Epidemiology and Outcomes of Late-Onset Neonatal Sepsis in Preterm Infants in a Tertiary Hospital
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
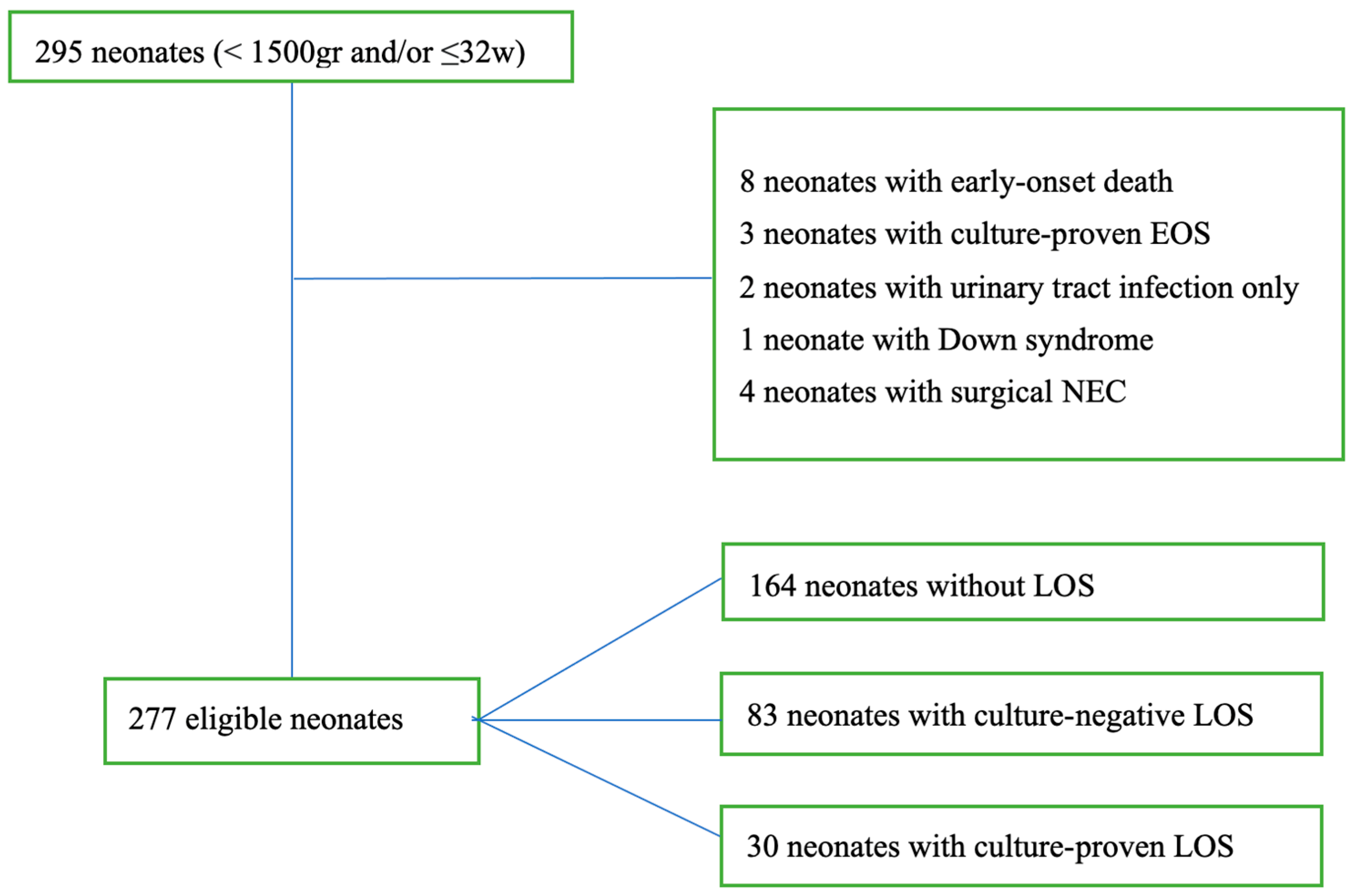
2.3. Definitions
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, Y.; Speer, C.P. Late-onset neonatal sepsis: Recent developments. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F257–F263. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Jefferies, A.L.; Yoon, E.W.; Lee, S.K.; Shah, P.S.; Canadian Neonatal, N. Risk Factors and Outcomes of Late-Onset Bacterial Sepsis in Preterm Neonates Born at <32 Weeks’ Gestation. Am. J. Perinatol. 2015, 32, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Coggins, S.A.; Glaser, K. Updates in Late-Onset Sepsis: Risk Assessment, Therapy, and Outcomes. Neoreviews 2022, 23, 738–755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yang, Z.; Shan, R.; Zhang, Y.; Yan, W.; Yang, Y.; Shah, P.S.; Lee, S.K.; Cao, Y.; Reduction of Infection in Neonatal Intensive Care Units using the Evidence-based Practice for Improving Quality Study Group. Neonatal Outcomes Following Culture-negative Late-onset Sepsis Among Preterm Infants. Pediatr. Infect. Dis. J. 2020, 39, 232–238. [Google Scholar] [CrossRef]
- Flannery, D.D.; Edwards, E.M.; Coggins, S.A.; Horbar, J.D.; Puopolo, K.M. Late-Onset Sepsis Among Very Preterm Infants. Pediatrics 2022, 150, e2022058813. [Google Scholar] [CrossRef]
- Greenberg, R.G.; Kandefer, S.; Do, B.T.; Smith, P.B.; Stoll, B.J.; Bell, E.F.; Carlo, W.A.; Laptook, A.R.; Sanchez, P.J.; Shankaran, S.; et al. Late-onset Sepsis in Extremely Premature Infants: 2000–2011. Pediatr. Infect. Dis. J. 2017, 36, 774–779. [Google Scholar] [CrossRef]
- Afonso, E.D.P.; Blot, S. Effect of gestational age on the epidemiology of late-onset sepsis in neonatal intensive care units—A review. Expert. Rev. Anti-Infect. Ther. 2017, 15, 917–924. [Google Scholar] [CrossRef]
- Tsai, M.H.; Hsu, J.F.; Chu, S.M.; Lien, R.; Huang, H.R.; Chiang, M.C.; Fu, R.H.; Lee, C.W.; Huang, Y.C. Incidence, clinical characteristics and risk factors for adverse outcome in neonates with late-onset sepsis. Pediatr. Infect. Dis. J. 2014, 33, e7–e13. [Google Scholar] [CrossRef]
- El Manouni El Hassani, S.; Berkhout, D.J.C.; Niemarkt, H.J.; Mann, S.; de Boode, W.P.; Cossey, V.; Hulzebos, C.V.; van Kaam, A.H.; Kramer, B.W.; van Lingen, R.A.; et al. Risk Factors for Late-Onset Sepsis in Preterm Infants: A Multicenter Case-Control Study. Neonatology 2019, 116, 42–51. [Google Scholar] [CrossRef]
- Shane, A.L.; Stoll, B.J. Neonatal sepsis: Progress towards improved outcomes. J. Infect. 2014, 68 (Suppl. S1), S24–S32. [Google Scholar] [CrossRef]
