Sodium Patterns and Their Variables in a Cohort of ELBW Infants in the First 10 Days of Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Characterstics and Fluid Administration Strategy
2.2. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the ELBW Cohort
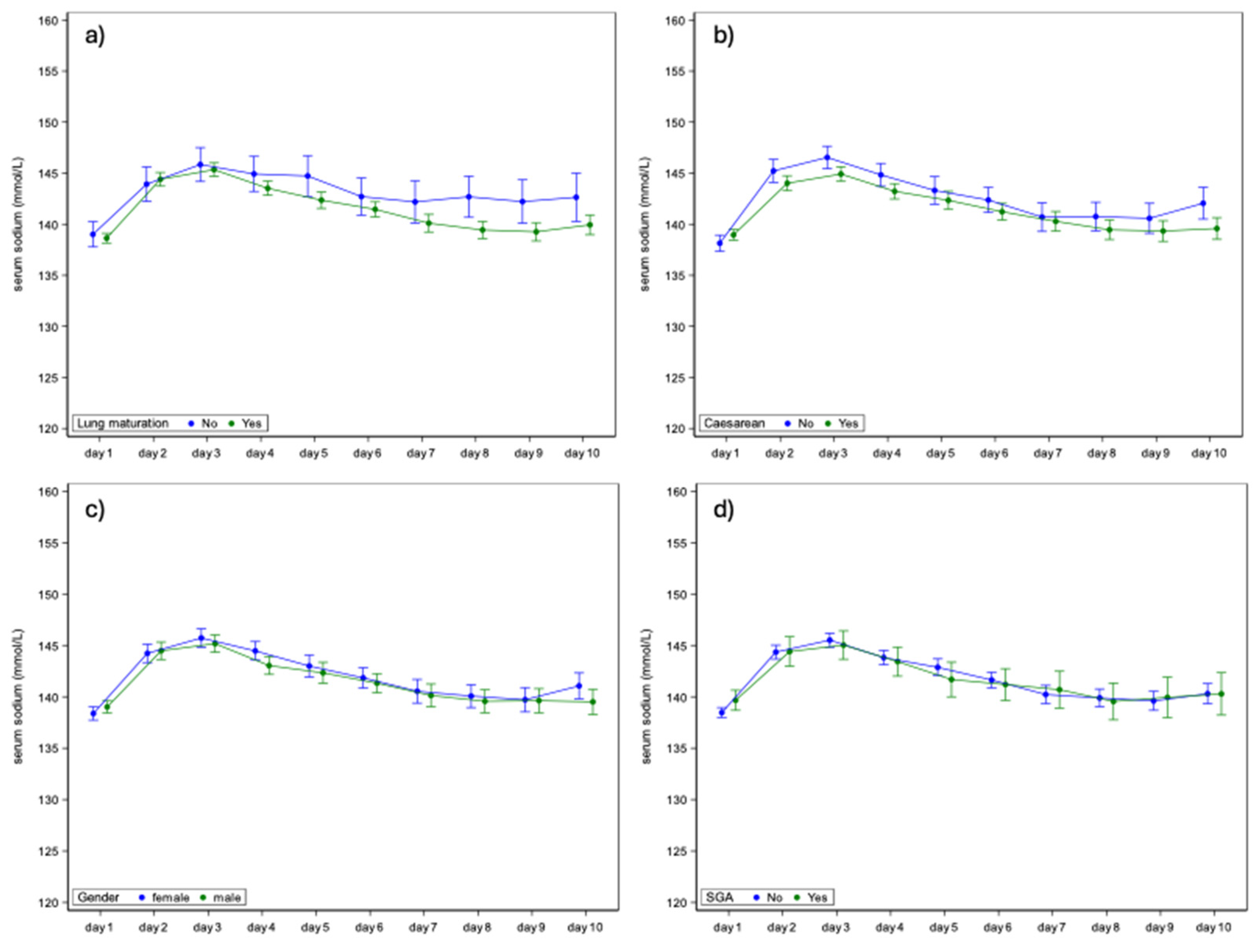
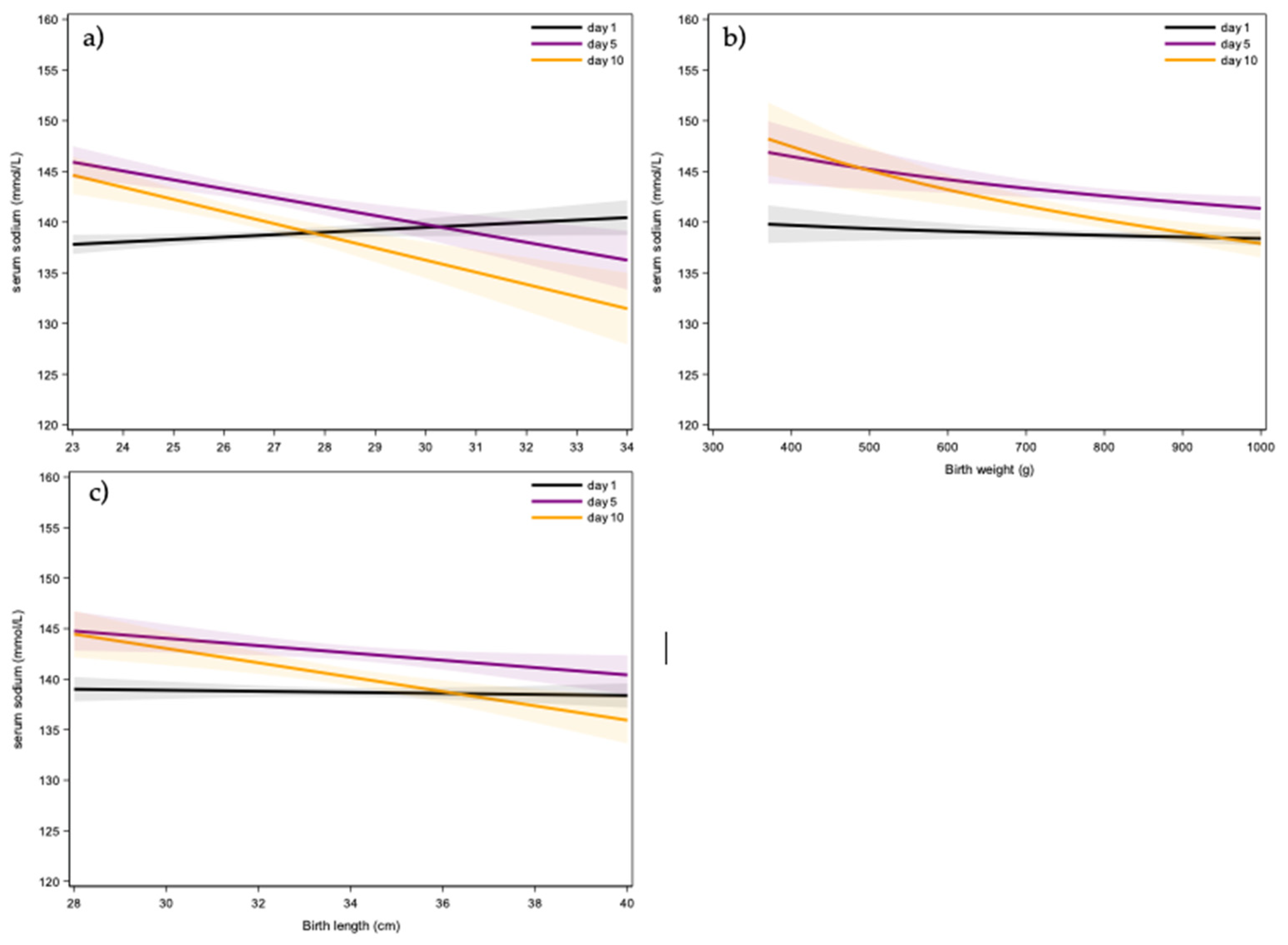
3.2. Perinatal Variables
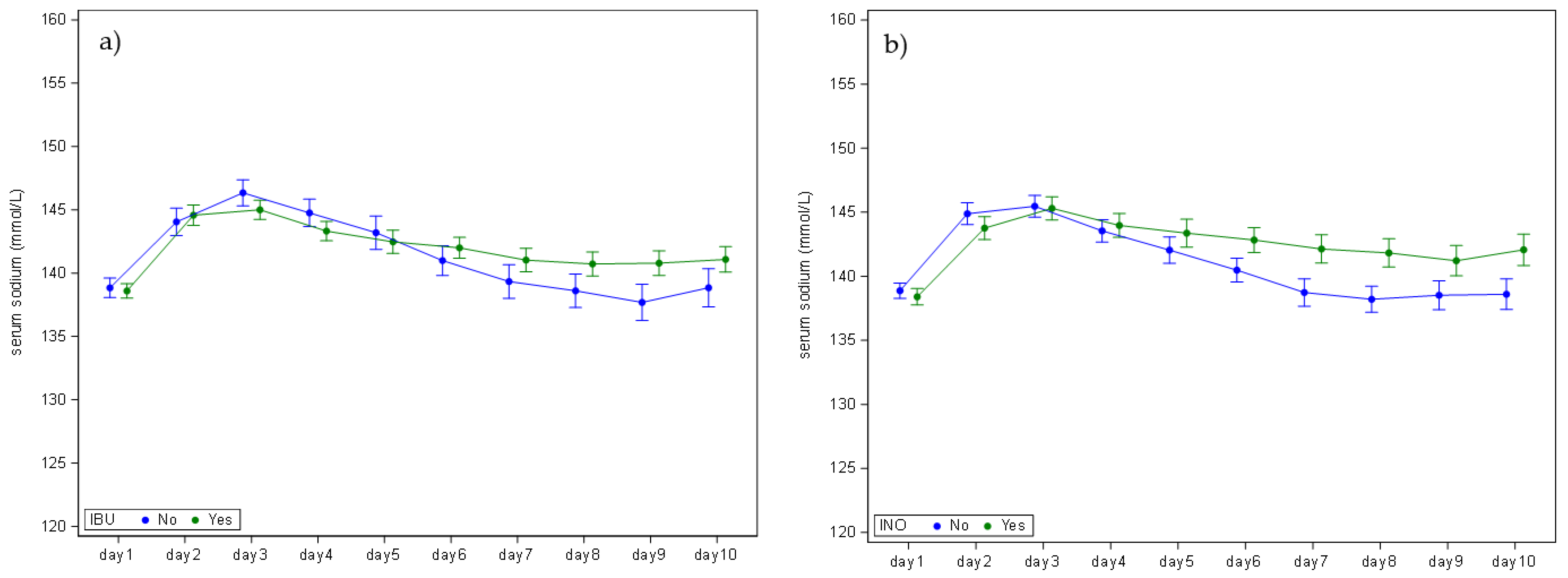
3.3. Neonatal Variables
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wada, M.; Kusuda, S.; Takahashi, N.; Nishida, H. Fluid and electrolyte balance in extremely preterm infants < 24 weeks of gestation in the first week of life. Pediatr. Int. 2008, 50, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Stritzke, A.; Thomas, S.; Amin, H.; Fusch, C.; Lodha, A. Renal consequences of preterm birth. Mol. Cell. Pediatr. 2017, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Monnikendam, C.S.; Mu, T.S.; Aden, J.K.; Lefkowitz, W.; Carr, N.R.; Aune, C.N.; Ahmad, K.A. Dysnatremia in extremely low birth weight infants is associated with multiple adverse outcomes. J. Perinatol. 2019, 39, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Pace, M.; van Sas, S.; Salaets, T.; Laenen, A.; Raaijmakers, A.; Allegaert, K. Hypo- and hypernatriemia in extremely low birth weight infants in the first 10 days of life: A review. Children 