Pediatric Thoracic MRI: Safer, Sharper and Smarter Diagnostics
Abstract
1. Introduction
2. Challenges of Thoracic MRI
3. Key Sequences and Techniques
3.1. Sequences
3.2. Techniques
3.3. Experimental Techniques
4. Clinical Indications for Pediatric Thoracic MRI
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed Tomography |
| DWI | Diffusion-Weighted Imaging |
| FSE | Fast Spin-Echo |
| GRE | Gradient-Echo |
| MRI | Magnetic Resonance Imaging |
| PREFUL | Phase-Resolved Functional Lung |
| SNR | Signal-to-Noise Ratio |
| TSE | Turbo Spin-Echo |
| UTE | Ultrashort Echo Time |
References
- Wagner, M.; Bowing, B.; Kuth, R.; Deimling, M.; Rascher, W.; Rupprecht, T. Low field thoracic MRI--A fast and radiation free routine imaging modality in children. Magn. Reson. Imaging 2001, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Wild, J.M.; Marshall, H.; Bock, M.; Schad, L.R.; Jakob, P.M.; Puderbach, M.; Molinari, F.; Van Beek, E.J.; Biederer, J. MRI of the lung (1/3): Methods. Insights Imaging 2012, 3, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Biederer, J.; Mirsadraee, S.; Beer, M.; Molinari, F.; Hintze, C.; Bauman, G.; Both, M.; Van Beek, E.J.; Wild, J.; Puderbach, M. MRI of the lung (3/3)-current applications and future perspectives. Insights Imaging 2012, 3, 373–386. [Google Scholar] [CrossRef]
- Delacoste, J.; Chaptinel, J.; Beigelman-Aubry, C.; Piccini, D.; Sauty, A.; Stuber, M. A double echo ultra short echo time (UTE) acquisition for respiratory motion-suppressed high resolution imaging of the lung. Magn. Reson. Med. 2018, 79, 2297–2305. [Google Scholar] [CrossRef]
- Munidasa, S.; Zanette, B.; Dumas, M.P.; Wee, W.; Braganza, S.; Li, D.; Ratjen, F.; Santyr, G. Comparison of 3D UTE free-breathing lung MRI with hyperpolarized (129)Xe MRI in pediatric cystic fibrosis. Magn. Reson. Med. 2025, 93, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Togao, O.; Obara, M.; van Cauteren, M.; Ohno, Y.; Doi, S.; Kuro-o, M.; Malloy, C.; Hsia, C.C.; Dimitrov, I. Ultra-short echo time (UTE) MR imaging of the lung: Comparison between normal and emphysematous lungs in mutant mice. J. Magn. Reson. Imaging 2010, 32, 326–333. [Google Scholar] [CrossRef]
- Togao, O.; Tsuji, R.; Ohno, Y.; Dimitrov, I.; Takahashi, M. Ultrashort echo time (UTE) MRI of the lung: Assessment of tissue density in the lung parenchyma. Magn. Reson. Med. 2010, 64, 1491–1498. [Google Scholar] [CrossRef]
- Friedlander, Y.; Munidasa, S.; Thakar, A.; Ragunayakam, N.; Venegas, C.; Kjarsgaard, M.; Zanette, B.; Capaldi, D.P.I.; Santyr, G.; Nair, P.; et al. Phase-Resolved Functional Lung (PREFUL) MRI to Quantify Ventilation: Feasibility and Physiological Relevance in Severe Asthma. Acad. Radiol. 2024, 31, 3416–3426. [Google Scholar] [CrossRef]
- Ouyang, T.; Tang, Y.; Klimes, F.; Vogel-Claussen, J.; Voskrebenzev, A.; Yang, Q. Phase-resolved Functional Lung (PREFUL) MRI May Reveal Distinct Pulmonary Perfusion Defects in Postacute COVID-19 Syndrome: Sex, Hospitalization, and Dyspnea Heterogeneity. J. Magn. Reson. Imaging 2025, 61, 851–862. [Google Scholar] [CrossRef]
