Mother–Infant Dyadic Neural Synchrony Measured Using EEG Hyperscanning and Validated Using Behavioral Measures
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Electroencephalography Hyperscanning and Circular Correlation Coefficient as a Neural Measure of Synchrony
3. Additional Measures
3.1. Welch Emotional Connection Scale (WECS)
3.2. Mother-to-Infant Bonding Scale (MIBS)
3.3. Edinburgh Postnatal Depression Screen (EPDS)
3.4. Statistical Analyses
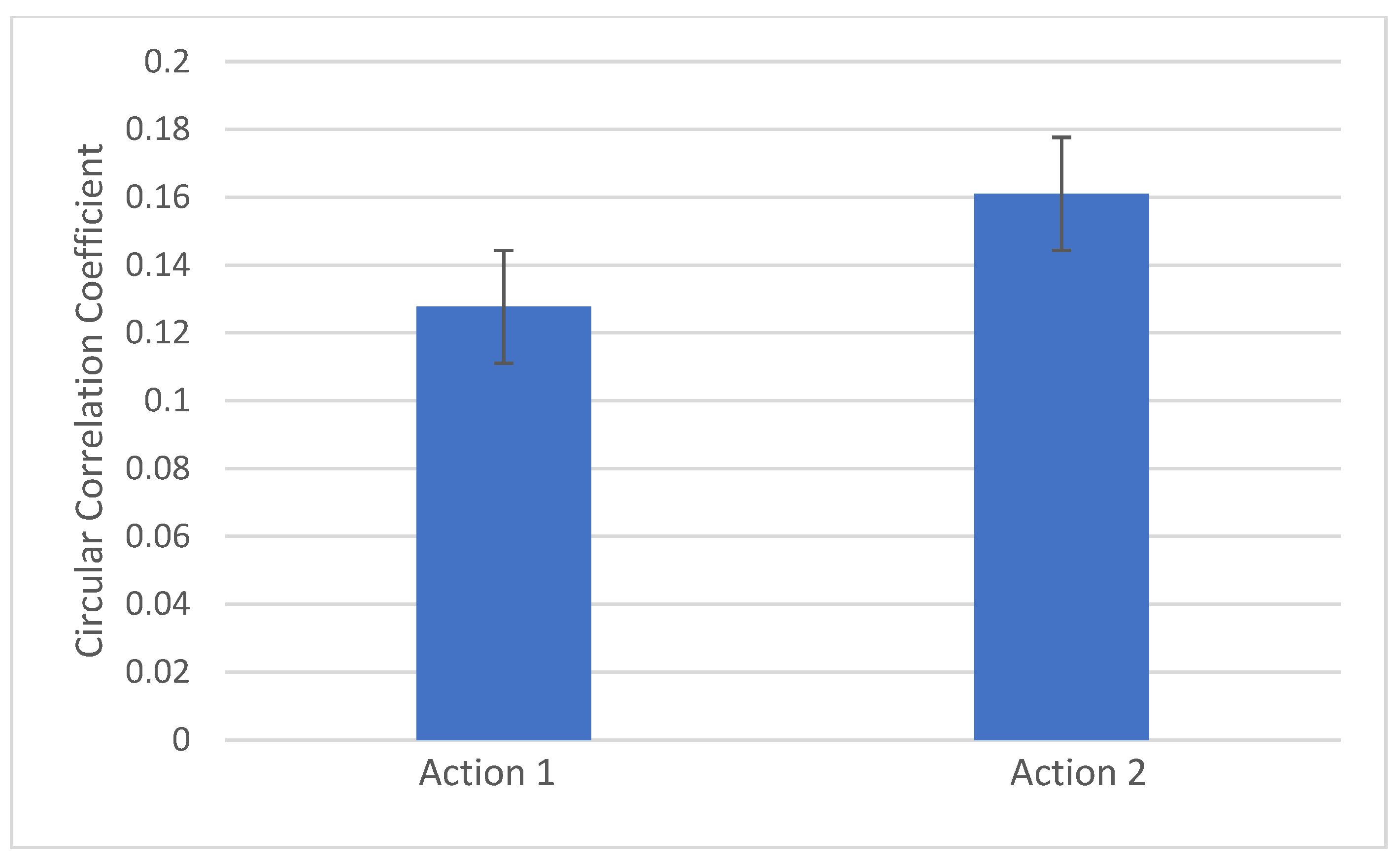
4. Results
5. Discussion
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, R.A.; Hoffenkamp, H.N.; Tooten, A.; Braeken, J.; Vingerhoets, A.J.; van Bakel, H.J. The quality of parent–infant interaction in the first 2 years after full-term and preterm birth. Parenting 2015, 15, 247–268. [Google Scholar] [CrossRef]
- Maupin, A.N.; Fine, J.G. Differential effects of parenting in preterm and full-term children on developmental outcomes. Early Hum. Dev. 2014, 90, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Neel, M.; Stark, A.; Maitre, N. Parenting style impacts cognitive and behavioural outcomes of former preterm infants: A systematic review. Child Care Health Dev. 2018, 44, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Treyvaud, K.; Doyle, L.W.; Lee, K.J.; Ure, A.; Inder, T.E.; Hunt, R.W.; Anderson, P.J. Parenting behavior at 2 years predicts school-age performance at 7 years in very preterm children. J. Child Psychol. Psychiatry 2016, 57, 814–821. [Google Scholar] [CrossRef]
- Feldman, R. Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J. Child Psychol. Psychiatry 2007, 48, 329–354. [Google Scholar] [CrossRef]
- Treyvaud, K.; Anderson, V.A.; Howard, K.; Bear, M.; Hunt, R.W.; Doyle, L.W.; Inder, T.E.; Woodward, L.; Anderson, P.J. Parenting behavior is associated with the early neurobehavioral development of very preterm children. Pediatrics 2009, 123, 555–561. [Google Scholar] [CrossRef]
- Quiñones-Camacho, L.E.; Hoyniak, C.P.; Wakschlag, L.S.; Perlman, S.B. Getting in synch: Unpacking the role of parent–child synchrony in the development of internalizing and externalizing behaviors. Dev. Psychopathol. 2022, 34, 1901–1913. [Google Scholar] [CrossRef]
- Schilbach, L.; Redcay, E. Synchrony Across Brains. Annu. Rev. Psychol. 2024, 76. [Google Scholar] [CrossRef]
- Bi, X.; Cui, H.; Ma, Y. Hyperscanning Studies on Interbrain Synchrony and Child Development: A Narrative Review. Neuroscience 2023, 530, 38–45. [Google Scholar] [CrossRef]
- Alves, C.R.L.; Gaspardo, C.M.; Altafim, E.R.P.; Linhares, M.B.M. Effectiveness of a longitudinal psychosocial intervention to strengthen mother–child interactions: The role of biological and contextual moderators. Child. Youth Serv. Rev. 2022, 133, 106333. [Google Scholar] [CrossRef]
- Berlin, L.J.; Brooks-Gunn, J.; McCarton, C.; McCormick, M.C. The effectiveness of early intervention: Examining risk factors and pathways to enhanced development. Prev. Med. 1998, 27, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.; Peters, R.; Lawrence, F. Family dynamics and child outcomes in early intervention: The role of developmental theory in the specification of effects. Early Child. Res. Q. 2003, 18, 446–467. [Google Scholar] [CrossRef]