- Zonnenberg, I.A.; van Dijk-Lokkart, E.M.; van den Dungen, F.A.M.; Vermeulen, R.J.; van Weissenbruch, M.M. Neurodevelopmental outcome at 2 years of age in preterm infants with late-onset sepsis. Eur. J. Pediatr. 2019, 178, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.J.; Aebischer, M.; Adams, M.; Natalucci, G.; Bonhoeffer, J.; Latzin, P.; Nelle, M.; Bucher, H.U.; Latal, B.; Swiss Neonatal, N.; et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics 2011, 128, e348–e357. [Google Scholar] [CrossRef] [PubMed]
- Cantey, J.B.; Wozniak, P.S.; Pruszynski, J.E.; Sanchez, P.J. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): A prospective interrupted time-series study. Lancet Infect. Dis. 2016, 16, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Ramasethu, J.; Kawakita, T. Antibiotic stewardship in perinatal and neonatal care. Semin. Fetal Neonatal Med. 2017, 22, 278–283. [Google Scholar] [CrossRef]
- Hou, S.; Yu, Y.; Wu, Y.; Cao, Y.; Zhang, J.; Liu, Z.; Guo, C.; Chen, Y.; Sun, X.; Li, M.; et al. Association Between Antibiotic Overexposure and Adverse Outcomes in Very-Low-Birth-Weight Infants Without Culture-Proven Sepsis or Necrotizing Enterocolitis: A Multicenter Prospective Study. Indian J. Pediatr. 2022, 89, 785–792. [Google Scholar] [CrossRef]
- Jean-Baptiste, N.; Benjamin, D.K., Jr.; Cohen-Wolkowiez, M.; Fowler, V.G., Jr.; Laughon, M.; Clark, R.H.; Smith, P.B. Coagulase-negative staphylococcal infections in the neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 2011, 32, 679–686. [Google Scholar] [CrossRef]
- Mobley, R.E.; Bizzarro, M.J. Central line-associated bloodstream infections in the NICU: Successes and controversies in the quest for zero. Semin. Perinatol. 2017, 41, 166–174. [Google Scholar] [CrossRef]
- Eichberger, J.; Resch, E.; Resch, B. Diagnosis of Neonatal Sepsis: The Role of Inflammatory Markers. Front. Pediatr. 2022, 10, 840288. [Google Scholar] [CrossRef]
- Parry, G.; Tucker, J.; Tarnow-Mordi, W.; UK Neonatal Staffing Study Collaborative Group. CRIB II: An update of the clinical risk index for babies score. Lancet 2003, 361, 1789–1791. [Google Scholar] [CrossRef]
- Vardhelli, V.; Murki, S.; Tandur, B.; Saha, B.; Oleti, T.P.; Deshabhotla, S.; Mohammed, Y.A.; Seth, S.; Siramshetty, S.; Kallem, V.R. Comparison of CRIB-II with SNAPPE-II for predicting survival and morbidities before hospital discharge in neonates with gestation ≤32 weeks: A prospective multicentric observational study. Eur. J. Pediatr. 2022, 181, 2831–2838. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef] [PubMed]
