A Systematic Review of the Oral Health Status of Hemophilic Patients
Abstract
1. Introduction
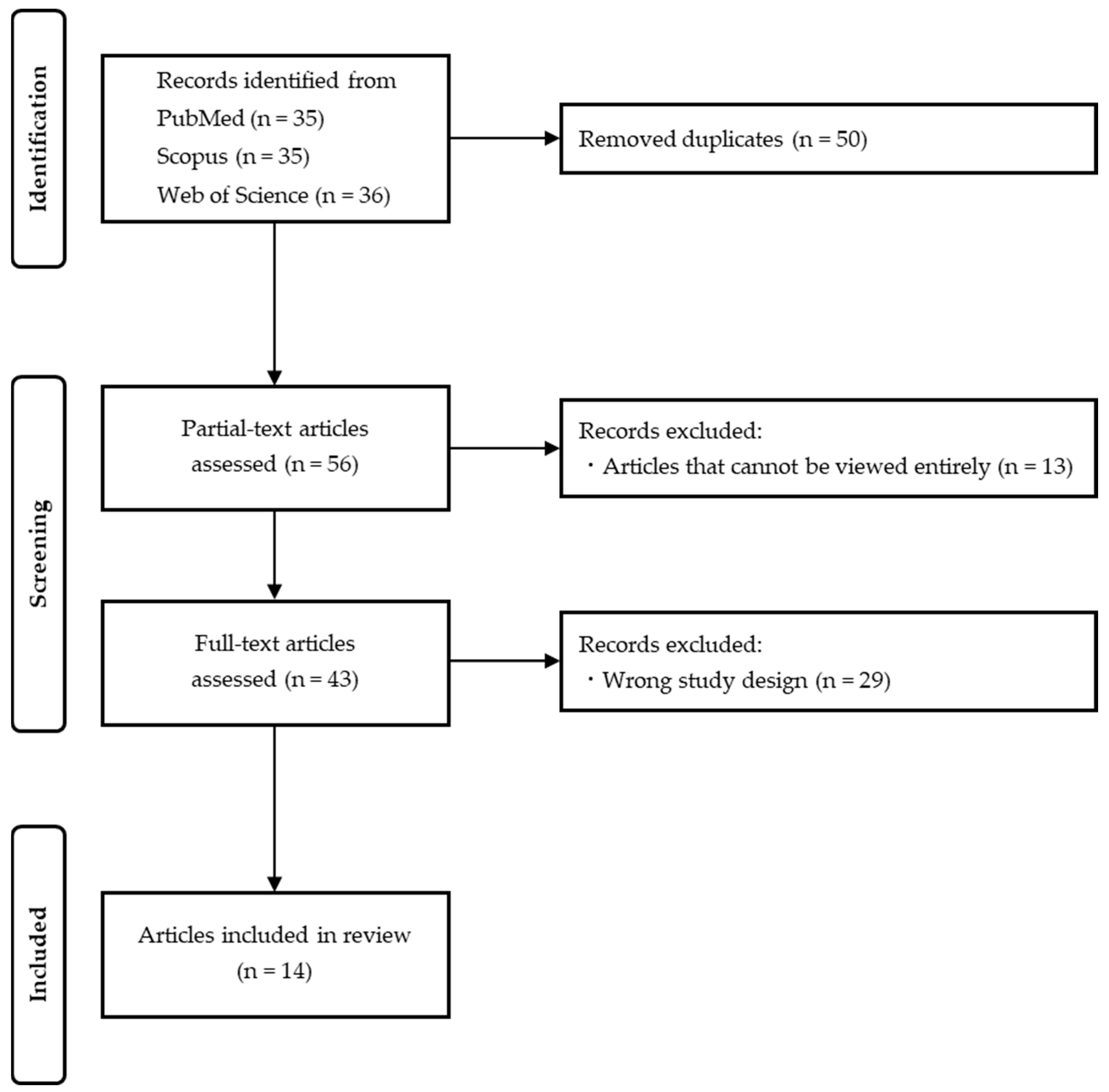
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- (P) Patients with congenital bleeding disorders, including hemophilia;
- (I) None;
- (C) Healthy control without systemic disease;
- (O) Oral health status including dental caries, periodontal diseases, and oral hygiene.
