Analysis of Pathogens in Respiratory Tract Infections and Their Effect on Disease Severity: Retrospective Data from a Tertiary Care German Children’s Hospital
Abstract
1. Introduction
1.1. Respiratory Infections
1.2. Epidemiological Background
2. Materials and Methods
2.1. Study Population
2.2. Applied Materials
2.3. Statistics
3. Results
3.1. Epidemiological Surveillance
3.1.1. Study Population
3.1.2. Incidence of the Most Common Pathogens in Childhood
3.1.3. Pathogen Incidence and Seasonality
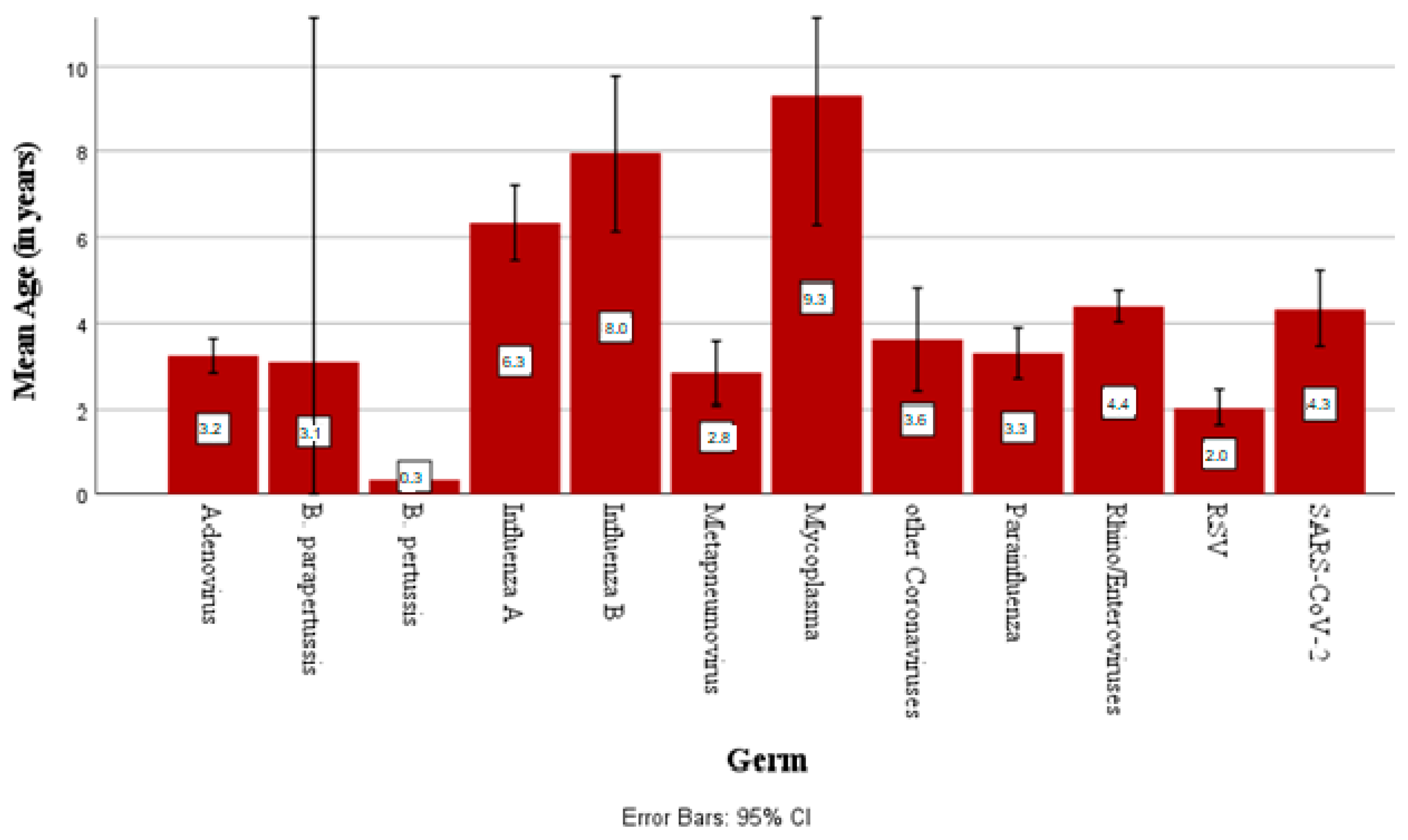
3.1.4. Pathogen Incidence by Age
3.2. Statistical Associations Between Parameters
3.2.1. Association Between Length of Stay and Pathogen Detection
3.2.2. Association of Oxygen Demand with Pathogen Detection
3.2.3. Association of Oxygen Demand with Duration of Inpatient Stay
3.2.4. Association of CRP Values with Pathogen Detection
3.2.5. Associations with Comorbidities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRP | C-reactive protein |
| CATREG | Regression go categorical data |
| CI | Confidence interval |
| e.g. | Exempli gratia, for example |
| LTIs | Lower respiratory tract infections |
| MERS | Middle East Respiratory Syndrome |
| mg/L | Milligrams per liter |
| PCR | Polymerase chain reaction |
| RSV | Respiratory syncytial virus |
| RTIs | Respiratory tract infections |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| UTIs | Upper respiratory tract infections |
References
- Zhu, G.; Xu, D.; Zhang, Y.; Wang, T.; Zhang, L.; Gu, W.; Shen, M. Epidemiological characteristics of four common respiratory viral infections in children. Virol. J. 2021, 18, 10. [Google Scholar] [CrossRef]
