharge from postoperative care should be evaluated using standardized criteria and formally documented.
For inpatients, discharge readiness requires an adequate level of consciousness, sufficient pain control, stable vital signs, and vital functions within the capabilities of the receiving ward.
For patients being discharged from the monitoring unit without further medical supervision, the criteria include the child being awake, oriented, exhibiting normal motor function, and having intact protective reflexes as initially observed. Stable, unassisted breathing with a peripheral oxygen saturation (SpO2) above 95% without supplemental oxygen for at least one hour, the absence of stridor, stable vital signs, and controlled pain (numerical rating scale [NRS] < 4) are prerequisites. Further prerequisites include the absence of PO(N)V, the ability to drink fluids, non-irritated and non-bleeding wound sites [101], and normothermia.
The revised Aldrete Score, which assesses activity, respiration, circulation, consciousness, and oxygen saturation, is a valuable tool for evaluating discharge readiness. However, it does not encompass pain or PO(N)V—both critical factors in evaluating pediatric recovery [101].
Continuous nerve blocks have demonstrated safety, efficacy, and high satisfaction rates in the treatment of acute pain among both patients and caregivers. This was found not only in perioperative and recovery unit settings, but also in outpatient care across various countries [102,103,104].
For infants under six months of age undergoing ambulatory procedures, strict adherence to a two-hour discharge criterion is not necessary, as it does not enhance safety, provided physiological readiness for discharge is achieved and patient selection is performed judiciously [105].
Note: The implementation of quality improvement measures—such as streamlined, structured handovers, standardized care protocols with checklists, and continuous healthcare provider training—remains integral to reducing translational gaps and enhancing safety and outcomes in postoperative pediatric recovery room care [4,106].
4. Conclusions
High-quality care in the pediatric recovery room is paramount, as it directly impacts perioperative safety, patient outcomes, overall hospital experience, and healthcare costs. Achieving this standard requires recovery units equipped with monitoring systems tailored to the specific needs of pediatric patients, and staffed by healthcare professionals with specialized training. Respect for the child’s privacy and the presence of parents are essential elements of patient-centered care.
Key strategies to optimize pediatric recovery include early oral fluid intake, effective and individualized pain management, and strategies to alleviate pain, anxiety, and stress. Additionally, the prevention and prompt treatment of postoperative nausea and vomiting and emergence delirium are critical for ensuring a smooth recovery.
Organizational measures, such as structured handovers, the implementation of standardized care protocols, ongoing professional development, quality improvement initiatives, and strategies for integrating new evidence, serve as foundational pillars for enhancing safety, accelerating recovery, and ensuring high levels of patient and caregiver satisfaction.
Author Contributions
Conceptualization, F.F.; writing—original draft preparation, L.K. and F.F.; writing—review and editing, L.K. and F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CHIPPS | Children’s and Infants’ Postoperative Pain Scale |
| CPSP | chronic post-surgical pain |
| ECG | electrocardiographic |
| ED | emergence delirium |
| EEG | electroencephalographic |
| LMA | laryngeal mask airway |
| NOPA | non-opioid analgesic |
| NSAID | nonsteroidal anti-inflammatory drugs |
| OSAS | obstructive sleep apnea syndrome |
| PO(N)V | postoperative nausea and/or vomiting |
| r-FLACC scale | revised Face, Legs, Activity, Cry, and Consolability scale |
References
- Murat, I.; Constant, I.; Maud’huy, H. Perioperative anaesthetic morbidity in children: A database of 24,165 anaesthetics over a 30-month period. Paediatr. Anaesth. 2004, 14, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Quan, H.; Bugar, J.M.; Lemaire, J.B.; Brant, R.; Ghali, W.A. Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J. Gen. Intern. Med. 2006, 21, 177–180. [Google Scholar] [CrossRef]
- Story, D.A.; Leslie, K.; Myles, P.S.; Fink, M.; Poustie, S.J.; Forbes, A.; Yap, S.; Beavis, V.; Kerridge, R.; REASON Investigators, Australian and New Zealand College of Anaesthetists Trials Group. Complications and mortality in older surgical patients in Australia and New Zealand (the REASON study): A multicentre, prospective, observational study. Anaesthesia 2010, 65, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.; Proctor, L.; Au, M.; Story, D.; Edwards, S.; Ludbrook, G. Incidence of early major adverse events after surgery in moderate-risk patients: Early postoperative adverse events. Br. J. Anaesth. 2020, 124, e9–e10. [Google Scholar] [CrossRef] [PubMed]
- General Comment No. 15 (2013) on the Right of the Child to the Enjoyment of the Highest Attainable Standard of Health (Art. 24). Available online: https://digitallibrary.un.org/record/778524?ln=en&v=pdf (accessed on 21 March 2025).