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias Assesment
3. Results
3.1. Comparison of the 14 Extracted Articles
3.2. Caries Status
3.3. Periodontal Status
3.4. Oral Hygiene Status
3.5. Risk of Bias Assessment
4. Discussion
4.1. Oral Health Status
4.2. Caries Status of Hemophilic Patients
4.3. Periodontal Status of Hemophilic Patients
4.4. Oral Hygiene Status of Hemophilic Patients
4.5. Dental Treatment of Hemophilic Patients
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bolton-Maggs, P.H.; Pasi, K.J. Haemophilias A and B. Lancet 2003, 361, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, A.C. Gene therapy for hemophilia. Hematol. Am. Soc. Hematol. Educ. Program 2022, 2022, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Brewer, A.K.; Mauser-Bunschoten, E.P.; Key, N.S.; Kitchen, S.; Llinas, A.; Ludlam, C.A.; Mahlangu, J.N.; Mulder, K.; Poon, M.C.; et al. Guidelines for the management of hemophilia. Haemophilia 2013, 19, e1–e47. [Google Scholar] [CrossRef]
- Struzycka, I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; De Stefano, J.A. Ultrasonic vs. hand instrumentation in periodontal therapy: Clinical outcomes. Periodontol. 2000 2016, 71, 113–127. [Google Scholar] [CrossRef]
- Worthington, H.V.; MacDonald, L.; Poklepovic Pericic, T.; Sambunjak, D.; Johnson, T.M.; Imai, P.; Clarkson, J.E. Home use of interdental cleaning devices, in addition to toothbrushing, for preventing and controlling periodontal diseases and dental caries. Cochrane Database Syst. Rev. 2019, 4, CD012018. [Google Scholar]
- Fiorillo, L. Oral Health: The First Step to Well-Being. Medicina 2019, 55, 676. [Google Scholar] [CrossRef]
- Rajantie, H.; Alapulli, H.; Mäkipernaa, A.; Ranta, S. Oral health care in children with haemophilia in Helsinki, Finland. Eur. Arch. Paediatr. Dent. 2013, 14, 339–343. [Google Scholar] [CrossRef]
- Scully, C.; Diz Dios, P.; Giangrande, P. Oral Care for People with Hemophilia or a Hereditary Bleeding Tendency, 2nd ed.; Treatment of Hemophilia Monograph No. 27; World Federation of Hemophilia: Montreal, QC, Canada, 2008. [Google Scholar]
- Kalsi, H.; Nanayakkara, L.; Pasi, K.J.; Bowles, L.; Hart, D.P. Access to primary dental care for patients with inherited bleeding disorders. Haemophilia 2012, 18, 510–515. [Google Scholar] [CrossRef]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020, 26 (Suppl. S6), 1–158. [Google Scholar] [CrossRef]
- Boyd, D.; Kinirons, M. Dental caries experience of children with haemophilia in Northern Ireland. Int. J. Paediatr. Dent. 1997, 7, 149–153. [Google Scholar] [CrossRef]
- Sonbol, H.; Pelargidou, M.; Lucas, V.S.; Gelbier, M.J.; Mason, C.; Roberts, G.J. Dental health indices and caries-related microflora in children with severe haemophilia. Haemophilia 2001, 7, 468–474. [Google Scholar] [CrossRef]
- Evangelista, L.M.; Lima, C.C.; Idalino, R.C.; Lima, M.D.; Moura, L.F. Oral health in children and adolescents with haemophilia. Haemophilia 2015, 21, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Jangra, B.; Goswami, M. Assessment of Dental Caries Experience and Periodontal Health Status among Children with Haemophilia in New Delhi, India—A Case Control Study. Oral Health Prev. Dent. 2017, 15, 131–137. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Training. Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook/current (accessed on 18 March 2025).