- Caballero, M.T.; Bianchi, A.M.; Nuno, A.; Ferretti, A.J.P.; Polack, L.M.; Remondino, I.; Rodriguez, M.G.; Orizzonte, L.; Vallone, F.; Bergel, E.; et al. Mortality Associated with Acute Respiratory Infections Among Children at Home. J. Infect. Dis. 2019, 219, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.F.; Da Silveira, H.L.; Queiroz, D.A.O.; Mantese, O.C.; Yokosawa, J. Respiratory virus infections in hospitalized and non-hospitalized children: Determinants of severe course of the disease. J. Infect. Dev. Ctries. 2022, 16, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Buttrini, M.; Farina, B.; Montecchini, S.; De Conto, F.; Chezzi, C. Respiratory Tract Infections and Laboratory Diagnostic Methods: A Review with A Focus on Syndromic Panel-Based Assays. Microorganisms 2022, 10, 1856. [Google Scholar] [CrossRef]
- Buchholz, U.; Lehfeld, A.S.; Tolksdorf, K.; Cai, W.; Reiche, J.; Biere, B.; Durrwald, R.; Buda, S. Respiratory infections in children and adolescents in Germany during the COVID-19 pandemic. J. Health Monit. 2023, 8, 20–38. [Google Scholar] [CrossRef]
- Ruan, Z.; Qi, J.; Qian, Z.M.; Zhou, M.; Yang, Y.; Zhang, S.; Vaughn, M.G.; LeBaige, M.H.; Yin, P.; Lin, H. Disease burden and attributable risk factors of respiratory infections in China from 1990 to 2019. Lancet Reg. Health West. Pac. 2021, 11, 100153. [Google Scholar] [CrossRef]
- WHO. Children Aged <5 Years with Acute Respiratory Infection Symptoms Taken to Facility (%). Available online: www.who.int/data/gho/indicator-metadata-registry/imr-details (accessed on 19 January 2025).