- Feldman, R.; Eidelman, A.I.; Sirota, L.; Weller, A. Comparison of skin-to-skin (kangaroo) and traditional care: Parenting outcomes and preterm infant development. Pediatrics 2002, 110, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; McDorman, S.A.; Romeo, R.R. How parent-child brain-to-brain synchrony can inform the study of child development. Child Dev. Perspect. 2024, 18, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M. Problems in the study of infant emotional development. Emot. Rev. 2011, 3, 131–137. [Google Scholar] [CrossRef]
- Lewis, M. Inside and outside: The relation between emotional states and expressions. Emot. Rev. 2011, 3, 189–196. [Google Scholar] [CrossRef]
- Relland, L.M.; Gehred, A.; Maitre, N.L. Behavioral and physiological signs for pain assessment in preterm and term neonates during a nociception-specific response: A systematic review. Pediatr. Neurol. 2019, 90, 13–23. [Google Scholar] [CrossRef]
- Lilley, C.M.; Craig, K.D.; Grunau, R.E. The expression of pain in infants and toddlers: Developmental changes in facial action. Pain. 1997, 72, 161–170. [Google Scholar] [CrossRef]
- Gvirts, H.; Perlmutter, R. What guides us to neurally and behaviorally align with anyone specific? A neurobiological model based on fNIRS hyperscanning studies. Neurosci. 2020, 26, 108–116. [Google Scholar] [CrossRef]
- Nguyen, T.; Bánki, A.; Markova, G.; Hoehl, S. Studying parent-child interaction with hyperscanning. Prog. Brain Res. 2020, 254, 1–24. [Google Scholar]
- Norton, E.S.; Manning, B.L.; Harriott, E.M.; Nikolaeva, J.I.; Nyabingi, O.S.; Fredian, K.M.; Page, J.M.; McWeeny, S.; Krogh-Jespersen, S.; MacNeill, L.A.; et al. Social EEG: A novel neurodevelopmental approach to studying brain-behavior links and brain-to-brain synchrony during naturalistic toddler-parent interactions. Dev. Psychobiol. 2022, 64, e22240. [Google Scholar] [CrossRef] [PubMed]
- Turk, E.; Vroomen, J.; Fonken, Y.; Levy, J.; van den Heuvel, M.I. In sync with your child: The potential of parent–child electroencephalography in developmental research. Dev. Psychobiol. 2022, 64, e22221. [Google Scholar] [CrossRef] [PubMed]
- Wass, S.V.; Whitehorn, M.; Marriott Haresign, I.; Phillips, E.; Leong, V. Interpersonal Neural Entrainment during Early Social Interaction. Trends Cogn. Sci. 2020, 24, 329–342. [Google Scholar] [CrossRef]
- Burgess, A.P. On the interpretation of synchronization in EEG hyperscanning studies: A cautionary note. Front. Hum. Neurosci. 2013, 7, 881. [Google Scholar] [CrossRef]
- Turk, E.; Endevelt-Shapira, Y.; Feldman, R.; van den Heuvel, M.I.; Levy, J. Brains in Sync: Practical Guideline for Parent-Infant EEG During Natural Interaction. Front. Psychol. 2022, 13, 833112. [Google Scholar] [CrossRef]
- Levy, J.; Lankinen, K.; Hakonen, M.; Feldman, R. The integration of social and neural synchrony: A case for ecologically valid research using MEG neuroimaging. Soc. Cogn. Affect. Neurosci. 2021, 16, 143–152. [Google Scholar] [CrossRef]