- Mielnik-Błaszczak, M. Evaluation of dentition status and oral hygiene in Polish children and adolescents with congenital haemorrhagic diatheses. Int. J. Paediatr. Dent. 1999, 9, 99–103. [Google Scholar] [CrossRef]
- Azhar, S.; Yazdanie, N.; Muhammad, N. Periodontal status and IOTN interventions among young hemophiliacs. Haemophilia 2006, 12, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Ziebolz, D.; Stühmer, C.; Hornecker, E.; Zapf, A.; Mausberg, R.F.; Chenot, J.F. Oral health in adult patients with congenital coagulation disorders-a case control study. Haemophilia 2011, 17, 527–531. [Google Scholar] [CrossRef]
- Salem, K.; Eshghi, P. Dental health and oral health-related quality of life in children with congenital bleeding disorders. Haemophilia 2013, 19, 65–70. [Google Scholar] [CrossRef]
- Othman, N.A.; Sockalingam, S.N.; Mahyuddin, A. Oral health status in children and adolescents with haemophilia. Haemophilia 2015, 21, 605–611. [Google Scholar] [CrossRef]
- Žaliūnienė, R.; Aleksejūnienė, J.; Brukienė, V.; Pečiulienė, V. Do hemophiliacs have a higher risk for dental caries than the general population? Medicina 2015, 51, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Pai, K.M.; Kurien, A.; Vineetha, R. Oral hygiene and dentition status in children and adults with hemophilia: A case-control study. Spec Care Dent. 2018, 38, 391–394. [Google Scholar]
- Kanjani, V.; Annigeri, R.G.; Hanagavadi, S.; Manjunath, M.R. Comparative analysis of oral health and treatment necessities in hemophilia individuals of Davangere population—A case control study. J. Family Med. Prim Care 2020, 9, 4774–4777. [Google Scholar]
- Kumar, M.; Pai, K.M.; Vineetha, R.; Kurien, A. Oral hygiene and dentition status in patients with congenital hemorrhagic disorders: A comparative study. Pesqui. Bras. Odontopediatria Clín. Integr. 2020, 20, e5392. [Google Scholar]
- Parvaie, P.; Shaygan Majd, H.; Ziaee, M.; Sharifzadeh, G.; Osmani, F. Evaluation of gum health status in hemophilia patients in Birjand (a case-control study). Am. J. Blood Res. 2020, 10, 54–59. [Google Scholar] [PubMed]
- Gupta, U.; Kumar, A.; Manjunath, B.C.; Aggarwal, S.; Singh, A.; Ahluwalia, R. Comparative Evaluation of the Oral Hygiene Status and Prevalence of Dental Caries in Hemophiliac and non-Hemophiliac Patients. Cardiometry 2022, 25, 1326–1331. [Google Scholar]
- Czajkowska, S.; Rupa-Matysek, J.; Gil, L.; Surdacka, A. Assessment of Oral Health and Healthy Habits in Adult Patients with Congenital Hemophilia. Eur J. Dent. 2023, 17, 161–172. [Google Scholar] [PubMed]
- Sharma, S.; Shahi, A.K.; Chandra, S.; Abdul, N.S.; Singh, B.; Singh, R.; Shivakumar, G.C. State of Dental Health and Management Needs of Young Hemophilic Patients: A Case-control Study. Int. J. Clin. Pediatr. Dent. 2023, 16, 380–387. [Google Scholar]
- Acar, G.; Aktaş, A. Assessment of jaw bone mineral density, resorption rates, and oral health in patients with severe hemophilia: A case-control study. Acta Odontol. Scand. 2024, 83, 132–139. [Google Scholar]
- Furuta, M.; Yamashita, Y. Oral Health and Swallowing Problems. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 216–222. [Google Scholar]
- Müller, L.K.; Jungbauer, G.; Jungbauer, R.; Wolf, M.; Deschner, J. Biofilm and Orthodontic Therapy. Monogr. Oral Sci. 2021, 29, 201–213. [Google Scholar] [PubMed]
- Andari, S.E.; Ghandour, L.A.; Chaaya, M.; Ghafari, J.G. Oral health status in a Lebanese geriatric population. East Mediterr. Health J. 2022, 28, 425–433. [Google Scholar] [PubMed]