- Available online: https://www.asahq.org/standards-and-practice-parameters/standards-for-postanesthesia-care (accessed on 21 January 2025).
- Sjoberg, C.; Ringdal, M.; Jildenstal, P. Postoperative Recovery in the Youngest: Beyond Technology. Children 2024, 11, 1021. [Google Scholar] [CrossRef]
- De Lourdes Levy, M.; Larcher, V.; Kurz, R.; Ethics Working Group of the Confederation of European Specialists in Paediatrics (CESP). Informed consent/assent in children. Statement of the Ethics Working Group of the Confederation of European Specialists in Paediatrics (CESP). Eur. J. Pediatr. 2003, 162, 629–633. [Google Scholar] [CrossRef]
- Olin, K.; Klinga, C.; Ekstedt, M.; Pukk-Harenstam, K. Exploring everyday work as a dynamic non-event and adaptations to manage safety in intraoperative anaesthesia care: An interview study. BMC Health Serv. Res. 2023, 23, 651. [Google Scholar] [CrossRef]
- Wensing, M. Implementation science in healthcare: Introduction and perspective. Z. Evid. Fortbild. Qual. Gesundhwes. 2015, 109, 97–102. [Google Scholar] [CrossRef]
- Proctor, E.K.; Powell, B.J.; McMillen, J.C. Implementation strategies: Recommendations for specifying and reporting. Implement. Sci. 2013, 8, 139. [Google Scholar] [CrossRef]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 50. [Google Scholar] [CrossRef]
- Klein, A.A.; Meek, T.; Allcock, E.; Cook, T.M.; Mincher, N.; Morris, C.; Nimmo, A.F.; Pandit, J.J.; Pawa, A.; Rodney, G.; et al. Recommendations for standards of monitoring during anaesthesia and recovery 2021: Guideline from the Association of Anaesthetists. Anaesthesia 2021, 76, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, M.; Duggar, B.; Hamrick, J.; Alonso, G.T.; Martin, L. Error traps in the perioperative management of children with type 1 diabetes. Paediatr. Anaesth. 2024, 34, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Riegger, L.Q.; Leis, A.M.; Golmirzaie, K.H.; Malviya, S. Risk Factors for Intraoperative Hypoglycemia in Children: A Multicenter Retrospective Cohort Study. Anesth. Analg. 2021, 132, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, T.S.; Snaebjornsdottir, S.; Sigurdsson, M.I. Incidence of hypoglycaemia in fasting children after induction of anaesthesia for elective procedures: A descriptive observational study. Eur. J. Anaesthesiol. 2023, 40, 950–952. [Google Scholar] [CrossRef]
- Abraham, M.B.; Karges, B.; Dovc, K.; Naranjo, D.; Arbelaez, A.M.; Mbogo, J.; Javelikar, G.; Jones, T.W.; Mahmud, F.H. ISPAD Clinical Practice Consensus Guidelines 2022: Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1322–1340. [Google Scholar] [CrossRef]
- Sawada, A.; Kamada, Y.; Hayashi, H.; Ichinose, H.; Sumita, S.; Yamakage, M. Effect of Intraoperative Glucose Infusion on Catabolism of Adipose Tissue and Muscle Protein in Patients Anesthetized With Remifentanil in Combination With Sevoflurane During Major Surgery: A Randomized Controlled Multicenter Trial. Anesth. Analg. 2016, 123, 869–876. [Google Scholar] [CrossRef]
- Nishina, K.; Mikawa, K.; Maekawa, N.; Asano, M.; Obara, H. Effects of exogenous intravenous glucose on plasma glucose and lipid homeostasis in anesthetized infants. Anesthesiology 1995, 83, 258–263. [Google Scholar] [CrossRef]
- Sjoberg, C.; Ringdal, M.; Lundqvist, P.; Jildenstal, P. How to Achieve Highly Professional Care in the Postoperative Ward: The Care of Infants and Toddlers. J. PeriAnesthesia Nurs. 2024, 40, 95–99. [Google Scholar] [CrossRef]
