Vipera Snakebite in Children: A Focus on Europe
Abstract
1. Introduction
2. Geographical and Epidemiologic Characteristics of Snakebite
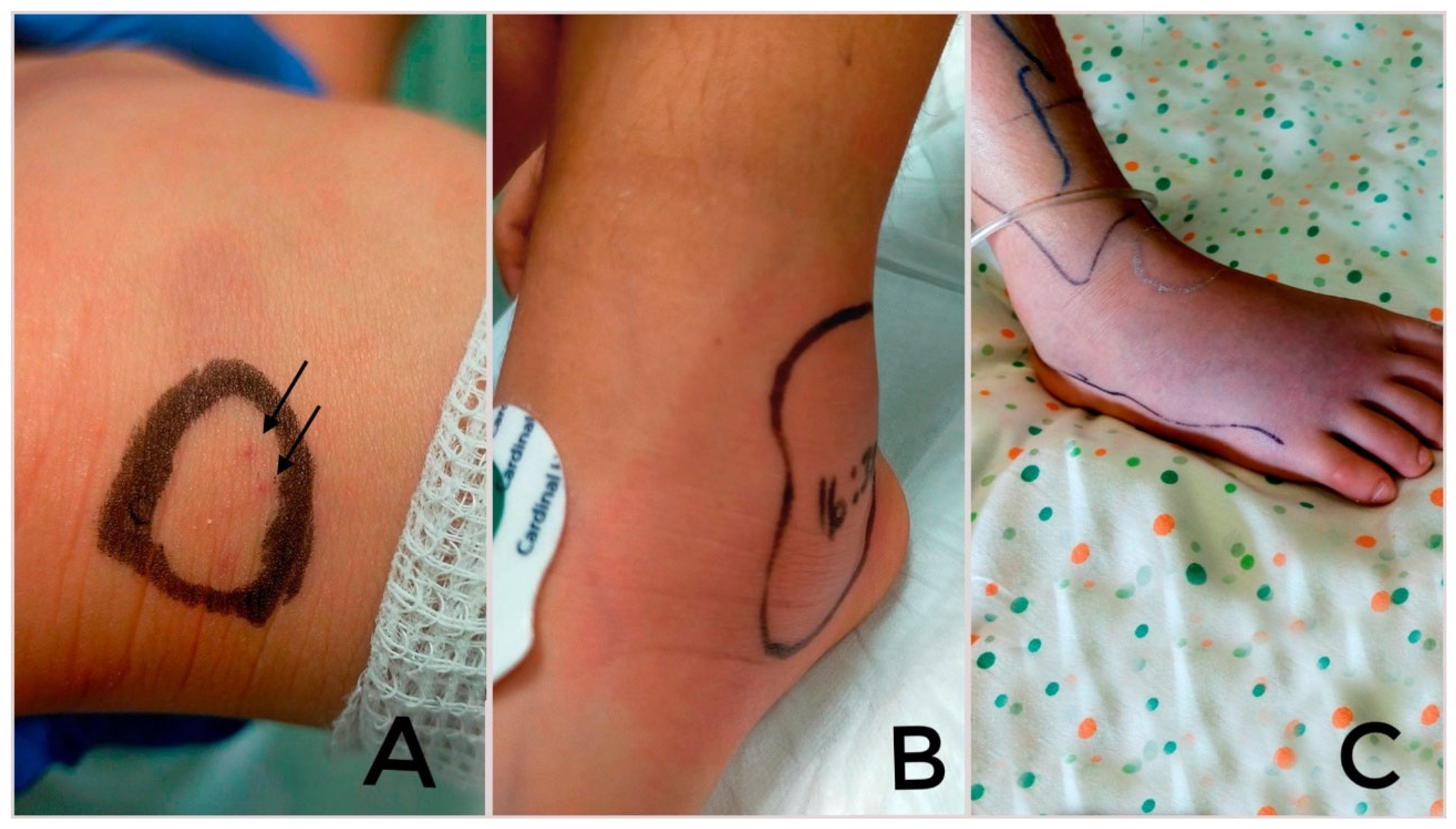
3. Pathogenesis and Clinical Characteristics of Snakebite in Children in Europe
- Phospholipase A2, responsible for hemolysis, neurotoxicity, myotoxicity, cardiotoxicity, cytotoxicity, anticoagulation, convulsions, hypotension and inflammation;
- Snake venom serine proteinases (thrombin-like enzymes) and snake venom metalloproteinases, leading to local and systemic hemorrhages, although some classes of snake venom metalloproteinases may also cause procoagulant and pro-inflammatory activities;
- Snake C-type lectin-like proteins, with anticoagulant and platelet-modulating activities;
- -
- Grade 0 (no envenoming): traces of bite and local signs.