- Lahart, A.C.; McPherson, C.C.; Gerber, J.S.; Warner, B.B.; Lee, B.R.; Newland, J.G. Application of an antibiotic spectrum index in the neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 2019, 40, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.S.; Hersh, A.L.; Kronman, M.P.; Newland, J.G.; Ross, R.K.; Metjian, T.A. Development and Application of an Antibiotic Spectrum Index for Benchmarking Antibiotic Selection Patterns Across Hospitals. Infect. Control Hosp. Epidemiol. 2017, 38, 993–997. [Google Scholar] [CrossRef]
- Klingenberg, C.; Kornelisse, R.F.; Buonocore, G.; Maier, R.F.; Stocker, M. Culture-Negative Early-Onset Neonatal Sepsis—At the Crossroad Between Efficient Sepsis Care and Antimicrobial Stewardship. Front. Pediatr. 2018, 6, 285. [Google Scholar] [CrossRef]
- Stocker, M.; van Herk, W.; El Helou, S.; Dutta, S.; Fontana, M.S.; Schuerman, F.; van den Tooren-de Groot, R.K.; Wieringa, J.W.; Janota, J.; van der Meer-Kappelle, L.H.; et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: A multicentre, randomised controlled trial (NeoPIns). Lancet 2017, 390, 871–881. [Google Scholar] [CrossRef]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Patel, R.M.; Ferguson, J.; McElroy, S.J.; Khashu, M.; Caplan, M.S. Defining necrotizing enterocolitis: Current difficulties and future opportunities. Pediatr. Res. 2020, 88 (Suppl. S1), 10–15. [Google Scholar] [CrossRef]
- Eichenwald, E.C.; Committee on Fetus and Newborn; Watterberg, K.L.; Aucott, S.; Benitz, W.E.; Cummings, J.J.; Goldsmith, J.; Poindexter, B.B.; Puopolo, K.; Stewart, D.L.; et al. Apnea of Prematurity. Pediatrics 2016, 137, e20153757. [Google Scholar] [CrossRef]
- Fenton, T.R.; Cormack, B.; Goldberg, D.; Nasser, R.; Alshaikh, B.; Eliasziw, M.; Hay, W.W.; Hoyos, A.; Anderson, D.; Bloomfield, F.; et al. “Extrauterine growth restriction” and “postnatal growth failure” are misnomers for preterm infants. J. Perinatol. 2020, 40, 704–714. [Google Scholar] [CrossRef]
- Greenbury, S.F.; Angelini, E.D.; Ougham, K.; Battersby, C.; Gale, C.; Uthaya, S.; Modi, N. Birthweight and patterns of postnatal weight gain in very and extremely preterm babies in England and Wales, 2008–2019: A cohort study. Lancet Child. Adolesc. Health 2021, 5, 719–728. [Google Scholar] [CrossRef]
- Verstraete, E.H.; De Coen, K.; Vogelaers, D.; Blot, S. Risk Factors for Health Care-Associated Sepsis in Critically Ill Neonates Stratified by Birth Weight. Pediatr. Infect. Dis. J. 2015, 34, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Ramasethu, J. Prevention of Health Care−Associated Infections in the NICU. NeoReviews 2020, 21, e546–e558. [Google Scholar] [CrossRef]