- Vogel-Claussen, J.; Kaireit, T.F.; Voskrebenzev, A.; Klimes, F.; Glandorf, J.; Behrendt, L.; Gutberlet, M.; Korz, C.; Speth, M.; Welte, T.; et al. Phase-resolved Functional Lung (PREFUL) MRI-derived Ventilation and Perfusion Parameters Predict Future Lung Transplant Loss. Radiology 2023, 307, e221958. [Google Scholar] [CrossRef]
- Geiger, J.; Zeimpekis, K.G.; Jung, A.; Moeller, A.; Kellenberger, C.J. Clinical application of ultrashort echo-time MRI for lung pathologies in children. Clin. Radiol. 2021, 76, 708.e9–708.e17. [Google Scholar] [CrossRef]
- Ringwald, F.G.; Wucherpfennig, L.; Hagen, N.; Mucke, J.; Kaletta, S.; Eichinger, M.; Stahl, M.; Triphan, S.M.F.; Leutz-Schmidt, P.; Gestewitz, S.; et al. Automated lung segmentation on chest MRI in children with cystic fibrosis. Front. Med. 2024, 11, 1401473. [Google Scholar] [CrossRef]
- Dournes, G.; Woods, J.C. Chronic Pediatric Lung Diseases: Counterpoint-A Growing Role for MRI. Am. J. Roentgenol. 2024, 224, e2432405. [Google Scholar] [CrossRef]
- Tepper, L.A.; Ciet, P.; Caudri, D.; Quittner, A.L.; Utens, E.M.; Tiddens, H.A. Validating chest MRI to detect and monitor cystic fibrosis lung disease in a pediatric cohort. Pediatr. Pulmonol. 2016, 51, 34–41. [Google Scholar] [CrossRef]
- Wyttenbach, R.; Vock, P.; Tschappeler, H. Cross-sectional imaging with CT and/or MRI of pediatric chest tumors. Eur. Radiol. 1998, 8, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Bhalla, A.S.; Jana, M. Pediatric Chest MRI: A Review. Indian. J. Pediatr. 2019, 86, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.C. Lung MRI: The future is now. Pediatr. Pulmonol. 2024, 60 (Suppl. 1), S73–S74. [Google Scholar] [CrossRef]
- Biederer, J.; Beer, M.; Hirsch, W.; Wild, J.; Fabel, M.; Puderbach, M.; Van Beek, E.J. MRI of the lung (2/3). Why ... when ... how? Insights Imaging 2012, 3, 355–371. [Google Scholar] [CrossRef]
- Bruegel, M.; Gaa, J.; Woertler, K.; Ganter, C.; Waldt, S.; Hillerer, C.; Rummeny, E.J. MRI of the lung: Value of different turbo spin-echo, single-shot turbo spin-echo, and 3D gradient-echo pulse sequences for the detection of pulmonary metastases. J. Magn. Reson. Imaging 2007, 25, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Biederer, J.; Reuter, M.; Both, M.; Muhle, C.; Grimm, J.; Graessner, J.; Heller, M. Analysis of artefacts and detail resolution of lung MRI with breath-hold T1-weighted gradient-echo and T2-weighted fast spin-echo sequences with respiratory triggering. Eur. Radiol. 2002, 12, 378–384. [Google Scholar] [CrossRef]
- Beckmann, N.; Tigani, B.; Mazzoni, L.; Fozard, J.R. MRI of lung parenchyma in rats and mice using a gradient-echo sequence. NMR Biomed. 2001, 14, 297–306. [Google Scholar] [CrossRef]
- Holverda, S.; Theilmann, R.J.; Sa, R.C.; Arai, T.J.; Hall, E.T.; Dubowitz, D.J.; Prisk, G.K.; Hopkins, S.R. Measuring lung water: Ex vivo validation of multi-image gradient echo MRI. J. Magn. Reson. Imaging 2011, 34, 220–224. [Google Scholar] [CrossRef]
- Sanchez, F.; Tyrrell, P.N.; Cheung, P.; Heyn, C.; Graham, S.; Poon, I.; Ung, Y.; Louie, A.; Tsao, M.; Oikonomou, A. Detection of solid and subsolid pulmonary nodules with lung MRI: Performance of UTE, T1 gradient-echo, and single-shot T2 fast spin echo. Cancer Imaging 2023, 23, 17. [Google Scholar] [CrossRef]