- Price, R.H.M.; Graham, C.; Ramalingam, S. Association between viral seasonality and meteorological factors. Sci. Rep. 2019, 9, 929. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Gavenciak, T.; Monrad, J.T.; Leech, G.; Sharma, M.; Mindermann, S.; Bhatt, S.; Brauner, J.; Kulveit, J. Seasonal variation in SARS-CoV-2 transmission in temperate climates: A Bayesian modelling study in 143 European regions. PLoS Comput. Biol. 2022, 18, e1010435. [Google Scholar] [CrossRef]
- Ljubin-Sternak, S.; Mestrovic, T.; Luksic, I.; Mijac, M.; Vranes, J. Seasonal Coronaviruses and Other Neglected Respiratory Viruses: A Global Perspective and a Local Snapshot. Front. Public Health 2021, 9, 691163. [Google Scholar] [CrossRef]
- Kramer, A.; Assadian, O.; Exner, M.; Hübner, N.O.; Simon, A. Infektionsschutz und spezielle Hygienemaßnahmen in klinischen Disziplinen. Krankenh-. Praxishyg. 2016, 337–549. [Google Scholar] [CrossRef]
- Assane, D.; Makhtar, C.; Abdoulaye, D.; Amary, F.; Djibril, B.; Amadou, D.; Niokhor, D.J.B.; Amadou, D.; Cheikh, L.; Ndongo, D.; et al. Viral and Bacterial Etiologies of Acute Respiratory Infections Among Children Under 5 Years in Senegal. Microbiol. Insights 2018, 11, 1178636118758651. [Google Scholar] [CrossRef] [PubMed]
- Akkoc, G.; Dogan, C.; Bayraktar, S.; Sahin, K.; Elevli, M. Evaluation of viral respiratory pathogens in children aged under five hospitalized with lower respiratory tract infections. North. Clin. Istanb. 2022, 9, 162–172. [Google Scholar] [CrossRef] [PubMed]
- RKI. Ausbrüche und Ausbruchsfälle mit COVID-19, Influenza und RSV-Infektionen—Ein Rückblick auf die Saison. Epidemiol. Bull. 2024, 39. Available online: https://edoc.rki.de/handle/176904/12264 (accessed on 27 March 2025).
- Unnewehr, M.; Kolditz, M.; Windisch, W.; Schaaf, B. Biomarkers in Diagnosis, Treatment and Prognosis of Infectious Lung Diseases. Pneumologie 2018, 72, 341–346. [Google Scholar] [CrossRef]
- Florin, T.A.; Ambroggio, L.; Brokamp, C.; Zhang, Y.; Rattan, M.; Crotty, E.; Belsky, M.A.; Krueger, S.; Epperson, T.N.t.; Kachelmeyer, A.; et al. Biomarkers and Disease Severity in Children with Community-Acquired Pneumonia. Pediatrics 2020, 145, e20193728. [Google Scholar] [CrossRef]