- Baek, J.; Kim, Y.M. The Impact of Parental Presence on Emergence Delirium in Pediatric Patients After General Anesthesia: A Systematic Review and Meta-analysis. J. PeriAnesthesia Nurs. 2024, 39, 475–483. [Google Scholar] [CrossRef]
- Kain, Z.N.; Mayes, L.C.; Caldwell-Andrews, A.A.; Karas, D.E.; McClain, B.C. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics 2006, 118, 651–658. [Google Scholar] [CrossRef]
- Kain, Z.N.; Caldwell-Andrews, A.A.; Mayes, L.C.; Weinberg, M.E.; Wang, S.M.; MacLaren, J.E.; Blount, R.L. Family-centered preparation for surgery improves perioperative outcomes in children: A randomized controlled trial. Anesthesiology 2007, 106, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Cote, C.J.; Zaslavsky, A.; Downes, J.J.; Kurth, C.D.; Welborn, L.G.; Warner, L.O.; Malviya, S.V. Postoperative apnea in former preterm infants after inguinal herniorrhaphy. A combined analysis. Anesthesiology 1995, 82, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Welborn, L.G.; de Soto, H.; Hannallah, R.S.; Fink, R.; Ruttimann, U.E.; Boeckx, R. The use of caffeine in the control of post-anesthetic apnea in former premature infants. Anesthesiology 1988, 68, 796–798. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W.; Caffeine for Apnea of Prematurity Trial, G. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W.; Caffeine for Apnea of Prematurity Trial, G. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2007, 357, 1893–1902. [Google Scholar] [CrossRef]
- Patino, M.; Sadhasivam, S.; Mahmoud, M. Obstructive sleep apnoea in children: Perioperative considerations. Br. J. Anaesth. 2013, 111 (Suppl. S1), i83–i95. [Google Scholar] [CrossRef]
- Albornoz, A.E.; Rana, M.; Hayes, J.; Englesakis, M.; Tsang, M.; Amin, R.; Gilfoyle, E.; Petre, M.A.; Campisi, P.; Aoyama, K. Perioperative clinical practice recommendations for pediatric tonsillectomy: A systematic review. Can. J. Anaesth. 2024, 71, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Webber, A.M.; Brennan, M. The high-risk pediatric patient for ambulatory surgery. Curr. Opin. Anaesthesiol. 2023, 36, 630–635. [Google Scholar] [CrossRef]
- Parry, M.; Glaisyer, H.R.; Bailey, P.M. Removal of LMA in children. Br. J. Anaesth. 1997, 78, 337–338. [Google Scholar] [CrossRef]
- Patterson, H.; Eady, J.; Sommerfield, A.; Sommerfield, D.; Hauser, N.; von Ungern-Sternberg, B.S. Patient positioning and its impact on perioperative outcomes in children: A narrative review. Paediatr. Anaesth. 2024, 34, 507–518. [Google Scholar] [CrossRef]
- Luedeke, C.M.; Rudolph, M.I.; Pulverenti, T.S.; Azimaraghi, O.; Grimm, A.M.; Jackson, W.M.; Jaconia, G.D.; Stucke, A.G.; Nafiu, O.O.; Karaye, I.M.; et al. Development and validation of a score for prediction of postoperative respiratory complications in infants and children (SPORC-C). Br. J. Anaesth. 2025, 134, 212–220. [Google Scholar] [CrossRef]
- Ledowski, T.; O’Dea, B.; Meyerkort, L.; Hegarty, M.; von Ungern-Sternberg, B.S. Postoperative Residual Neuromuscular Paralysis at an Australian Tertiary Children’s Hospital. Anesthesiol. Res. Pract. 2015, 2015, 410248. [Google Scholar] [CrossRef] [PubMed]