- Patel, A.S.; Jalihal, S.; Ankola, A.V.; Santhosh, V.N.; Ragu, K.; Thakker, J.; Coutinho, D.; Kabra, L. Dental caries, oral hygiene status and deleterious habits among migrant construction workers of Belagavi, India. J. Prev. Med. Hyg. 2024, 65, E65–E72. [Google Scholar]
- Banihashem Rad, S.A.; Esteves-Oliveira, M.; Maklennan, A.; Douglas, G.V.A.; Castiglia, P.; Campus, G. Oral health inequalities in immigrant populations worldwide: A scoping review of dental caries and periodontal disease prevalence. BMC Public Health 2024, 24, 1968. [Google Scholar] [CrossRef]
- Govindaraju, L.; Gurunathan, D. Comparison of the Oral Hygiene Status in Children With and Without Juvenile Diabetes—A Comparative Study. Indian J. Dent. Res. 2023, 34, 410–412. [Google Scholar] [PubMed]
- de Castilho, A.R.F.; Mialhe, F.L.; de Barbosa, T.S.; Puppin-Rontani, R.M. Influence of Family Environment on Children’s Oral Health: A Systematic Review. J. Pediatr. (Rio J.) 2013, 89, 116–123. [Google Scholar] [CrossRef]
- Karamehmedovic, E.; Bajric, E.; Virtanen, J.I. Oral Health Behaviour of Nine-Year-Old Children and Their Parents in Sarajevo. Int. J. Environ. Res. Public Health 2021, 18, 3235. [Google Scholar] [CrossRef]
- Olak, J.; Nguyen, M.S.; Nguyen, T.T.; Nguyen, B.B.T.; Saag, M. The Influence of Mothers’ Oral Health Behaviour and Perception Thereof on the Dental Health of Their Children. EPMA J. 2018, 9, 187–193. [Google Scholar]
- Arora, A.; Nargundkar, S.; Fahey, P.; Joshua, H.; John, J.R. Social Determinants and Behavioural Factors Influencing Toothbrushing Frequency among Primary School Children in Rural Australian Community of Lithgow, New South Wales. BMC Res. Notes 2020, 13, 403. [Google Scholar]
- Touger-Decker, R.; van Loveren, C. Sugars and dental caries. Am. J. Clin. Nutr. 2003, 78, 881S–892S. [Google Scholar]
- Amato, J.N.; de Sousa Eskenazi, E.M.; Massaoka, C.; de Araújo de Assis, C.R.; Castelo, P.M. Relation between caries experience and the consumption of sweetened drinks and processed food in children: A population-based study. Int. J. Dent. Hyg. 2023, 21, 561–568. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Oral Health Survey: Basic Methods, 4th ed.; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Verrusio, C.; Iorio-Siciliano, V.; Blasi, A.; Leuci, S.; Adamo, D.; Nicolò, M. The effect of orthodontic treatment on periodontal tissue inflammation: A systematic review. Quintessence Int. 2018, 49, 69–77. [Google Scholar]
- Gasner, N.S.; Schure, R.S. Periodontal Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Solomon, S.M.; Timpu, D.; Forna, D.A.; Stefanache, M.A.; Martu, S.; Stoleriu, S. AFM comparative study of root surface morphology after three methods of scaling. Mater. Plast. 2016, 53, 546–549. [Google Scholar]
- Zaliuniene, R.; Peciuliene, V.; Brukiene, V.; Aleksejuniene, J. Hemophilia and oral health. Stomatologija 2014, 16, 127–131. [Google Scholar] [PubMed]
- Thornhill, M.H.; Gibson, T.B.; Durkin, M.J.; Dayer, M.J.; Lockhart, P.B.; O′Gara, P.T.; Baddour, L.M. Prescribing of antibiotic prophylaxis to prevent infective endocarditis. J. Am. Dent. Assoc. 2020, 151, 835–845.e31. [Google Scholar] [CrossRef] [PubMed]
- Farrkh, A.; Garrison, E.; Closmann, J.J. Dental surgical management of the patient with hemophilia. Gen. Dent. 2016, 64, 14–17. [Google Scholar]
- Raso, S.; Napolitano, M.; Sirocchi, D.; Siragusa, S.; Hermans, C. The important impact of dental care on haemostatic treatment burden in patients with mild haemophilia. Haemophilia 2022, 28, 996–999. [Google Scholar] [CrossRef]