- Beck, C.; Gallagher, K.; Taylor, L.A.; Goldstein, J.A.; Mithal, L.B.; Gernand, A.D. Chorioamnionitis and Risk for Maternal and Neonatal Sepsis: A Systematic Review and Meta-analysis. Obs. Gynecol. 2021, 137, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Puopolo, K.M.; Hansen, N.I.; Lorch, S.A.; DeMauro, S.B.; Greenberg, R.G.; Cotten, C.M.; Sanchez, P.J.; Bell, E.F.; Eichenwald, E.C.; et al. Neurodevelopmental outcomes following neonatal late-onset sepsis and blood culture-negative conditions. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 467–473. [Google Scholar] [CrossRef]
- Bedetti, L.; Corso, L.; Miselli, F.; Guidotti, I.; Toffoli, C.; Miglio, R.; Roversi, M.F.; Muttini, E.D.C.; Pugliese, M.; Bertoncelli, N.; et al. Neurodevelopmental Outcome after Culture-Proven or So-Called Culture-Negative Sepsis in Preterm Infants. J. Clin. Med. 2024, 13, 1140. [Google Scholar] [CrossRef]
- Huncikova, Z.; Vatne, A.; Stensvold, H.J.; Lang, A.M.; Stoen, R.; Brigtsen, A.K.; Salvesen, B.; Oymar, K.A.A.; Ronnestad, A.; Klingenberg, C.; et al. Late-onset sepsis in very preterm infants in Norway in 2009-2018: A population-based study. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 478–484. [Google Scholar] [CrossRef]
- El Rafei, R.; Jarreau, P.H.; Norman, M.; Maier, R.F.; Barros, H.; Reempts, P.V.; Pedersen, P.; Cuttini, M.; Zeitlin, J.; Group, E.R. Variation in very preterm extrauterine growth in a European multicountry cohort. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 316–323. [Google Scholar] [CrossRef]
- Gounaris, A.; Sokou, R.; Theodoraki, M.; Gounari, E.; Panagiotounakou, P.; Antonogeorgos, G.; Ioakeimidis, G.; Parastatidou, S.; Konstantinidi, A.; Grivea, I.N. “Aggressive” Feeding of Very Preterm Neonates and Body Mass Index at School Age. Nutrients 2021, 13, 1901. [Google Scholar] [CrossRef]
- Hornik, C.P.; Fort, P.; Clark, R.H.; Watt, K.; Benjamin, D.K., Jr.; Smith, P.B.; Manzoni, P.; Jacqz-Aigrain, E.; Kaguelidou, F.; Cohen-Wolkowiez, M. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum. Dev. 2012, 88 (Suppl. S2), S69–S74. [Google Scholar] [CrossRef]
- Modi, N.; Dore, C.J.; Saraswatula, A.; Richards, M.; Bamford, K.B.; Coello, R.; Holmes, A. A case definition for national and international neonatal bloodstream infection surveillance. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F8–F12. [Google Scholar] [CrossRef]
- Piantino, J.H.; Schreiber, M.D.; Alexander, K.; Hageman, J. Culture negative sepsis and systemic inflammatory response syndrome in neonates. Neoreviews 2013, 14, 294–305. [Google Scholar] [CrossRef]