2025, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- van Donge, T.; Allegaert, K.; Gotta, V.; Smits, A.; Levtchenko, E.; Mekahli, D.; van den Anker, J.; Pfister, M. Characterizing dynamics of serum creatinine and creatinine clearance in extremely low birth weight neonates during the first 6 weeks of life. Pediatr. Nephrol. 2021, 36, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; Hildebrand, H.; Singh, K.; Turner, M.A. The publication quality of laboratory values in clinical studies in neonates. Pediatr. Res. 2023, 94, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Devlieger, H.; De Pourcq, L.; Casneuf, A.; Vanhole, C.; de Zegher, F.; Jaeken, J.; Eggermont, E. Standard two-compartment formulation for total parenteral nutrition in the neonatal intensive care unit: A fluid tolerance based system. Clin. Nutr. 1993, 12, 282–286. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS Software, version 9.4; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- Gawlowski, Z.; Aladangady, N.; Coen, P.G. Hypernatraemia in preterm infants born at less than 27 weeks gestation. J. Paediatr. Child. Health 2006, 42, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Segar, J.L. A physiological approach to fluid and electrolyte management of the preterm infant: Review. J. Neonatal Perinat. Med. 2020, 13, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Costarino, A.T.; Gruskay, J.A.; Corcoran, L.; Polin, R.A.; Baumgart, S. Sodium restriction versus daily maintenance replacement in very low birth weight premature neonates: A randomized, blind therapeutic trial. J. Pediatr. 1992, 120, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Eibensteiner, F.; Laml-Wallner, G.; Thanhaeuser, M.; Ristl, R.; Ely, S.; Jilma, B.; Berger, A.; Haiden, N. ELBW infants receive inadvertent sodium load above the recommended intake. Pediatr. Res. 2020, 88, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Boubred, F.; Herlenius, E.; Bartocci, M.; Jonsson, B.; Vanpée, M. Extremely preterm infants who are small for gestational age have a high risk of early hypophosphatemia and hypokalemia. Acta Paediatr. 2015, 104, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Stritzke, A.; Ismail, R.; Rose, M.S.; Lyon, A.W.; Tenton, T.R. Cord-Blood Derived Chemistry Reference Values in Preterm Infants for Sodium, Chloride, Potassium, Glucose, and Creatinine. Am. J. Perinatol. 2024, 41, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, N.; Shoji, H.; Ikeda, N.; Murano, Y.; Okuno, T.; Kantake, M.; Yokomizo, T.; Shimizu, T. The impact of cyclooxygenase inhibitor use on urinary prostaglandin metabolites in preterm infants. Pediatr. Neonatol. 