- von Ungern-Sternberg, B.S.; Hammer, J.; Schibler, A.; Frei, F.J.; Erb, T.O. Decrease of functional residual capacity and ventilation homogeneity after neuromuscular blockade in anesthetized young infants and preschool children. Anesthesiology 2006, 105, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Lorinc, A.N.; Lawson, K.C.; Niconchuk, J.A.; Modes, K.B.; Moore, J.D.; Brenn, B.R. Residual Weakness and Recurarization After Sugammadex Administration in Pediatric Patients: A Case Series. A A Pract. 2020, 14, e01225. [Google Scholar] [CrossRef] [PubMed]
- Heier, T.; Caldwell, J.E. Impact of hypothermia on the response to neuromuscular blocking drugs. Anesthesiology 2006, 104, 1070–1080. [Google Scholar] [CrossRef]
- Bindu, B.; Bindra, A.; Rath, G. Temperature management under general anesthesia: Compulsion or option. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 306–316. [Google Scholar] [CrossRef]
- Bjertnaes, L.J.; Naesheim, T.O.; Reierth, E.; Suborov, E.V.; Kirov, M.Y.; Lebedinskii, K.M.; Tveita, T. Physiological Changes in Subjects Exposed to Accidental Hypothermia: An Update. Front. Med. 2022, 9, 824395. [Google Scholar] [CrossRef]
- Zanelli, S.; Buck, M.; Fairchild, K. Physiologic and pharmacologic considerations for hypothermia therapy in neonates. J. Perinatol. 2011, 31, 377–386. [Google Scholar] [CrossRef]
- Zhao, J.; Le, Z.; Chu, L.; Gao, Y.; Zhang, M.; Fan, J.; Ma, D.; Hu, Y.; Lai, D. Risk factors and outcomes of intraoperative hypothermia in neonatal and infant patients undergoing general anesthesia and surgery. Front. Pediatr. 2023, 11, 1113627. [Google Scholar] [CrossRef]
- Akin, A.; Esmaoglu, A.; Boyaci, A. Postoperative shivering in children and causative factors. Paediatr. Anaesth. 2005, 15, 1089–1093. [Google Scholar] [CrossRef]
- Nemeth, M.; Miller, C.; Brauer, A. Perioperative Hypothermia in Children. Int. J. Environ. Res. Public Health 2021, 18, 7541. [Google Scholar] [CrossRef] [PubMed]
- Laptook, A.R.; Salhab, W.; Bhaskar, B.; Neonatal Research, N. Admission temperature of low birth weight infants: Predictors and associated morbidities. Pediatrics 2007, 119, e643–e649. [Google Scholar] [CrossRef]
- Eberhart, L.H.; Morin, A.M.; Guber, D.; Kretz, F.J.; Schauffelen, A.; Treiber, H.; Wulf, H.; Geldner, G. Applicability of risk scores for postoperative nausea and vomiting in adults to paediatric patients. Br. J. Anaesth. 2004, 93, 386–392. [Google Scholar] [CrossRef]
- Edler, A.A.; Mariano, E.R.; Golianu, B.; Kuan, C.; Pentcheva, K. An analysis of factors influencing postanesthesia recovery after pediatric ambulatory tonsillectomy and adenoidectomy. Anesth. Analg. 2007, 104, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef] [PubMed]
- Roher, K.; Fideler, F. Perioperative Complications in Pediatric Anesthesia. Anasthesiol. Intensiv. Notfallmed Schmerzther. 2022, 57, 563–576. [Google Scholar] [CrossRef]
- Cox, R.G. Alternative therapies and postoperative vomiting. Paediatr. Anaesth. 2016, 26, 782–783. [Google Scholar] [CrossRef]
- Abraham, J. Acupressure and acupuncture in preventing and managing postoperative nausea and vomiting in adults. J. Perioper. Pract. 2008, 18, 543–551. [Google Scholar] [CrossRef]
- Chauvin, C.; Schalber-Geyer, A.S.; Lefebvre, F.; Bopp, C.; Carrenard, G.; Marcoux, L.; Mayer, J.F.; Schwaab, C.; Joshi, G.P.; Diemunsch, P. Early postoperative oral fluid intake in paediatric day case surgery influences the need for opioids and postoperative vomiting: A controlled randomized trialdagger. Br. J. Anaesth. 2017, 118, 407–414. [Google Scholar] [CrossRef]
- Johns, D.E.; Gerling, V.; Pasker-de Jong, P.C. Ice pops in the recovery room: Effects on postoperative nausea and vomiting. Br. J. Anaesth. 2017, 118, 637–638. [Google Scholar] [CrossRef]