- Josephson, C.D.; Caliendo, A.M.; Easley, K.A.; Knezevic, A.; Shenvi, N.; Hinkes, M.T.; Patel, R.M.; Hillyer, C.D.; Roback, J.D. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: A prospective cohort study. JAMA Pediatr. 2014, 168, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Kadambari, S.; Whittaker, E.; Lyall, H. Postnatally acquired cytomegalovirus infection in extremely premature infants: How best to manage? Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.Y.; Synnes, A.; Roberts, A.; Deshpandey, A.; Dow, K.; Yoon, E.W.; Lee, K.S.; Dobson, S.; Lee, S.K.; Shah, P.S.; et al. Association Between Antibiotic Use and Neonatal Mortality and Morbidities in Very Low-Birth-Weight Infants Without Culture-Proven Sepsis or Necrotizing Enterocolitis. JAMA Pediatr. 2016, 170, 1181–1187. [Google Scholar] [CrossRef]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E.; Committee on Fetus and Newborn; Committee on Infectious Diseases. Management of Neonates Born at ≤34 6/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182896. [Google Scholar] [CrossRef]
- Dimopoulou, V.; Klingenberg, C.; Naver, L.; Nordberg, V.; Berardi, A.; El Helou, S.; Fusch, G.; Bliss, J.M.; Lehnick, D.; Guerina, N.; et al. Antibiotic exposure for culture-negative early-onset sepsis in late-preterm and term newborns: An international study. Pediatr. Res. 2024, 1–7. [Google Scholar] [CrossRef]
- Kouni, S.; Tsolia, M.; Roilides, E.; Dimitriou, G.; Tsiodras, S.; Skoutelis, A.; Kourkouni, E.; Gkentzi, D.; Iosifidis, E.; Spyridis, N.; et al. Establishing nationally representative central line-associated bloodstream infection surveillance data for paediatric patients in Greece. J. Hosp. Infect. 2019, 101, 53–59. [Google Scholar] [CrossRef]
- Dong, Y.; Glaser, K.; Speer, C.P. Late-onset sepsis caused by Gram-negative bacteria in very low birth weight infants: A systematic review. Expert. Rev. Anti-Infect. Ther. 2019, 17, 177–188. [Google Scholar] [CrossRef]
- Harrison, M.L.; Dickson, B.F.R.; Sharland, M.; Williams, P.C.M. Beyond Early- and Late-onset Neonatal Sepsis Definitions: What are the Current Causes of Neonatal Sepsis Globally? A Systematic Review and Meta-analysis of the Evidence. Pediatr. Infect. Dis. J. 2024, 43, 1182–1190. [Google Scholar] [CrossRef]
- Oldendorff, F.; Nordberg, V.; Giske, C.G.; Naver, L. A decade of neonatal sepsis in Stockholm, Sweden: Gram-positive pathogens were four times as common as Gram-negatives. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 959–968. [Google Scholar] [CrossRef]
- Kostlin-Gille, N.; Hartel, C.; Haug, C.; Gopel, W.; Zemlin, M.; Muller, A.; Poets, C.F.; Herting, E.; Gille, C. Epidemiology of Early and Late Onset Neonatal Sepsis in Very Low Birthweight Infants: Data From the German Neonatal Network. Pediatr. Infect. Dis. J. 2021, 40, 255–259. [Google Scholar] [CrossRef]
- Powell, J.; Beirne, I.; Minihan, B.; O’Connell, N.H.; Sharma, S.; Dunworth, M.; Philip, R.K.; Dunne, C.P. Neonatal bacteraemia in Ireland: A ten-year single-institution retrospective review. PLoS ONE 2024, 19, e0306855. [Google Scholar] [CrossRef]