2024, 65, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Agakidou, E.; Chatziioannadis, I.; Kontou, A.; Stathopoulou, T.; Chotas, W.; Sarafidis, K. An update on pharmacologic management of neonatal hypotension: When, why, and which medication. Children 2024, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, G.; Kavvadia, V.; Marcou, M.; Greenough, A. Antenatal steroids and fluid balance in very low birth weight infants. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F509–F513. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, E.; Fenelli, T.; Visentin, S.; Trevisanuto, D.; Zanardo, V. Consequences in infants that were intrauterine growth restricted. J. Pregnancy 2011, 2011, 364381. [Google Scholar] [CrossRef] [PubMed]
- Salloum, J.A.; Garten, L.; Bührer, C. Diuresis-led volume replacement strategy in extremely low birth weight infants. Acta Paediatr. 2024, 113, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Jochum, F.; Moltu, S.J.; Senterre, T.; Nomayo, A.; Goulet, O.; Iacobelli, S.; ESPGHAN/ESPEN/ESPR/CSPEN working group on parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Fluid and electrolytes. Clin. Nutr. 2018, 37, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
| Variable | Statistic | All | |
|---|---|---|---|
| Birth weight (g) | N | 211 | |
| Mean | 807 | ||
| Std | 135 | ||
| Median | 830 | ||
| IQR | (715; 910) | ||
| Range | (370; 1000) | ||