- Leber, A.L.; Everhart, K.; Daly, J.A.; Hopper, A.; Harrington, A.; Schreckenberger, P.; McKinley, K.; Jones, M.; Holmberg, K.; Kensinger, B. Multicenter Evaluation of BioFire FilmArray Respiratory Panel 2 for Detection of Viruses and Bacteria in Nasopharyngeal Swab Samples. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Rogers, B.B.; Shankar, P.; Jerris, R.C.; Kotzbauer, D.; Anderson, E.J.; Watson, J.R.; O’Brien, L.A.; Uwindatwa, F.; McNamara, K.; Bost, J.E. Impact of a rapid respiratory panel test on patient outcomes. Arch. Pathol. Lab. Med. 2015, 139, 636–641. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Brydges, C.R. Effect Size Guidelines, Sample Size Calculations, and Statistical Power in Gerontology. Innov. Aging 2019, 3, igz036. [Google Scholar] [CrossRef]
- Kim, T.K.; Park, J.H. More about the basic assumptions of t-test: Normality and sample size. Korean J. Anesthesiol. 2019, 72, 331–335. [Google Scholar] [CrossRef]
- Thabane, L.; Mbuagbaw, L.; Zhang, S.; Samaan, Z.; Marcucci, M.; Ye, C.; Thabane, M.; Giangregorio, L.; Dennis, B.; Kosa, D.; et al. A tutorial on sensitivity analyses in clinical trials: The what, why, when and how. BMC Med. Res. Methodol. 2013, 13, 92. [Google Scholar] [CrossRef]
- Giardina, F.A.M.; Piralla, A.; Ferrari, G.; Zavaglio, F.; Cassaniti, I.; Baldanti, F. Molecular Epidemiology of Rhinovirus/Enterovirus and Their Role on Cause Severe and Prolonged Infection in Hospitalized Patients. Microorganisms 2022, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- McMorrow, M.L.; Moline, H.L.; Toepfer, A.P.; Halasa, N.B.; Schuster, J.E.; Staat, M.A.; Williams, J.V.; Klein, E.J.; Weinberg, G.A.; Clopper, B.R.; et al. Respiratory Syncytial Virus-Associated Hospitalizations in Children <5 Years: 2016–2022. Pediatrics 2024, 154, e2023065623. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.K.; Na, J.Y.; Kim, J.; Oh, J.W.; Kim, Y.J.; Choi, Y.J. Age-Specific Characteristics of Adult and Pediatric Respiratory Viral Infections: A Retrospective Single-Center Study. J. Clin. Med. 2022, 11, 3197. [Google Scholar] [CrossRef]
- Mariani, M.; Parodi, A.; Minghetti, D.; Ramenghi, L.A.; Palmero, C.; Ugolotti, E.; Medici, C.; Saffioti, C.; Castagnola, E. Early and Late Onset Neonatal Sepsis: Epidemiology and Effectiveness of Empirical Antibacterial Therapy in a III Level Neonatal Intensive Care Unit. Antibiotics 2022, 11, 284. [Google Scholar] [CrossRef]