- Aldecoa, C.; Bettelli, G.; Bilotta, F.; Sanders, R.D.; Audisio, R.; Borozdina, A.; Cherubini, A.; Jones, C.; Kehlet, H.; MacLullich, A.; et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur. J. Anaesthesiol. 2017, 34, 192–214. [Google Scholar] [CrossRef]
- Voepel-Lewis, T.; Malviya, S.; Tait, A.R. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth. Analg. 2003, 96, 1625–1630. [Google Scholar] [CrossRef]
- Vlajkovic, G.P.; Sindjelic, R.P. Emergence delirium in children: Many questions, few answers. Anesth. Analg. 2007, 104, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ringblom, J.; Wahlin, I.; Proczkowska, M. A psychometric evaluation of the Pediatric Anesthesia Emergence Delirium scale. Paediatr. Anaesth. 2018, 28, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Sikich, N.; Lerman, J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology 2004, 100, 1138–1145. [Google Scholar] [CrossRef]
- Somaini, M.; Engelhardt, T.; Fumagalli, R.; Ingelmo, P.M. Emergence delirium or pain after anaesthesia—How to distinguish between the two in young children: A retrospective analysis of observational studies. Br. J. Anaesth. 2016, 116, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.S.S.; Mammen, P.M.; Shankar, S.R.; Viswanathan, S.A.; Rebekah, G.; Russell, S.; Earnest, R.; Chikkala, S.M. Pediatric Anesthesia Emergence Delirium Scale: A diagnostic meta-analysis. World J. Clin. Pediatr. 2022, 11, 196–205. [Google Scholar] [CrossRef]
- Locatelli, B.G.; Ingelmo, P.M.; Emre, S.; Meroni, V.; Minardi, C.; Frawley, G.; Benigni, A.; Di Marco, S.; Spotti, A.; Busi, I.; et al. Emergence delirium in children: A comparison of sevoflurane and desflurane anesthesia using the Paediatric Anesthesia Emergence Delirium scale. Paediatr. Anaesth. 2013, 23, 301–308. [Google Scholar] [CrossRef]
- Ringblom, J.; Wahlin, I.; Proczkowska, M.; Korhonen, L.; Arestedt, K. Measurement Properties of the Pediatric Anesthesia Emergence Delirium Scale: A Confirmatory Factor Analysis-Based Study. Paediatr. Anaesth. 2025, 35, 155–162. [Google Scholar] [CrossRef]
- Aono, J.; Mamiya, K.; Manabe, M. Preoperative anxiety is associated with a high incidence of problematic behavior on emergence after halothane anesthesia in boys. Acta Anaesthesiol. Scand. 1999, 43, 542–544. [Google Scholar] [CrossRef]
- Mason, K.P. Paediatric emergence delirium: A comprehensive review and interpretation of the literature. Br. J. Anaesth. 2017, 118, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Muzzi, E.; Ronfani, L.; Bossini, B.; Lezcano, C.; Orzan, E.; Barbi, E. Effects of Intraoperative Auditory Stimulation on Pain and Agitation on Awakening After Pediatric Adenotonsillectomy: A Randomized Clinical Trial. JAMA Otolaryngol.-Head Neck Surg. 2021, 147, 638–645. [Google Scholar] [CrossRef]
- Xiao, Y.; Jin, X.; Zhang, Y.; Huang, T.; Zhou, L.; Gao, J. Efficacy of propofol for the prevention of emergence agitation after sevoflurane anaesthesia in children: A meta-analysis. Front. Surg. 2022, 9, 1031010. [Google Scholar] [CrossRef]
- Ng, K.T.; Sarode, D.; Lai, Y.S.; Teoh, W.Y.; Wang, C.Y. The effect of ketamine on emergence agitation in children: A systematic review and meta-analysis. Paediatr. Anaesth. 2019, 29, 1163–1172. [Google Scholar] [CrossRef]