| Length (cm) | N | 200 | |
| Mean | 34 | ||
| Std | 2.4 | ||
| Median | 34 | ||
| IQR | (32; 36) | ||
| Range | (28; 40) | ||
| Growth category | AGA | n/N (%) | 170/211 (80.6%) |
| SGA | n/N (%) | 41/211 (19.4%) | |
| Gestational age (weeks) | N | 211 | |
| Mean | 26.7 | ||
| Std | 1.9 | ||
| Median | 26 | ||
| IQR | (25; 28) | ||
| Range | (23; 34) | ||
| Sex | Female | n/N (%) | 99/211 (46.9%) |
| Male | n/N (%) | 111/211 (52.6%) | |
| Unknown | n/N (%) | 1/211 (0.5%) | |
| Neonatal death | No | n/N (%) | 183/211 (86.7%) |
| Yes | n/N (%) | 28/211 (13.3%) | |
| Lung maturation | No | n/N (%) | 28/207 (13.5%) |
| Yes | n/N (%) | 179/207 (86.5%) | |
| Caesarean | No | n/N (%) | 63/209 (30.1%) |
| Yes | n/N (%) | 146/209 (69.9%) | |
| Ibuprofen | No | n/N (%) | 79/209 (37.8%) |
| Yes | n/N (%) | 130/209 (62.2%) | |
| Inotropic agents | No | n/N (%) | 111/208 (53.4%) |
| Yes | n/N (%) | 97/208 (46.6%) | |
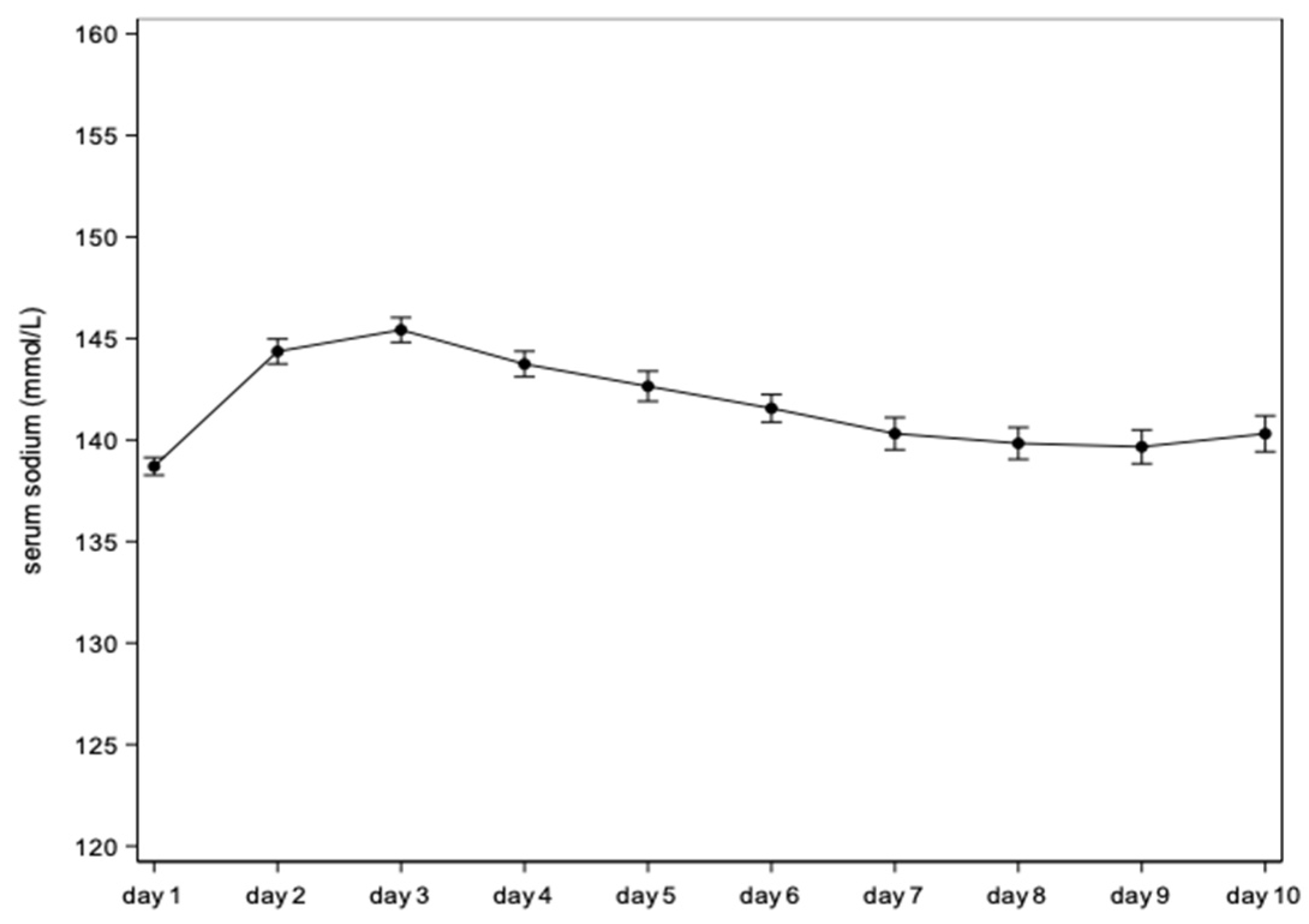
| Day | Mean Estimate (95% CI) | Mean Difference vs. Day 1 (95% CI) | p-Value |
|---|---|---|---|
| Day 1 | 138.7 (138.3; 139.1) | 0 | . |
| Day 2 | 144.4 (143.7; 145.0) | 5.7 (5.0; 6.3) | <0.0001 |
| Day 3 | 145.4 (144.8; 146.0) | 6.7 (6.0; 7.5) | <0.0001 |
| Day 4 | 143.7 (143.1; 144.4) | 5.0 (4.3; 5.8) | <0.0001 |
| Day 5 | 142.6 (141.9; 143.4) | 3.9 (3.1; 4.8) | <0.0001 |