- Havdal, L.B.; Boas, H.; Bekkevold, T.; Bakken Kran, A.M.; Rojahn, A.E.; Stordal, K.; Debes, S.; Dollner, H.; Nordbo, S.A.; Barstad, B.; et al. Risk factors associated with severe disease in respiratory syncytial virus infected children under 5 years of age. Front. Pediatr. 2022, 10, 1004739. [Google Scholar] [CrossRef]
- Ebner, W.; Brandis, M.; Hauer, T.; Rüden, H.; Daschner, F. Sinnvolle und nicht sinnvolle Hygienemaßnahmen in der Pädiatrie. Monatsschr. Kinderheilkd. 2000, 148, 1017–1023. [Google Scholar] [CrossRef]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L.; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am. J. Infect. Control. 2024, 35 (Suppl. S2), S65–S164. [Google Scholar] [CrossRef]
- Bhati, D.; Deogade, M.S.; Kanyal, D. Improving Patient Outcomes Through Effective Hospital Administration: A Comprehensive Review. Cureus 2023, 15, e47731. [Google Scholar] [CrossRef]
- Kunaratanapruk, S.; Silpapojakul, K. Unnecessary hospital infection control practices in Thailand: A survey. J. Hosp. Infect. 1998, 40, 55–59. [Google Scholar] [CrossRef]
- Bekhof, J.; Wessels, M.; Ten Velde, E.; Hoekstra, M.; Langenhorst, V.; Bruijnesteijn, L.; Brand, P.L.P.; Ruijs, G. Room Sharing in Hospitalized Children with Bronchiolitis and the Occurrence of Hospital-Acquired Infections: A Prospective Cohort Study. Hosp. Pediatr. 2019, 9, 415–422. [Google Scholar] [CrossRef] [PubMed]
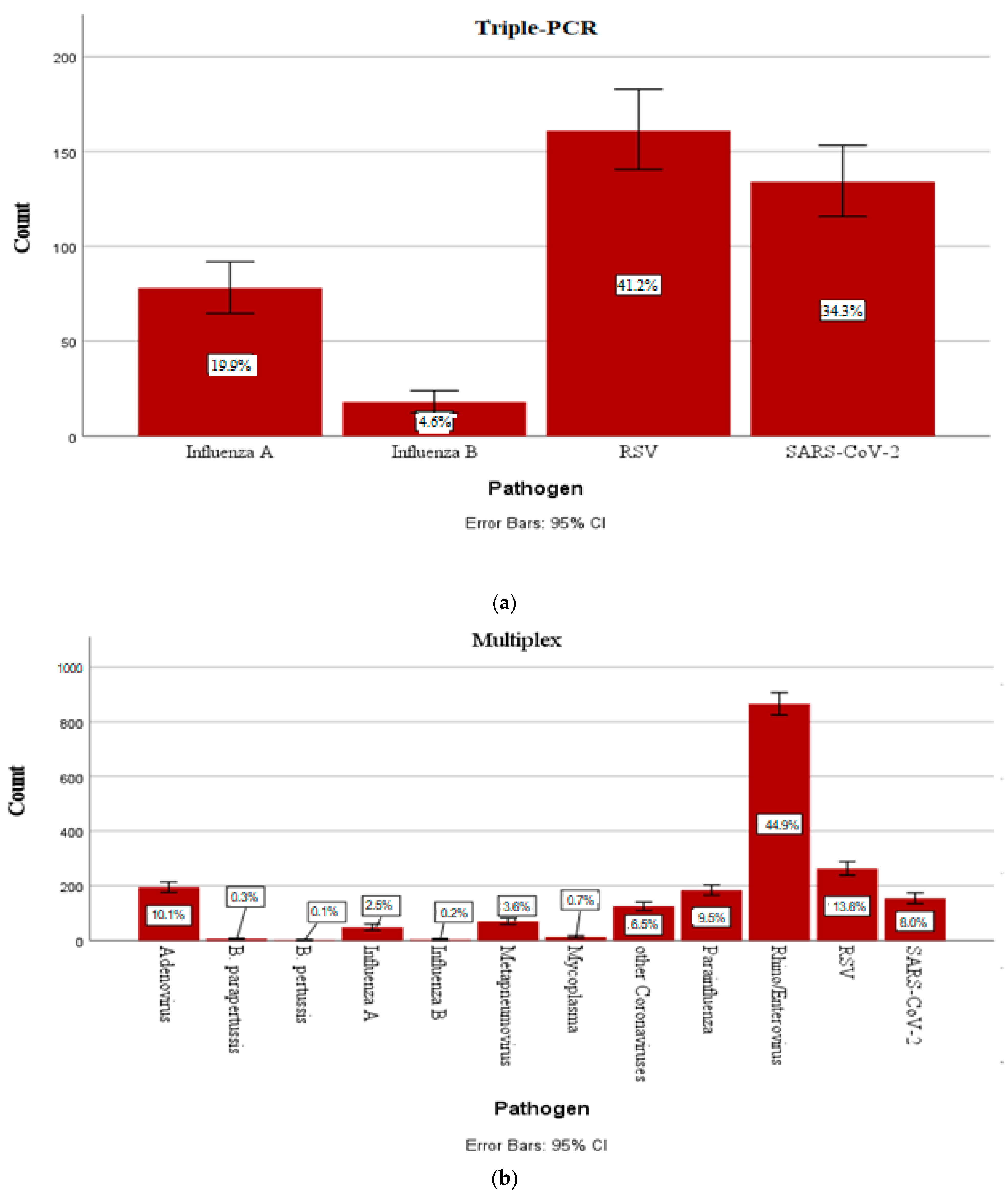

| Viruses | Influenza virus A/B, RSV, metapneumovirus, parainfluenza virus 1–4, adenovirus, coronavirus NL63/229E/OC43/HKU1, rhino/enterovirus, MERS coronavirus, SARS-CoV-2 |
| Bacteria | Mycoplasma pneumoniae, Chlamydophila pneumoniae, Bordetella pertussis, Bordetella parapertussis |
| Pathogen | Count | Mean Length of Stay (Days) | Standard Deviation (95% CI) |
|---|---|---|---|
| Adenovirus | 195 | 4.37 | 2.20 |
| Other coronaviruses | 125 | 4.22 | 2.69 |
| B. parapertussis | 6 | 5.17 | 1.72 |
| B. pertussis | 2 | 5.5 | 3.54 |
| Influenza A | 126 | 3.96 | 2.46 |
| Influenza B | 21 | 3.71 | 1.88 |
| Negative | 2564 | 8.08 | 11.88 |
| Metapneumovirus | 70 | 4.70 | 2.54 |
| Mycoplasma | 13 | 4.15 | 1.40 |
| Parainfluenza | 184 | 4.19 | 3.11 |
| Rhino/enteroviruses | 866 | 5.03 | 7.64 |
| RSV | 424 | 5.5 | 2.74 |
| SARS-CoV-2 | 288 | 5.60 | 9.04 |
| Total | 4884 | 6.54 | 9.28 |
| Pathogen Combinations | Count | Incidence Rate (%) | Mean Length of Stay in Days (95% CI) |
|---|---|---|---|
| Adenovirus/B. parapertussis | 1 | 0.3 | 8.00 |
| Adenovirus/other coronaviruses | 11 | 3.4 | 3.90 ± 1.86 |