- -
- Grade 1 (mild envenoming): local edema and absence of systemic symptoms.
- -
- Grade 2 (moderate envenoming): extensive regional edema, hypotension, vomiting, diarrhea.
- -
- Grade 3 (severe envenoming): persistent hypotension or shock, hemorrhage.
4. Long-Term Consequences of Snakebite in Children
5. Diagnosis of Snakebite Envenomation
6. Pediatric Management of Snakebite
6.1. Pre-Hospital Care
6.2. Hospital Care
6.3. Immunotherapy
6.4. Adverse Reactions to Antivenom
6.5. Ancillary Therapy
7. Pediatric Viper-Bite Cases in Europe in Literature
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| V. | Vipera |
| PLA2 | Phospholipase A2 |
| Ig | Immunoglobulin |
| LMWE | Low-molecular-weight heparin |
References
- Septelici, D.; Carbone, G.; Cipri, A.; Esposito, S. Management Strategies for Common Animal Bites in Pediatrics: A Narrative Review on the Latest Progress. Microorganisms 2024, 12, 924. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.-P.; Saz-Parkinson, Z.; Amate Blanco, J.M. Epidemiology of Snakebite in Europe: Comparison of Data from the Literature and Case Reporting. Toxicon 2013, 76, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.R.; Crevani, M.; Avella, I.; Cerullo, A.; Dorne, J.C.M.; Paolino, G.; Zattera, C. A guide to the clinical management of Vipera snakebite in Italy. Toxins 2024, 16, 255. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, M.P.; Geeta, M.G.; Krishnakumar, P.; Rajesh, T.V.; George, B. Snake bite mortality in children: Beyond bite to needle time. Arch. Dis. Child. 2017, 102, 445–449. [Google Scholar]
- Warrell, D.A. Guidelines for the Management of Snake-Bites; World Health Organization: New Delhi, India; SEARO: New Delhi, India, 2010. [Google Scholar]
- Paolino, G.; Di Nicola, M.R.; Avella, I.; Mercuri, S.R. Venomous Bites, Stings and Poisoning by European Vertebrates as an Overlooked and Emerging Medical Problem: Recognition, Clinical Aspects and Therapeutic Management. Life 2023, 13, 1228. [Google Scholar] [CrossRef]
- Marano, M.; Pisani, M.; Zampini, G.; Pontrelli, G.; Roversi, M. Acute Exposure to European Viper Bite in Children: Advocating for a Pediatric Approach. Toxins 2021, 13, 330. [Google Scholar] [CrossRef]
- Sassoè-Pognetto, M.; Cavalcante, R.; Paonessa, M. Acute compartment syndrome and fasciotomy after a viper bite in Italy: A case report. Ital. J. Pediatr. 2024, 50, 70. [Google Scholar] [CrossRef]
- Oliveira, I.S.; Pucca, M.B.; A Cerni, F.; Vieira, S.; Sachett, J.; de Farias, A.S.; Lacerda, M.; Murta, F.; Baia-Da-Silva, D.; Rocha, T.A.H.; et al. Snakebite envenoming in Brazilian children: Clinical aspects, management and outcomes. J. Trop. Pediatr. 2023, 69, fmad010. [Google Scholar]
- Collinson, S.; Lamb, T.; Cardoso, I.A.; Diggle, P.J.; Lalloo, D.G. A systematic review of variables associated with snakebite risk in spatial and temporal analyses. Trans. R. Soc. Trop. Med. Hyg. 2025, trae131. [Google Scholar] [CrossRef]
- Kasturiratne, A.; Wickremasinghe, A.R.; De Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; De Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar]
- Ahmed, S.M.; Ahmed, M.; Nadeem, A.; Mahajan, J.; Choudhary, A.; Pal, J. Emergency Treatment of a Snake Bite: Pearls from Literature. J. Emerg. Trauma. Shock 2008, 1, 97–105. [Google Scholar]