- Hoang, T.; Dowdy, R.A.E. Review of Inherited Coagulation Disorders. Anesth. Prog. 2024, 71, 87–95. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
|
|
|
|
| Author | Bleeding Diathesis Group | Control Group | Evaluation Items |
|---|---|---|---|
| Mielnik-Błaszczak M, 1999 [18] | Male patients aged 4–18 years (n = 80) Hemophilia A (n = 70) Hemophilia B (n = 7) von Willebrand’s disease (n = 3) | Male patients aged 4–18 years (n = 80) | Caries status Oral hygiene status |
| Azhar S, 2006 [19] | Hemophilic volunteers suffering from severe form of disease, i.e., clotting factor level < 2% and dependent on transfusions in case of surgery (n = 52) Average age was 16.6 (SD ± 3.2) years | Controls with age and gender matched (n = 192) Average age was 16.7 (SD ± 3.0) years | Caries status Peridontal status |
| Ziebolz D, 2011 [20] | Patients suffering from congenital coagulation disease (n = 15) Hemophilia A (n = 8) von Willebrand’s disease (n = 7) Average age was 39.2 (SD ± 8.3) years | Healthy patients receiving routine dental check-up were selected randomly as controls (n = 31) Average age was 36.4 (SD ± 9.6) years | Caries status Peridontal status Oral hygiene status |
| Salem K, 2013 [21] | Patients with congenital bleeding disorders aged 2–15 years (n = 46) Average age was 7.6 (SD ± 4.2) years | Children in same age and gender demographics were selected as control group (n = 46) Average age was 7.5 (SD ± 3.4) years | Caries status Oral hygiene status |
| Othman NA, 2015 [22] | Hemophilia patients aged 7–16 years with no systemic disease other than haemophilia (n = 50) Hemophilia A (n = 41) Hemophilia B (n = 8) Other type of Hemophilia (n = 1) Average age was 11.7 (SD ± 0.4) years | Control subjects were selected during oral health screening program and were matched in terms of age (n = 50) Average age was 12.0 (SD ± 0.2) years | Caries status Peridontal status Oral hygiene status |
| Žaliūnienė R, 2015 [23] | Patients 4 years or older listed in register of hemophilia patients (n = 76) | Control group was chosen from general population by randomly selecting subjects (n = 79) | Caries status Oral hygiene status |
| Kumar M, 2018 [24] | Patients diagnosed with hemophilia (n = 100) Hemophilia A (n = 86) Hemophilia B (n = 14) Average age was 20.0 years | Age-matched volunteers (n = 100) Average age was 20.1 years | Caries status Oral hygiene status |
| Kanjani V, 2020 [25] | Patients who had registered with Hemophilia Society (n = 50) Hemophilia A (n = 36) Hemophilia B (n = 14) Average age was 17.7 (SD ± 10.5) years | Healthy individuals matched with hemophilic individuals in terms of age and gender (n = 50) Average age was 17.7 (SD ± 10.5) years | Caries status Oral hygiene status |
| Kumar M, 2020 [26] | Patients diagnosed with hemophilia (A or B) who were registered with Hemophilia Society (n = 11) Average age was 19.4 years | Gender-matched healthy volunteers (n = 11) Average age was 19.5 years | Caries status Oral hygiene status |
| Parvaie P, 2020 [27] | Group selected by non-probability sampling method from patients with hemophilia referred to hemophilia center (n = 89) Hemophilia A (n = 73) Hemophilia B (n = 12) Other type of Hemophilia (n = 4) Average age was 26.6 (SD ± 14.8) years | Patients referred to dental clinic who were matched for age, sex (n = 89) Average age was 27.5 (SD ± 15.1) years | Periodontal status |
| Gupta U, 2022 [28] | Subjects with hemophilia aged 7 to 30 years (n = 300) Hemophilia A (n = 269) Hemophilia B (n = 31) Average age was 18.5 (SD ± 6.2) years | Controls recruited from among relatives who came along with hemophilic patients (n = 300) Average age was 19.2 (SD ± 6.1) years | Caries status Oral hygiene status |