- Zhang, Y.; Kwon, W.; Lee, H.Y.; Ko, S.M.; Kim, S.H.; Lee, W.Y.; Yong, S.J.; Jung, S.H.; Byun, C.S.; Lee, J.; et al. Imaging Assessment of Visceral Pleural Surface Invasion by Lung Cancer: Comparison of CT and Contrast-Enhanced Radial T1-Weighted Gradient Echo 3-Tesla MRI. Korean J. Radiol. 2021, 22, 829–839. [Google Scholar] [CrossRef]
- Dang, S.; Han, D.; Duan, H.; Jiang, Y.; Aihemaiti, A.; Yu, N.; Yu, Y.; Duan, X. The value of T2-weighted MRI contrast ratio combined with DWI in evaluating the pathological grade of solid lung adenocarcinoma. Clin. Radiol. 2024, 79, 279–286. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, L.; Shi, G.; Xu, Q.; Wang, Q.; Zhu, H.; Feng, H.; Wang, L.; Zhang, N.; Xue, M.; et al. Pulmonary Functional Imaging for Lung Adenocarcinoma: Combined MRI Assessment Based on IVIM-DWI and OE-UTE-MRI. Front. Oncol. 2021, 11, 677942. [Google Scholar] [CrossRef]
- Guneyli, S.; Tor, M.; Hassoy, H.; Aygun, M.S.; Altinmakas, E.; Dik Altintas, S.; Savas, R. Spin-echo and diffusion-weighted MRI in differentiation between progressive massive fibrosis and lung cancer. Diagn. Interv. Radiol. 2021, 27, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Landini, N. MRI and zero or ultra-short echo-time sequences in secondary interstitial lung diseases: Current applicability and future perspectives. Eur. Radiol. 2025, 35, 2955–2957. [Google Scholar] [CrossRef] [PubMed]
- Janos, S.; Schooler, G.R.; Ngo, J.S.; Davis, J.T. Free-breathing unsedated MRI in children: Justification and techniques. J. Magn. Reson. Imaging 2019, 50, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, L.; Cross, R.; O’Brien, K.J.; Xue, H.; Kellman, P.; Hansen, M.S. Free-breathing motion-corrected late-gadolinium-enhancement imaging improves image quality in children. Pediatr. Radiol. 2016, 46, 983–990. [Google Scholar] [CrossRef]
- Zanette, B.; Schrauben, E.M.; Munidasa, S.; Goolaub, D.S.; Singh, A.; Coblentz, A.; Stirrat, E.; Couch, M.J.; Grimm, R.; Voskrebenzev, A.; et al. Clinical Feasibility of Structural and Functional MRI in Free-Breathing Neonates and Infants. J. Magn. Reson. Imaging 2022, 55, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Tischendorf, P.; Kunnemann, M.D.; Krahling, T.; Lange, J.H.; Heindel, W.; Beck, L. Thoracic MRI in Pediatric Oncology: Feasibility and Image Quality of Post-Contrast Free-Breathing Radial 3D T1 Weighted Imaging. Biomedicines 2025, 13, 2302. [Google Scholar] [CrossRef]
- MacKenzie, J.D.; Vasanawala, S.S. State-of-the-art in pediatric body and musculoskeletal magnetic resonance imaging. Semin. Ultrasound CT MR 2010, 31, 86–99. [Google Scholar] [CrossRef]
- Glockner, J.F.; Hu, H.H.; Stanley, D.W.; Angelos, L.; King, K. Parallel MR imaging: A user’s guide. Radiographics 2005, 25, 1279–1297. [Google Scholar] [CrossRef]
- Kozak, B.M.; Jaimes, C.; Kirsch, J.; Gee, M.S. MRI Techniques to Decrease Imaging Times in Children. Radiographics 2020, 40, 485–502. [Google Scholar] [CrossRef]