- Markova, G.; Nguyen, T.; Hoehl, S. Neurobehavioral Interpersonal Synchrony in Early Development: The Role of Interactional Rhythms. Front. Psychol. 2019, 10, 2078. [Google Scholar] [CrossRef]
- Neel, M.L.; Jeanvoine, A.; Key, A.; Stark, A.R.; Norton, E.S.; Relland, L.M.; Hay, K.; Maitre, N.L. Behavioral and neural measures of infant responsivity increase with maternal multisensory input in non-irritable infants. Brain Behav. 2023, 13, e3253. [Google Scholar] [CrossRef]
- Hane, A.; LaCoursiere, J.N.; Mitsuyama, M.; Wieman, S.; Ludwig, R.; Kwon, K.Y.; Browne, J.; Austin, J.; Myers, M.; Welch, M. The Welch Emotional Connection Screen. (WECS): Validation of a brief mother-infant relational health screen. Acta Paediatr. 2019, 108, 615–625. [Google Scholar] [CrossRef]
- Debnath, R.; Buzzell, G.A.; Morales, S.; Bowers, M.E.; Leach, S.C.; Fox, N.A. The Maryland Analysis of Developmental EEG (MADE) Pipeline. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bell, M.A. Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology 2002, 39, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Marshall, P.J.; Bar-Haim, Y.; Fox, N.A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2002, 113, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, G.; Drake, F.L. PYTHON 2.6 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Frosch, C.A.; Fagan, M.A.; Lopez, M.A.; Middlemiss, W.; Chang, M.; Hane, A.A.; Welch, M.G. Validation study showed that ratings on the Welch Emotional Connection Screen at infant age six months are associated with child behavioural problems at age three years. Acta Paediatr. 2019, 108, 889–895. [Google Scholar] [CrossRef]
- Taylor, A.; Atkins, R.; Kumar, R.; Adams, D.; Glover, V. A new Mother-to-Infant Bonding Scale: Links with early maternal mood. Arch. Women’s Ment. Health 2005, 8, 45–51. [Google Scholar] [CrossRef]
- Bienfait, M.; Maury, M.; Haquet, A.; Faillie, J.-L.; Franc, N.; Combes, C.; Daudé, H.; Picaud, J.-C.; Rideau, A.; Cambonie, G. Pertinence of the self-report mother-to-infant bonding scale in the neonatal unit of a maternity ward. Early Hum. Dev. 2011, 87, 281–287. [Google Scholar] [CrossRef]
- Wittkowski, A.; Wieck, A.; Mann, S. An evaluation of two bonding questionnaires: A comparison of the Mother-to-Infant Bonding Scale with the Postpartum Bonding Questionnaire in a sample of primiparous mothers. Arch. Women’s Ment. Health 2007, 10, 171–175. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of Postnatal Depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Nguyen, T.; Abney, D.H.; Salamander, D.; Bertenthal, B.I.; Hoehl, S. Proximity and touch are associated with neural but not physiological synchrony in naturalistic mother-infant interactions. Neuroimage 2021, 244, 118599. [Google Scholar] [CrossRef]