| Adenovirus/other coronaviruses/parainfluenzavirus | 1 | 1.3 | 4.00 |
| Adenovirus/other coronaviruses/parainfluenzavirus/rhino/enterovirus/RSV | 1 | 0.3 | 7.00 |
| Adenovirus/other coronaviruses/parainfluenzavirus/rhino/enterovirus/RSV | 1 | 0.3 | 3.00 |
| Adenovirus/metapneumovirus | 1 | 0.3 | 7.00 |
| Adenovirus/metapneumovirus/rhino/enterovirus | 1 | 0.3 | 3.00 |
| Adenovirus/parainfluenzavirus | 11 | 3.4 | 4.54 ± 1.57 |
| Adenovirus/parainfluenzavirus/rhino/enterovirus | 4 | 1.2 | 2.00 |
| Adenovirus/rhino/enterovirus | 49 | 15.2 | 4.53 ± 2.12 |
| Adenovirus/rhino/enterovirus/RSV | 6 | 1.9 | 4.83 ± 2.48 |
| Adenovirus/rhino/enterovirus/SARS-CoV2 | 1 | 0.3 | 4.00 |
| Adenovirus/RSV | 3 | 0.9 | 6.33 ± 2.08 |
| Adenovirus/SARS-CoV2 | 4 | 1.2 | 3.50 ± 1.73 |
| B. parapertussis/rhino/enterovirus | 2 | 0.6 | 5.50 ± 0.70 |
| B. parapertussis/RSV | 1 | 0.3 | 4.00 |
| B. pertussis/rhino/enterovirus | 1 | 0.3 | 3.00 |
| Other coronaviruses/influenza A | 1 | 0.3 | 2.00 |
| Other coronaviruses/other coronaviruses | 1 | 0.3 | 3.00 |
| Other coronaviruses/metapneumovirus | 1 | 0.3 | 13.00 |
| Other coronaviruses/parainfluenzavirus | 2 | 0.6 | 3.00 |
| Other coronaviruses/parainfluenzavirus/rhino/enterovirus | 2 | 0.6 | 4.50 |
| Other coronaviruses/parainfluenzavirus/rhino/enterovirus/SARS-CoV2 | 1 | 0.3 | 3.00 |
| Other coronaviruses/rhino/enterovirus | 20 | 6.2 | 3.95 ± 2.06 |
| Other coronaviruses/rhino/enterovirus/RSV | 3 | 0.9 | 3.00 ± 1.00 |
| Other coronaviruses/RSV | 9 | 2.8 | 4.22 ± 2.53 |
| Other coronaviruses/SARS-CoV2 | 1 | 0.3 | 4.00 |
| Other coronaviruses/RSV | 1 | 0.3 | 3.00 |
| Influenza A/parainfluenzavirus | 1 | 0.3 | 2.00 |
| Influenza A/RSV | 9 | 2.8 | 6.33 ± 1.93 |
| Influenza A/SARS-CoV2 | 5 | 1.5 | 3.60 ± 0.89 |
| Influenza A/rhino/enterovirus | 5 | 1.5 | 3.80 ± 1.79 |
| Influenza B/SARS-CoV2 | 1 | 0.3 | 4.00 |
| Metapneumovirus/rhino/enterovirus | 7 | 2.2 | 5.86 ± 4.67 |
| Metapneumovirus/RSV | 1 | 0.3 | 6.00 |
| Metapneumovirus/SARS-CoV2 | 6 | 1.9 | 4.33 ± 1.50 |
| Mycoplasma/rhino/enterovirus | 4 | 1.2 | 3.75 ± 0.96 |
| Mycoplasma/SARS-CoV2 | 1 | 0.3 | 4.00 |
| Parainfluenzavirus/parainfluenzavirus | 1 | 0.3 | 6.00 |
| Parainfluenzavirus/rhino/enterovirus | 37 | 11.5 | 3.95 ± 2.24 |
| Parainfluenzavirus/rhino/enterovirus/RSV | 4 | 1.2 | 6.50 ± 1.91 |
| Parainfluenzavirus/rhino/enterovirus/SARS-CoV2 | 1 | 0.3 | 9.00 |