- Zhao, N.; Zeng, J.; Fan, L.; Zhang, C.; Wu, Y.; Wang, X.; Gao, F.; Yu, C. The Effect of Alfentanil on Emergence Delirium Following General Anesthesia in Children: A Randomized Clinical Trial. Paediatr. Drugs 2022, 24, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Park, J.H.; Lee, J.S.; Choi, T.; Kim, M.S. Effects of intravenous fentanyl around the end of surgery on emergence agitation in children: Systematic review and meta-analysis. Paediatr. Anaesth. 2017, 27, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Qi, T.S.; Ng, A. Emergence delirium: An overview with an emphasis on the use of electroencephalography in its management. Anesth. Pain. Med. 2024, 19, S87–S95. [Google Scholar] [CrossRef]
- Petre, M.A.; Levin, D.N.; Englesakis, M.; Maynes, J.T.; Pechlivanoglou, P.; Aoyama, K. Dexmedetomidine vs. total intravenous anaesthesia in paediatric emergence delirium: A network meta-analysis. Eur. J. Anaesthesiol. 2021, 38, 1111–1123. [Google Scholar] [CrossRef]
- Koo, C.H.; Koo, B.W.; Han, J.; Lee, H.T.; Lim, D.; Shin, H.J. The effects of intraoperative magnesium sulfate administration on emergence agitation and delirium in pediatric patients: A systematic review and meta-analysis of randomized controlled trials. Paediatr. Anaesth. 2022, 32, 522–530. [Google Scholar] [CrossRef]
- Bernhart, K.; Becke-Jakob, K.; Lehmann, T.; Harnik, M.; Seiler, S.; Meissner, W.; Stuber, F.; Stamer, U.M. Analgesic use and favourable patient-reported outcome measures after paediatric surgery: An analysis of registry data. Br. J. Anaesth. 2023, 130, 74–82. [Google Scholar] [CrossRef]
- Buttner, W.; Finke, W.; Hilleke, M.; Reckert, S.; Vsianska, L.; Brambrink, A. Development of an observational scale for assessment of postoperative pain in infants. Anasthesiol Intensiv. Notfallmed Schmerzther 1998, 33, 353–361. [Google Scholar] [CrossRef]
- Malviya, S.; Voepel-Lewis, T.; Burke, C.; Merkel, S.; Tait, A.R. The revised FLACC observational pain tool: Improved reliability and validity for pain assessment in children with cognitive impairment. Paediatr. Anaesth. 2006, 16, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Crellin, D.J.; Harrison, D.; Santamaria, N.; Babl, F.E. Systematic review of the Face, Legs, Activity, Cry and Consolability scale for assessing pain in infants and children: Is it reliable, valid, and feasible for use? Pain 2015, 156, 2132–2151. [Google Scholar] [CrossRef]
- Zielinski, J.; Morawska-Kochman, M.; Zatonski, T. Pain assessment and management in children in the postoperative period: A review of the most commonly used postoperative pain assessment tools, new diagnostic methods and the latest guidelines for postoperative pain therapy in children. Adv. Clin. Exp. Med. 2020, 29, 365–374. [Google Scholar] [CrossRef]
- Furuta, M.; Suzuki, Y.; Sun, N.; Aoyama, K. Chronic post-surgical pain in pediatric population. J. Anesth. 2022, 36, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Rabbitts, J.A.; Fisher, E.; Rosenbloom, B.N.; Palermo, T.M. Prevalence and Predictors of Chronic Postsurgical Pain in Children: A Systematic Review and Meta-Analysis. J. Pain 2017, 18, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, B.N.; Frederiksen, S.D.; Wang, V.; Park, C.S.; Gordon, G.; Brar, G.; Rasic, N.; Stinson, J.N.; Birnie, K.A.; Rabbitts, J.A. Prognostic factors of chronic postsurgical pain in children and adolescents: A systematic review and meta-analysis. Reg. Anesth. Pain Med. 2025, 50, 144–152. [Google Scholar] [CrossRef]