| Day 6 | 141.6 (140.9; 142.2) | 2.9 (2.0; 3.7) | <0.0001 |
| Day 7 | 140.3 (139.5; 141.1) | 1.6 (0.7; 2.5) | 0.0005 |
| Day 8 | 139.8 (139.0; 140.6) | 1.1 (0.2; 2.0) | 0.0141 |
| Day 9 | 139.7 (138.8; 140.5) | 1.0 (0.0; 1.9) | 0.0422 |
| Day 10 | 140.3 (139.4; 141.2) | 1.6 (0.6; 2.6) | 0.0015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Sas, S.; Pace, M.; Salaets, T.; Laenen, A.; Raaijmakers, A.; Allegaert, K. Sodium Patterns and Their Variables in a Cohort of ELBW Infants in the First 10 Days of Life. Children 2025, 12, 337. https://doi.org/10.3390/children12030337
van Sas S, Pace M, Salaets T, Laenen A, Raaijmakers A, Allegaert K. Sodium Patterns and Their Variables in a Cohort of ELBW Infants in the First 10 Days of Life. Children. 2025; 12(3):337. https://doi.org/10.3390/children12030337
Chicago/Turabian Stylevan Sas, Stijn, Myrna Pace, Thomas Salaets, Annouschka Laenen, Anke Raaijmakers, and Karel Allegaert. 2025. "Sodium Patterns and Their Variables in a Cohort of ELBW Infants in the First 10 Days of Life" Children 12, no. 3: 337. https://doi.org/10.3390/children12030337
APA Stylevan Sas, S., Pace, M., Salaets, T., Laenen, A., Raaijmakers, A., & Allegaert, K. (2025). Sodium Patterns and Their Variables in a Cohort of ELBW Infants in the First 10 Days of Life. Children, 12(3), 337. https://doi.org/10.3390/children12030337