| Characteristics (n 277) | No LOS (n 164) | CNLOS (n 83) | CPLOS (n 30) | p-Value |
|---|---|---|---|---|
| Prenatal steroids, n (%) | 126 (76.8) | 64 (77.1) | 22 (73.3) | 0.921 |
| Cesarean section, n (%) | 125 (76.2) | 65 (78.3) | 19 (63.3) | 0.247 |
| In-hospital delivery, n (%) | 126 (76.8) | 72 (86.7) | 26 (86.7) | 0.12 |
| DM, n (%) | 20 (12.2) | 7 (8.4) | 2 (6.7) | 0.25 |
| Infection, n (%) | 68 (41.5) | 40 (48.2) | 21 (70) | 0.006 |
| IUGR, n (%) | 31 (18.9) | 19 (22.9) | 2 (6.7) | 0.139 |
| Resuscitation at birth, n (%) | 17 (10.4) | 16 (19.3) | 13 (43.3) | <0.001 |
| No LOS (n 164) | CNLOS (n 83) | CPLOS (n 30) | p-Value | |
|---|---|---|---|---|
| Male n (%) | 73 (44.5) | 47 (54.2) | 14 (46.7) | 0.351 |
| BW (gr), mean (SD) | 1508.3 (237.5) | 1105.24 (269.65) | 916.66 (312.65) | <0.001 |
| BW < 1500 gr, n (%) | 85 (51.8) | 81 (97.5) | 28 (93.3) | |
| BW < 1000 gr, n (%) | 4 (2.4) | 35 (42.2) | 22 (73.3) | |
| GA (wks), mean (SD) | 31.24 (1.5) | 28.5 (2.52) | 26.93 (2.43) | |
| GA < 29 wks, n (%) | 9 (5.5) | 47 (56.6) | 23 (76.7) | <0.001 |
| Neonatal course | ||||
| CRIB ΙΙ median (ΙQR) | 3 (2–4) | 8 (4–10) | 10 (8.75–13.25) | <0.001 |
| ΤPΝ, n (%) | 155 (94.5) | 83 (100) | 30 (100) | 0.041 |
| TPN duration in days, median (ΙQR) | 6 (5–8) | 12 (10–18) | 19 (12.75–28) | <0.001 |
| Full enteral feeding in days, median (ΙQR) | 9 (8–10) | 14 (10–18.5) | 21 (15–29) | <0.001 |
| Umbilical catheter, n (%) | 74 (45.1) | 68 (81.9) | 29 (96.6) | <0.001 |
| PICC, n (%) | 4 (2.4) | 32 (38.6) | 11 (36.7) | <0.001 |
| Possible EOS, n (%) | 47 (28.65) | 53 (63.9) | 27 (90) | <0.001 |
| DOT1, mean (SD) | 14.15 (3.35) | 14.89 (3.31) | 14.4 (3.64) | 0.182 |
| ASI/AD-1, mean (SD) | 6.5 (1.04) | 6.25 (1.1) | 5.87 (5.5) | 0.045 |
| O2 therapy duration in days, median (ΙQR) | 4 (2–9) | 48 (14–73) | 60 (13.75–114) | <0.001 |
| MV, n (%) | 56 (34.14) | 54 (65.1) | 27 (90) | <0.001 |
| MV duration in days, median (ΙQR) | 1 (1–3) | 5 (2–14) | 6 (3–35) | <0.001 |
| RDS, n (%) | 108 (65.9) | 80 (96.4) | 30 (100) | <0.001 |
| PDA, n (%) | 7 (4.3) | 17 (20.5) | 11 (36.7) | <0.001 |
| Jaundice n (%) | 156 (95.1) | 81 (97.6) | 30 (100) | 0.329 |
| Duration of phototherapy in days, mean (SD) | 2.4 (1.28) | 3.52 (1.77) | 4.4 (1.9) | <0.001 |
| Apnea of prematurity, n (%) | 41 (25) | 65 (78.3) | 25 (83.3) | <0.001 |
| Need for PRBC transfusions, n (%) | 86 (52.4) | 82 (98.8) | 30 (100) | <0.001 |
| Neonatal outcome | ||||
| IVH (I-IV), n (%) | 25 (15.2) | 24 (28.9) | 13 (43.3) | <0.001 |
| PVL, n (%) | 18 (11) | 31 (37.35) | 15 (50) | <0.001 |
| BPD, n (%) | 18 (11) | 51 (61.4) | 19 (63.3) | <0.001 |