- Ziesenitz, V.C.; Welzel, T.; van Dyk, M.; Saur, P.; Gorenflo, M.; van den Anker, J.N. Efficacy and Safety of NSAIDs in Infants: A Comprehensive Review of the Literature of the Past 20 Years. Paediatr. Drugs 2022, 24, 603–655. [Google Scholar] [CrossRef]
- Wong, I.; St John-Green, C.; Walker, S.M. Opioid-sparing effects of perioperative paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Paediatr. Anaesth. 2013, 23, 475–495. [Google Scholar] [CrossRef]
- Messerer, B.; Grogl, G.; Stromer, W.; Jaksch, W. Pediatric perioperative systemic pain therapy: Austrian interdisciplinary recommendations on pediatric perioperative pain management. Schmerz 2014, 28, 43–64. [Google Scholar] [CrossRef]
- Birmingham, P.K.; Tobin, M.J.; Fisher, D.M.; Henthorn, T.K.; Hall, S.C.; Cote, C.J. Initial and subsequent dosing of rectal acetaminophen in children: A 24-h pharmacokinetic study of new dose recommendations. Anesthesiology 2001, 94, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.R.; Nicholson, A.; Cardwell, M.E.; Siviter, G.; Smith, A.F. Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database Syst. Rev. 2013, 2013, CD003591. [Google Scholar] [CrossRef] [PubMed]
- Ringsten, M.; Kredo, T.; Ebrahim, S.; Hohlfeld, A.; Bruschettini, M. Diclofenac for acute postoperative pain in children. Cochrane Database Syst. Rev. 2023, 12, CD015087. [Google Scholar] [CrossRef]
- Yoon, E.; Babar, A.; Choudhary, M.; Kutner, M.; Pyrsopoulos, N. Acetaminophen-Induced Hepatotoxicity: A Comprehensive Update. J. Clin. Transl. Hepatol. 2016, 4, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Thibault, C.; Pelletier, E.; Nguyen, C.; Trottier, E.D.; Dore-Bergeron, M.J.; DeKoven, K.; Roy, A.M.; Piche, N.; Delisle, J.F.; Morin, C.; et al. The Three W’s of Acetaminophen In Children: Who, Why, and Which Administration Mode. J. Pediatr. Pharmacol. Ther. 2023, 28, 20–28. [Google Scholar] [CrossRef]
- Stamer, U.M.; Stammschulte, T.; Erlenwein, J.; Koppert, W.; Freys, S.; Meissner, W.; Ahrens, P.; Brede, E.M.; Lindig, M.; Dusch, M.; et al. Recommendations for the perioperative use of dipyrone: Expert recommendation of the working group on acute pain of the German Pain Society, the scientific working group on pain medicine of the German Society for Anesthesiology and Intensive Care Medicine and the surgical working group on acute pain of the German Society for Surgery with participation of representatives of the Drug Commission of the German Medical Association. Schmerz 2019, 33, 287–294. [Google Scholar] [CrossRef]
- Zahn, J.; Eberl, S.; Rodle, W.; Rascher, W.; Neubert, A.; Toni, I. Metamizole Use in Children: Analysis of Drug Utilisation and Adverse Drug Reactions at a German University Hospital between 2015 and 2020. Paediatr. Drugs 2022, 24, 45–56. [Google Scholar] [CrossRef]
- Sharp, D.; Jaffrani, A. A PRISMA Systematic Review on the Safety and Efficacy of Patient-Controlled Analgesia (PCA) in Pediatrics. J. Pediatr. Nurs. 2021, 61, 219–223. [Google Scholar] [CrossRef]
- Walker, S.M. Neonatal pain. Paediatr. Anaesth. 2014, 24, 39–48. [Google Scholar] [CrossRef]
- Schultz-Machata, A.M.; Becke, K.; Weiss, M. Nalbuphine in pediatric anesthesia. Anaesthesist 2014, 63, 135–143. [Google Scholar] [CrossRef]