- Piazza, E.H.L.; Hasson, U.; Lew-Williams, C. Infant and Adult Brains Are Coupled to the Dynamics of Natural Communication. Psychol. Sci. 2020, 31, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Naoi, N.; Minagawa-Kawai, Y.; Kobayashi, A.; Takeuchi, K.; Nakamura, K.; Yamamoto, J.-i.; Shozo, K. Cerebral responses to infant-directed speech and the effect of talker familiarity. Neuroimage 2012, 59, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, C.P.; Neel, M.L.; Jeanvoine, A.; Maitre, N.L. Investigation of mothers’ elicited infant-directed speech and singing for preterm infants. Pediatr. Res. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wass, S.V.; Noreika, V.; Georgieva, S.; Clackson, K.; Brightman, L.; Nutbrown, R.; Covarrubias, L.S.; Leong, V. Parental neural responsivity to infants’ visual attention: How mature brains influence immature brains during social interaction. PLoS Biol. 2018, 16, e2006328. [Google Scholar] [CrossRef]
- Wan, M.W.; Downey, D.; Strachan, H.; Elliott, R.; Williams, S.R.; Abel, K.M. The neural basis of maternal bonding. PLoS ONE 2014, 9, e88436. [Google Scholar] [CrossRef]

| Infant Data (N = 47) | N, Unless Noted * | %, Unless Noted * |
|---|---|---|
| Female | 24 | 51 |
| Gestational age in weeks (median, IQR) * | 39 | (39, 40) |
| Corrected Age in days (median, IQR) * | 94 | (78, 114) |
| Race | ||
| White | 36 | 77 |
| Black or African-American | 2 | 4 |
| Asian | 2 | 4 |
| More than one race | 7 | 15 |
| Ethnicity | ||
| Hispanic | 3 | 6 |
| Not Hispanic | 41 | 87 |
| No response | 3 | 6 |
| WECS facial expressiveness (median, IQR) * | 1.7 | (1.4, 2.0) |
| WECS sensitivity to parent (median, IQR) * | 2.5 | (2.2, 2.8) |
| WECS vocal communication (median, IQR)* | 2.0 | (1.9, 2.2) |
| Maternal Data (N = 47) | ||
| Maternal Education | ||
| Partial College or Trade School | 6 | 13 |
| College Graduation | 15 | 32 |
| Graduate Education | 26 | 55 |
| MIBS Score (median, IQR) * | 1 | (0, 2) |
| EPDS Score (median, IQR) * | 3 | (1, 5) |
| WECS Facial 1 | WECS Facial 2/3 | |
|---|---|---|
| Difference between CCorr Action 2–Action 1 * | 0.012 | 0.096 |
| WECS Vocal 1 | WECS Vocal 2/3 | |
| Difference between CCorr Action 2–Action 1 * | 0.008 | 0.038 |
| WECS sensitivity 1 | WECS sensitivity 2/3 | |
|---|---|---|
| Difference between CCorr Action 3–Action 1 * | −0.072 | 0.019 |
| WECS facial 1 | WECS facial 2/3 | |
| Difference between CCorr Action 3–Action 1 | −0.023 | 0.031 |
| WECS vocal 1 | WECS vocal 2/3 | |
| Difference between CCorr Action 3–Action 1 | −0.003 | 0.056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neel, M.L.; Jeanvoine, A.; Kjeldsen, C.P.; Maitre, N.L. Mother–Infant Dyadic Neural Synchrony Measured Using EEG Hyperscanning and Validated Using Behavioral Measures. Children 2025, 12, 115. https://doi.org/10.3390/children12020115
Neel ML, Jeanvoine A, Kjeldsen CP, Maitre NL. Mother–Infant Dyadic Neural Synchrony Measured Using EEG Hyperscanning and Validated Using Behavioral Measures. Children. 2025; 12(2):115. https://doi.org/10.3390/children12020115
Chicago/Turabian StyleNeel, Mary Lauren, Arnaud Jeanvoine, Caitlin P. Kjeldsen, and Nathalie L. Maitre. 2025. "Mother–Infant Dyadic Neural Synchrony Measured Using EEG Hyperscanning and Validated Using Behavioral Measures" Children 12, no. 2: 115. https://doi.org/10.3390/children12020115
APA StyleNeel, M. L., Jeanvoine, A., Kjeldsen, C. P., & Maitre, N. L. (2025). Mother–Infant Dyadic Neural Synchrony Measured Using EEG Hyperscanning and Validated Using Behavioral Measures. Children, 12(2), 115. https://doi.org/10.3390/children12020115