| ROP (≥ III), n (%) | 5 (3.1) | 15 (18.1) | 10 (40) | <0.001 |
| ΕUGR, n (%) | 46 (28.5) | 25 (31.6) | 11 (52.38) | 0.197 |
| Hospitalization in days, median (ΙQR) | 28 (23–39) | 63 (43–85) | 69.5 (33–121.3) | <0.001 |
| Mortality, n (%) | 3 (1.8) | 4 (4.8) | 9 (30) | <0.001 |
| CNLOS (n 83) | CPLOS (n 30) | p-Value | |
|---|---|---|---|
| Number of episodes, mean (SD) | 1.57 (1.71) | 1.068 (0.25) | 0.039 |
| DOT2, mean (SD) | 20(14–33) | 20 (14–35) | 0.935 |
| ASI/AD-2, mean (SD) | 10.53 (2.57) | 11.04 (3.52) | 0.097 |
| Umblical catheter duration (d), mean (SD) | 11.96 (4.45) | 14.17 (7.33) | 0.083 |
| PICC duration (d), median (ΙQR) | 14 (9–16.5) | 14 (8–33) | 0.461 |
| MV duration (d), median (ΙQR) | 5 (2–14) | 6 (3–35) | 0.435 |
| Number of PRBC transfusions, median (ΙQR) | 3 (2–7) | 6 (4–12) | 0.009 |
| Thrombopenia, n (%) | 8 (9.6) | 12 (40) | <0.001 |
| Use of inotropes, n (%) | 28 (33.7) | 21(70) | 0.006 |
| Mortality, n (%) | 4 (4.8) | 9 (30) | <0.001 |
| Microorganism | n | % | Blood | CSF |
|---|---|---|---|---|
| Gram-positive | 12 | 36.4 | 12 | 0 |
| 12 | 36.4 | 12 | 0 |
| Staphylococcus epidermidis | 9 | 27.3 | ||
| Staphylococcus haemoliticus | 1 | 3.0 | ||
| Staphylococcus warneri | 2 | 6.1 | ||
| Gram-negative | 14 | 42.4 | ||
| 3 | 9.1 | 3 | 0 |
| 2 | 6.1 | 1 | 1 |
| 2 | 6.1 | 2 | 0 |
| 2 | 6.1 | 2 | 0 |
| 2 | 6.1 | 2 | 0 |
| 1 | 3.0 | 1 | 0 |
| 1 | 3.0 | 1 | 0 |
| Fungi | 7 | 21.2 | ||
| 4 | 12.1 | 4 | 0 |
| 2 | 6.1 | 2 | 0 |
| 1 | 3.0 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaffe, K.; Syrogiannopoulos, G.A.; Petinaki, E.; Goudesidou, M.; Kalaitzi, A.; Gounaris, A.; Grivea, I.N. Epidemiology and Outcomes of Late-Onset Neonatal Sepsis in Preterm Infants in a Tertiary Hospital. Children 2025, 12, 532. https://doi.org/10.3390/children12050532
Kaffe K, Syrogiannopoulos GA, Petinaki E, Goudesidou M, Kalaitzi A, Gounaris A, Grivea IN. Epidemiology and Outcomes of Late-Onset Neonatal Sepsis in Preterm Infants in a Tertiary Hospital. Children. 2025; 12(5):532. https://doi.org/10.3390/children12050532
Chicago/Turabian StyleKaffe, Katerina, George A. Syrogiannopoulos, Efthimia Petinaki, Maria Goudesidou, Anna Kalaitzi, Antonios Gounaris, and Ioanna N. Grivea. 2025. "Epidemiology and Outcomes of Late-Onset Neonatal Sepsis in Preterm Infants in a Tertiary Hospital" Children 12, no. 5: 532. https://doi.org/10.3390/children12050532
APA StyleKaffe, K., Syrogiannopoulos, G. A., Petinaki, E., Goudesidou, M., Kalaitzi, A., Gounaris, A., & Grivea, I. N. (2025). Epidemiology and Outcomes of Late-Onset Neonatal Sepsis in Preterm Infants in a Tertiary Hospital. Children, 12(5), 532. https://doi.org/10.3390/children12050532