- Aldamluji, N.; Burgess, A.; Pogatzki-Zahn, E.; Raeder, J.; Beloeil, H.; PROSPECT Working Group Collaborators. PROSPECT guideline for tonsillectomy: Systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 2021, 76, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.H.; Kim, K.N.; Kim, J.Y.; Song, S.M. The effects of intranasal dexmedetomidine premedication in children: A systematic review and meta-analysis. Can. J. Anaesth. 2017, 64, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Burjek, N.E.; Hafeman, M.; Guthrie, D.; Desai, A.; Jin, Z.; Brockel, M.; Moore, R. Perioperative use of gabapentinoids in pediatric patients. Anesthesiol. Perioper. Sci. 2023, 1, 21. [Google Scholar] [CrossRef]
- Pillai Riddell, R.R.; Bucsea, O.; Shiff, I.; Chow, C.; Gennis, H.G.; Badovinac, S.; DiLorenzo-Klas, M.; Racine, N.M.; Ahola Kohut, S.; Lisi, D.; et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst. Rev. 2023, 6, CD006275. [Google Scholar] [CrossRef]
- Vittinghoff, M.; Lonnqvist, P.A.; Mossetti, V.; Heschl, S.; Simic, D.; Colovic, V.; Dmytriiev, D.; Holzle, M.; Zielinska, M.; Kubica-Cielinska, A.; et al. Postoperative pain management in children: Guidance from the pain committee of the European Society for Paediatric Anaesthesiology (ESPA Pain Management Ladder Initiative). Paediatr. Anaesth. 2018, 28, 493–506. [Google Scholar] [CrossRef]
- Vittinghoff, M.; Lonnqvist, P.A.; Mossetti, V.; Heschl, S.; Simic, D.; Colovic, V.; Hozle, M.; Zielinska, M.; Maria, B.J.; Oppitz, F.; et al. Postoperative Pain Management in children: Guidance from the Pain Committee of the European Society for Paediatric Anaesthesiology (ESPA Pain Management Ladder Initiative) Part II. Anaesth. Crit. Care Pain Med. 2024, 43, 101427. [Google Scholar] [CrossRef]
- Fung, A.C.; Tsang, J.T.; Chung, P.H.; Kak-Yuen Wong, K. Does Chewing Gum Lead to Earlier Postoperative Gastrointestinal Recovery in Children? A Systematic Review and Meta-analysis. J. Pediatr. Surg. 2024, 59, 268–274. [Google Scholar] [CrossRef]
- Rafeeqi, T.; Pearson, E.G. Enhanced recovery after surgery in children. Transl. Gastroenterol. Hepatol. 2021, 6, 46. [Google Scholar] [CrossRef]
- Aldrete, J.A. The post-anesthesia recovery score revisited. J. Clin. Anesth. 1995, 7, 89–91. [Google Scholar] [CrossRef]
- Stein, A.L.; Baumgard, D.; Del Rio, I.; Tutiven, J.L. Updates in Pediatric Regional Anesthesia and Its Role in the Treatment of Acute Pain in the Ambulatory Setting. Curr. Pain. Headache Rep. 2017, 21, 11. [Google Scholar] [CrossRef]
- Finneran, J.J.t.; Ilfeld, B.M. Continuous peripheral nerve blocks for analgesia following painful ambulatory surgery: A review with focus on recent developments in infusion technology. Curr. Opin. Anaesthesiol. 2023, 36, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Gurnaney, H.; Kraemer, F.W.; Maxwell, L.; Muhly, W.T.; Schleelein, L.; Ganesh, A. Ambulatory continuous peripheral nerve blocks in children and adolescents: A longitudinal 8-year single center study. Anesth. Analg. 2014, 118, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Uffman, J.C.; Kim, S.S.; Quan, L.N.; Shelton, T.; Beltran, R.J.; Jatana, K.R.; Chiang, T.; Tobias, J.D. Adverse Events in Infants Less Than 6 Months of Age After Ambulatory Surgery and Diagnostic Imaging Requiring Anesthesia. Pediatr. Qual. Saf. 2022, 7, e574. [Google Scholar] [CrossRef] [PubMed]
- Kurth, C.D.; Tyler, D.; Heitmiller, E.; Tosone, S.R.; Martin, L.; Deshpande, J.K. National pediatric anesthesia safety quality improvement program in the United States. Anesth. Analg. 2014, 119, 112–121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).