- Marano, M.; Pisani, M.; Stoppa, F.; Di Nardo, M.; Pirozzi, N.; Luca, E.; Pulitanò, S.; Conti, G.; Marzano, L.; De Luca, D.; et al. Antitoxin use and pediatric intensive care for viper bites in Rome, Italy. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 485–492. [Google Scholar] [PubMed]
- Del Brutto, O.H.; Del Brutto, V.J. Neurological Complications of Venomous Snake Bites: A Review. Acta Neurol. Scand. 2012, 125, 363–372. [Google Scholar]
- McDiarmid, R.W.; Campbell, J.A.; Touré, T. Snake Species of the World: A Taxonomic and Geographic Reference; Herpetologists’ League: Washington, DC, USA, 1999; Volume 1, 511p. [Google Scholar]
- Guillon, M.; Guiller, G.; DeNardo, D.F.; Lourdais, O. Microclimate Preferences Correlate with Contrasted Evaporative Water Loss in Parapatric Vipers at Their Contact Zone. Can. J. Zool. 2014, 92, 81–86. [Google Scholar]
- Di Nicola, M.R.; Pontara, A.; Kass, G.E.N.; Kramer, N.I.; Avella, I.; Pampena, R.; Mercuri, S.R.; Dorne, J.L.C.M.; Paolino, G. Vipers of Major Clinical Relevance in Europe: Taxonomy, Venom Composition, Toxicology and Clinical Management of Human Bites. Toxicology 2021, 453, 152724. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Di Nicola, M.; Pontara, A.; Didona, D.; Moliterni, E.; Mercuri, S.; Grano, M.; Borgianni, N.; Kumar, R.; Pampena, R. Vipera snakebite in Europe: A systematic review of a neglected disease. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2247–2260. [Google Scholar] [CrossRef]
- Pozio, E. Venomous snake bites in Italy: Epidemiological and clinical aspects. Trop. Med. Parasitol. 1988, 39, 62–66. [Google Scholar]
- Le Geyt, J.; Pach, S.; Gutiérrez, J.M.; Habib, A.G.; Maduwage, K.P.; Hardcastle, T.C.; Hernández Diaz, R.; Avila-Aguero, M.L.; Ya, K.T.; Williams, D.; et al. Paediatric Snakebite Envenoming: Recognition and Management of Cases. Arch. Dis. Child. 2021, 106, 14–19. [Google Scholar]
- Gasanov, S.E.; Dagda, R.K.; Rael, E.D. Snake Venom Cytotoxins, Phospholipase A2s, and Zn2+-dependent Metalloproteinases: Mechanisms of Action and Pharmacological Relevance. J. Clin. Toxicol. 2014, 4, 1000181. [Google Scholar] [CrossRef]
- Ayvazyan, N.; Ghukasyan, G.; Ghulikyan, L.; Kirakosyan, G.; Sevoyan, G.; Voskanyan, A.; Karabekyan, Z. The Contribution of Phospholipase A2 and Metalloproteinases to the Synergistic Action of Viper Venom on the Bioenergetic Profile of Vero Cells. Toxins 2022, 14, 724. [Google Scholar] [CrossRef]
- Avella, I.; Calvete, J.J.; Sanz, L.; Wüster, W.; Licata, F.; Quesada-Bernat, S.; Rodríguez, Y.; Martínez-Freiría, F. Interpopulational variation and ontogenetic shift in the venom composition of Lataste’s viper (Vipera latastei, Boscá 1878) from northern Portugal. J. Proteom. 2022, 263, 104613. [Google Scholar] [CrossRef] [PubMed]
- Marano, M.; Di Giuseppe, M.; Pro, S.; Pisani, M.; Montibeller, M.; Bottari, G.; Nunziata, J.; Cecchetti, C. Vipera Aspis Bite Neurotoxicity: Two Pediatric Cases in Central Italy. Clin. Toxicol. 2020, 58, 849–850. [Google Scholar] [CrossRef]
- Chippaux, J.P. Epidemiology of snakebites in Europe: A systematic review of the literature. Toxicon 2012, 59, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, G.; Duregotti, E.; Locatelli, C.A.; Giampreti, A.; Lonati, D.; Rossetto, O.; Pirazzini, M. Variability in venom composition of European viper subspecies limits the cross-effectiveness of antivenoms. Sci. Rep. 2018, 8, 9818. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.; Jürgensen, J.A.; Føns, S.; Haack, A.M.; Friis, R.U.W.; Dam, S.H.; Bush, S.P.; White, J.; Laustsen, A.H. Snakebite Envenoming Diagnosis and Diagnostics. Front. Immunol. 2021, 12, 661457. [Google Scholar] [CrossRef]