| Czajkowska S, 2023 [29] | Patients, aged between 18 and 70 years, in whom congenital hemophilia A or B was diagnosed (n = 77) Hemophilia A (n = 64) Hemophilia B (n = 13) Mean age was 35 years | Control group consisted of healthy volunteers, matched according to age and gender (n = 50) Mean age was 29.5 years | Caries status Peridontal status Oral hygiene status |
| Sharma S, 2023 [30] | Young male individuals suffering from hemophilia and registered with Hemophilia Society (n = 200) | Young, healthy male individuals matched with case group with respect to age and gender (n = 200) | Caries status Oral hygiene status |
| Acar G, 2024 [31] | Patients diagnosed with severe hemophilia A or B (n = 48) Hemophilia A (n = 38) Hemophilia B (n = 10) Average age was 37.6 years | Control group of 49 individuals with same characteristics but without systemic diseases (n = 49) Average age was 42.0 years | Caries status Peridontal status Oral hygiene status |
| Author | Results | Quick Summary Compared To Control Group | ||
|---|---|---|---|---|
| Caries Status | Periodondal Status | Oral Hygiene Status | ||
| Mielnik-Błaszczak M, 1999 [18] | No statistically significant differences were found in caries severity between the sick and healthy children. The value of OHI was significantly lower in in the sick group. | ! | Not listed | − |
| Azhar S, 2006 [19] | The DMFT value was higher in the sick group. A high proportion of patients showed inflammation compared to controls when their periodontal disease was assessed using the MGI. | − | − | Not listed |
| Ziebolz D, 2011 [20] | The median DMFT values of patients and healthy controls were not significantly different. There was a statistically significant difference in periodontal bone loss, but the observed difference is not clinically meaningful. Patients had significantly better oral hygiene (modified Quigley–Hein Index). | ! | ! | + |
| Salem K, 2013 [21] | Patients were significantly more caries-free, with less decayed teeth in primary-permanent dentition. There was no significant difference in the value of OHI-S. | + | Not listed | ! |
| Othman NA, 2015 [22] | No significant difference was found between haemophilia patients and controls for both primary (dft, dt, ft) and permanent (DMFT, DT, FT) teeth. The mean MGI for haemophilia patients was significantly lower than in controls. Although no significant difference was found in OHI-S, a significantly higher proportion of haemophilia patients had a better oral hygiene status compared to the controls. | ! | + | + |
| Žaliūnienė R, 2015 [23] | In the deciduous dentition, the overall caries experience (dft) significantly statistically differed between the hemophilic patients (2.6 ± 2.6) and their matched healthy controls (6.1 ± 2.5). Although the mean and SD of dental plaque levels were higher in children with hemophilia, this difference was not statistically significant. On the other hand, hemophilic adults had significantly higher dental plaque levels compared to the control subjects. | + | Not listed | ! |
| Kumar M, 2018 [24] | The mean dmft/DMFT scores were exactly the same for both the groups and not statistically significant. There was a statistically significant difference in OHI-S scores, with the hemophilic subjects exhibiting a poorer oral hygiene status when compared to the healthy group. | ! | Not listed | − |
| Kanjani V, 2020 [25] | No significant distinction in DMFT was observed between the groups. When oral hygiene status was compared, a fair oral hygiene status was found in both hemophilic and healthy individuals. | ! | Not listed | ! |