| Parainfluenzavirus/RSV | 4 | 1.2 | 4.50 ± 1.00 |
| Parainfluenzavirus/SARS-CoV2 | 2 | 0.6 | 3.50 ± 3.54 |
| Rhino/enterovirus/RSV | 71 | 22.0 | 5.94 ± 3.52 |
| Rhino/enterovirus/SARS-CoV2 | 16 | 5.0 | 3.18 ± 0.98 |
| RSV/SARS-CoV2 | 6 | 1.9 | 3.83 ± 1.83 |
| Total | 323 | 100.0 | 4.67 ± 2.62 |
| Oxygen Demand | |||
|---|---|---|---|
| Pathogen Combinations | Yes | No | Total |
| Adenovirus/other coronaviruses | 0 | 1 | 1 |
| Adenovirus/other coronaviruses/parainfluenzavirus | 0 | 1 | 1 |
| Adenovirus/metapneumovirus | 1 | 0 | 1 |
| Adenovirus/parainfluenzavirus | 0 | 3 | 3 |
| Adenovirus/parainfluenzavirus/rhino/enterovirus | 0 | 1 | 1 |
| Adenovirus/rhino/enterovirus | 1 | 9 | 10 |
| Adenovirus/rhino/enterovirus/RSV | 1 | 1 | 2 |
| B. parapertussis/rhino/enterovirus | 1 | 1 | 2 |
| B. parapertussis/RSV | 0 | 1 | 1 |
| B. pertussis/rhino/enterovirus | 0 | 1 | 1 |
| Other coronaviruses/ influenza A | 0 | 1 | 1 |
| Other coronaviruses/other coronaviruses | 0 | 1 | 1 |
| Other coronaviruses/metapneumovirus | 1 | 0 | 1 |
| Other coronaviruses/rhino/enterovirus | 0 | 2 | 2 |
| Other coronaviruses/RSV | 0 | 1 | 1 |
| Influenza A/RSV | 7 | 2 | 9 |
| Influenza A/SARS-CoV2 | 0 | 4 | 4 |
| Influenza A/rhino/enterovirus | 0 | 5 | 5 |
| Influenza B/SARS-CoV2 | 1 | 0 | 1 |
| Metapneumovirus/rhino/enterovirus | 1 | 3 | 4 |
| Metapneumovirus/RSV | 1 | 0 | 1 |
| Metapneumovirus/SARS-CoV2 | 1 | 0 | 1 |
| Parainfluenzavirus/parainfluenzavirus | 1 | 0 | 1 |
| Parainfluenzavirus/rhino/enterovirus | 1 | 15 | 16 |
| Parainfluenzavirus/rhino/enterovirus/RSV | 1 | 0 | 1 |
| Parainfluenzavirus/RSV | 2 | 0 | 2 |
| Rhino/enterovirus/RSV | 9 | 6 | 15 |
| Rhino/enterovirus/SARS-CoV2 | 0 | 2 | 2 |
| RSV/SARS-CoV2 | 1 | 2 | 3 |
| Total | 31 | 63 | 94 |
| Pathogen Combinations | Mean Max. CRP (in mg/L) 95% CI |
|---|---|
| Adenovirus/other coronaviruses | 18.50 ± 19.41 |
| Adenovirus/other coronaviruses/parainfluenzavirus | 49.0 |
| Adenovirus/metapneumovirus | 4.0 |
| Adenovirus/parainfluenzavirus | 50.42 ± 51.85 |
| Adenovirus/parainfluenzavirus/rhino/enterovirus | 11.50 ± 13.51 |
| Adenovirus/rhino/enterovirus | 41.17 ± 62.17 |
| Adenovirus/rhino/enterovirus/RSV | 62.00 ± 57.02 |
| Adenovirus/rhino/enterovirus/SARS-CoV2 | 10.20 |
| Adenovirus/RSV | 33.45 ± 4.17 |
| Adenovirus/SARS-CoV2 | 25.25 ± 29.36 |
| B. parapertussis/rhino/enterovirus | 141.00 ± 192.33 |