- Tunjić Pejak, D.; Nesek Adam, V.; Srzić, I. Venomous Snakebites in Croatia, Clinical Presentation, Diagnosis and Treatment. Acta Clin. Croat. 2022, 61, 59–66. [Google Scholar] [CrossRef]
- Claudet, I.; Maréchal, C.; Gurrera, E.; Cordier, L.; Honorat, R.; Grouteau, E. Risk factors for high-grade envenomations after French viper bites in children. Pediatr. Emerg. Care 2012, 28, 650–654. [Google Scholar] [CrossRef]
- Hsu, C.-P.; Chuang, J.-F.; Hsu, Y.-P.; Wang, S.-Y.; Fu, C.-Y.; Yuan, K.-C.; Chen, C.-H.; Kang, S.-C.; Liao, C.-H. Predictors of the development of post-snakebite compartment syndrome. Scand. J. Trauma. Resusc. Emerg. Med. 2015, 23, 97. [Google Scholar] [CrossRef]
- Lonati, D.; Giampreti, A.; Rossetto, O.; Petrolini, V.M.; Vecchio, S.; Buscaglia, E.; Mazzoleni, M.; Chiara, F.; Aloise, M.; Gentilli, A.; et al. Neurotoxicity of European viperids in Italy: Pavia Poison Control Centre case series 2001–2011. Clin. Toxicol. 2014, 52, 269–276. [Google Scholar] [CrossRef]
- Liapis, K.; Charitaki, E.; Psaroulaki, A. Case Report: Spherocytic Hemolytic Anemia after Envenomation by Long-Nosed Viper (Vipera ammodytes). Am. J. Trop. Med. Hyg. 2019, 101, 1442–1445. [Google Scholar] [CrossRef]
- Audebert, F.; Sorkine, M.; Bon, C. Envenoming by Viper Bites in France: Clinical Gradation and Biological Quantification by ELISA. Toxicon 1992, 30, 599–609. [Google Scholar] [CrossRef]
- Boels, D.; Courtois, A.; Paradis, C.; Caillet, P.; Labadie, M. First Step in Assessment of VipGrade®, a Computerized Clinical Decision System to Assess Vipera Envenomation Grading: A Single-Center Interrater Reliability Study. Clin. Toxicol. 2022, 60, 514–520. [Google Scholar]
- Boels, D.; Hamel, J.F.; Bretaudeau Deguigne, M.; Harry, P. European Viper Envenomings: Assessment of ViperfavTM and Other Symptomatic Treatments. Clin. Toxicol. 2012, 50, 189–196. [Google Scholar]
- Waiddyanatha, S.; Silva, A.; Siribaddana, S.; Isbister, G.K. Long-term Effects of Snake Envenoming. Toxins 2019, 11, 193. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Nagendra, L.; Tyagi, P. Snakebite Envenomation and Endocrine Dysfunction 2021. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Muniyapillai, T.; S, M.; R, A.; Kulothungan, K.; Thirunavukkarasu, S.; George, N. Clinical Profile and Outcomes of Pediatric Snakebite Envenomation: A Three-Year Retrospective Study from a Rural Tertiary Care Center in South India. Cureus 2024, 16, e73976. [Google Scholar]
- Ozay, G.; Bosnak, M.; Ece, A.; Davutoglu, M.; Dikici, B.; Gurkan, F.; Bosnak, V.; Haspolat, K. Clinical characteristics of children with snakebite poisoning and management of complications in the pediatric intensive care unit. Pediatr. Int. 2005, 47, 669–675. [Google Scholar]
- Parker-Cote, J.; Meggs, W.J. First Aid and Pre-Hospital Management of Venomous Snakebites. Trop. Med. Infect. Dis. 2018, 3, 45. [Google Scholar] [CrossRef]
- León, G.; Vargas, M.; Segura, Á.; Herrera, M.; Villalta, M.; Sánchez, A.; Solano, G.; Gómez, A.; Sánchez, M.; Estrada, R.; et al. Current technology for the industrial manufacture of snake antivenoms. Toxicon 2018, 151, 63–73. [Google Scholar]