| Kumar M, 2020 [26] | The DMFT score did not vary significantly between the groups. Higher OHI-S scores and a poor oral hygiene status were observed more in the hemophilia group than in the healthy controls. | ! | Not listed | − |
| Parvaie P, 2020 [27] | Although the mean of the MGI and the Periodontal Index were higher in hemophilic patients than in healthy individuals, this difference was not statistically significant. | Not listed | − | Not listed |
| Gupta U, 2022 [28] | The caries prevalence was higher in hemophilic patients than in controls, and the DMFT score was significantly higher in those with hemophilia. The mean debris, calculus, and overall OHI score were significantly higher in those with hemophilia. | − | Not listed | − |
| Czajkowska S, 2023 [29] | The incidence of dental caries in patients with hemophilia was higher compared to that of healthy patients. The BOP score in hemophilia patients was higher, which shows a significant difference. A comparison regarding oral hygiene status based on the Approximal Plaque Index showed that the oral hygiene status of hemophilia patients was poor. | − | − | − |
| Sharma S, 2023 [30] | Hemophilic people had a considerably greater incidence of dental caries. In addition, their DMFT/DEFT and OHI-S scores were barely poorer than those of healthy people. | − | Not listed | − |
| Acar G, 2024 [31] | No significant difference was found between the patient and control groups in terms of the DMFT. The GI and gingival bleeding time index scores, which indicate the inflammatory response of the periodontium, were found to be significantly higher in the patient group with hemophilia than in the healthy control group. In addition, patients with hemophilia had significantly higher DI-S, CI-S, and OHI-S scores than those of the control group. | ! | − | − |
| Study | Risk of Bias Domains | ||||
|---|---|---|---|---|---|
| Selection Bias | Performance Bias | Detection Bias | Reporting Bias | Attrition Bias | |
| Mielnik-Błaszczak M, 1999 [18] | ! | + | + | + | + |
| Azhar S, 2006 [19] | ! | ! | − | + | + |
| Ziebolz D, 2011 [20] | − | + | + | + | + |
| Salem K, 2013 [21] | ! | + | + | + | + |
| Othman NA, 2015 [22] | ! | ! | + | + | + |
| Žaliūnienė R, 2015 [23] | + | + | + | ! | − |
| Kumar M, 2018 [24] | ! | ! | ! | + | + |
| Kanjani V, 2020 [25] | ! | ! | ! | + | + |
| Kumar M, 2020 [26] | − | ! | ! | + | + |
| Parvaie P, 2020 [27] | + | + | + | ! | + |
| Gupta U, 2022 [28] | + | + | + | + | + |
| Czajkowska S, 2023 [29] | + | + | ! | + | + |
| Sharma S, 2023 [30] | + | + | + | + | + |
| Acar G, 2024 [31] | ! | + | ! | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akitomo, T.; Kaneki, A.; Mitsuhata, C.; Nomura, R. A Systematic Review of the Oral Health Status of Hemophilic Patients. Children 2025, 12, 490. https://doi.org/10.3390/children12040490
Akitomo T, Kaneki A, Mitsuhata C, Nomura R. A Systematic Review of the Oral Health Status of Hemophilic Patients. Children. 2025; 12(4):490. https://doi.org/10.3390/children12040490
Chicago/Turabian StyleAkitomo, Tatsuya, Ami Kaneki, Chieko Mitsuhata, and Ryota Nomura. 2025. "A Systematic Review of the Oral Health Status of Hemophilic Patients" Children 12, no. 4: 490. https://doi.org/10.3390/children12040490
APA StyleAkitomo, T., Kaneki, A., Mitsuhata, C., & Nomura, R. (2025). A Systematic Review of the Oral Health Status of Hemophilic Patients. Children, 12(4), 490. https://doi.org/10.3390/children12040490