| B. parapertussis/RSV | 1.00 |
| Other coronaviruses/influenza A | 1.00 |
| Other coronaviruses/other coronaviruses | 1.00 |
| Other coronaviruses/parainfluenzavirus | 5.00 |
| Other coronaviruses/rhino/enterovirus | 32.29 ± 50.80 |
| Other coronaviruses/RSV | 9.00 |
| Influenza A/parainfluenzavirus | 36.00 |
| Influenza A/RSV | 40.9 ± 60.95 |
| Influenza A/SARS-CoV2 | 8.60 ± 7.37 |
| Influenza A/rhino/enterovirus | 13.80 ± 14.92 |
| Influenza B/SARS-CoV2 | 49.00 |
| Metapneumovirus/rhino/enterovirus | 12.80 ± 13.81 |
| Metapneumovirus/RSV | 32.00 |
| Metapneumovirus/SARS-CoV2 | 7.83 ± 5.27 |
| Mycoplasma/rhino/enterovirus | 59.75 ± 35.07 |
| Mycoplasma/SARS-CoV2 | 16.10 |
| Parainfluenzavirus/parainfluenzavirus | 33.00 |
| Parainfluenzavirus/rhino/enterovirus | 16.44 ± 21.75 |
| Parainfluenzavirus/rhino/enterovirusRSV | 12.60 ± 14.99 |
| Parainfluenzavirus/rhino/enterovirus/SARS-CoV2 | 12.00 |
| Parainfluenzavirus/RSV | 56.00 ± 29.70 |
| Parainnfluenzavirus/SARS-CoV2 | 2.50 ± 0.71 |
| Rhino/enterovirus/RSV | 39.21 ± 51.04 |
| Rhino/enterovirus/SARS-CoV2 | 10.04 ± 10.29 |
| RSV/SARS-CoV2 | 12.10 ± 21.31 |
| Total | 29.38 ± 45.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strempas, P.; Weberruss, H.; Bollinger, T.; Rupprecht, T. Analysis of Pathogens in Respiratory Tract Infections and Their Effect on Disease Severity: Retrospective Data from a Tertiary Care German Children’s Hospital. Children 2025, 12, 438. https://doi.org/10.3390/children12040438
Strempas P, Weberruss H, Bollinger T, Rupprecht T. Analysis of Pathogens in Respiratory Tract Infections and Their Effect on Disease Severity: Retrospective Data from a Tertiary Care German Children’s Hospital. Children. 2025; 12(4):438. https://doi.org/10.3390/children12040438
Chicago/Turabian StyleStrempas, Petros, Heidi Weberruss, Thomas Bollinger, and Thomas Rupprecht. 2025. "Analysis of Pathogens in Respiratory Tract Infections and Their Effect on Disease Severity: Retrospective Data from a Tertiary Care German Children’s Hospital" Children 12, no. 4: 438. https://doi.org/10.3390/children12040438
APA StyleStrempas, P., Weberruss, H., Bollinger, T., & Rupprecht, T. (2025). Analysis of Pathogens in Respiratory Tract Infections and Their Effect on Disease Severity: Retrospective Data from a Tertiary Care German Children’s Hospital. Children, 12(4), 438. https://doi.org/10.3390/children12040438