- Kurtović, T.; Lang Balija, M.; Brvar, M.; Dobaja Borak, M.; Mateljak Lukačević, S.; Halassy, B. Comparison of Preclinical Properties of Several Available Antivenoms in the Search for Effective Treatment of Vipera ammodytes and Vipera berus Envenoming. Toxins 2021, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Fernández, J.; Sanz, L.; Angulo, Y.; Sasa, M.; Gutiérrez, J.M.; Calvete, J.J. Venomous snakes of Costa Rica: Biological and medical implications of their venom proteomic profiles analyzed through the strategy of snake venomics. J. Proteom. 2014, 105, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Lamb, T.; De Haro, L.; Lonati, D.; Brvar, M.; Eddleston, M. Antivenom for European Vipera species envenoming. Clin. Toxicol. 2017, 55, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Nuchpraryoon, I.; Garner, P. Interventions for preventing reactions to snake antivenom. Cochrane Database Syst. Rev. 2000, 1999, CD002153. [Google Scholar] [PubMed]
- Waiddyanatha, S.; Silva, A.; Wedasingha, S.; Siribaddana, S.; Isbister, G.K. Incidence of serum sickness following Indian polyvalent antivenom therapy in a cohort of snake-envenomed patients in rural Sri Lanka. Clin. Toxicol. 2023, 61, 518–523. [Google Scholar] [CrossRef]
- Mender, M.M.; Bolton, F.; Berry, C.; Young, M. Antivenom: An immunotherapy for the treatment of snakebite envenoming in sub-Saharan Africa. Adv. Protein Chem. Struct. Biol. 2022, 129, 435–477. [Google Scholar]
- Theakston, R.D. An objective approach to antivenom therapy and assessment of first-aid measures in snake bite. Ann. Trop. Med. Parasitol. 1997, 91, 857–865. [Google Scholar]
- Jollivet, V.; Hamel, J.F.; de Haro, L.; Labadie, M.; Sapori, J.M.; Cordier, L.; Villa, A.; Nis-se, P.; Puskarczyk, E.; Berthelon, L.; et al. European viper envenomation recorded by French poison control centers: A clinical assessment and management study. Toxicon 2015, 108, 97–103. [Google Scholar] [CrossRef]
- Claudet, I.; Grouteau, E.; Cordier, L.; Franchitto, N.; Bréhin, C. Hyperglycemia is a risk factor for high-grade envenomations after European viper bites (Vipera spp.) in children. Clin. Toxicol. 2016, 54, 34–39. [Google Scholar] [CrossRef]
- Karabuva, S.; Vrkić, I.; Brizić, I.; Ivić, I.; Lukšić, B. Venomous snakebites in children in southern Croatia. Toxicon 2016, 112, 8–15. [Google Scholar]
- Claudet, I.; Germain, H. VipGrade® electronic clinical tool: Retrospective evaluation on a paediatric cohort of European viper bites. Clin. Toxicol. 2024, 62, 726–732. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlandi, G.; Rossi, N.; Chiarelli, F.; Di Filippo, P. Vipera Snakebite in Children: A Focus on Europe. Children 2025, 12, 393. https://doi.org/10.3390/children12030393
Orlandi G, Rossi N, Chiarelli F, Di Filippo P. Vipera Snakebite in Children: A Focus on Europe. Children. 2025; 12(3):393. https://doi.org/10.3390/children12030393
Chicago/Turabian StyleOrlandi, Greta, Nadia Rossi, Francesco Chiarelli, and Paola Di Filippo. 2025. "Vipera Snakebite in Children: A Focus on Europe" Children 12, no. 3: 393. https://doi.org/10.3390/children12030393
APA StyleOrlandi, G., Rossi, N., Chiarelli, F., & Di Filippo, P. (2025). Vipera Snakebite in Children: A Focus on Europe. Children, 12(3), 393. https://doi.org/10.3390/children12030393