- Chavhan, G.B.; Babyn, P.S.; Singh, M.; Vidarsson, L.; Shroff, M. MR imaging at 3.0 T in children: Technical differences, safety issues, and initial experience. Radiographics 2009, 29, 1451–1466. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Q.; Song, Y.; Tao, X.; Chen, J.; Zhao, J.; Jiang, Z. Comparison of lung lesion assessment using free-breathing dynamic contrast-enhanced 1.5-T MRI with a golden-angle radial stack-of-stars VIBE sequence and CT. Acta Radiol. 2024, 65, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Henzler, T.; Dietrich, O.; Krissak, R.; Wichmann, T.; Lanz, T.; Reiser, M.F.; Schoenberg, S.O.; Fink, C. Half-Fourier-acquisition single-shot turbo spin-echo (HASTE) MRI of the lung at 3 Tesla using parallel imaging with 32-receiver channel technology. J. Magn. Reson. Imaging 2009, 30, 541–546. [Google Scholar] [CrossRef]
- Yoon, J.H.; Nickel, M.D.; Peeters, J.M.; Lee, J.M. Rapid Imaging: Recent Advances in Abdominal MRI for Reducing Acquisition Time and Its Clinical Applications. Korean J. Radiol. 2019, 20, 1597–1615. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.G.; Lee, J.M.; Lee, S.M.; Kang, H.J.; Lee, E.S.; Hur, B.Y.; Yoon, J.H.; Kim, E.; Doneva, M. High Acceleration Three-Dimensional T1-Weighted Dual Echo Dixon Hepatobiliary Phase Imaging Using Compressed Sensing-Sensitivity Encoding: Comparison of Image Quality and Solid Lesion Detectability with the Standard T1-Weighted Sequence. Korean J. Radiol. 2019, 20, 438–448. [Google Scholar] [CrossRef]
- Feng, L.; Benkert, T.; Block, K.T.; Sodickson, D.K.; Otazo, R.; Chandarana, H. Compressed sensing for body MRI. J. Magn. Reson. Imaging 2017, 45, 966–987. [Google Scholar] [CrossRef]
- Chandarana, H.; Feng, L.; Ream, J.; Wang, A.; Babb, J.S.; Block, K.T.; Sodickson, D.K.; Otazo, R. Respiratory Motion-Resolved Compressed Sensing Reconstruction of Free-Breathing Radial Acquisition for Dynamic Liver Magnetic Resonance Imaging. Investig. Radiol. 2015, 50, 749–756. [Google Scholar] [CrossRef]
- Zhang, T.; Yousaf, U.; Hsiao, A.; Cheng, J.Y.; Alley, M.T.; Lustig, M.; Pauly, J.M.; Vasanawala, S.S. Clinical performance of a free-breathing spatiotemporally accelerated 3-D time-resolved contrast-enhanced pediatric abdominal MR angiography. Pediatr. Radiol. 2015, 45, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, Y.; Isoda, H.; Maetani, Y.S.; Arizono, S.; Shimada, K.; Togashi, K. MRI artifact reduction and quality improvement in the upper abdomen with PROPELLER and prospective acquisition correction (PACE) technique. Am. J. Roentgenol. 2008, 191, 1154–1158. [Google Scholar] [CrossRef]
- Chen, L.; Liu, D.; Zhang, J.; Xie, B.; Zhou, X.; Grimm, R.; Huang, X.; Wang, J.; Feng, L. Free-breathing dynamic contrast-enhanced MRI for assessment of pulmonary lesions using golden-angle radial sparse parallel imaging. J. Magn. Reson. Imaging 2018, 48, 459–468. [Google Scholar] [CrossRef]
- Sodhi, K.S.; Bhatia, A.; Rana, P.; Mathew, J.L. Impact of Radial Percentage K-Space Filling and Signal Averaging on Native Lung MRI Image Quality in 3D Radial UTE Acquisition: A Pilot Study. Acad. Radiol. 2023, 30, 2557–2565. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, Y.H.; Cheon, J.E.; Lee, S.M.; Cho, H.H.; Shin, S.M.; Kim, W.S.; Kim, I.O. Improved abdominal MRI in non-breath-holding children using a radial k-space sampling technique. Pediatr. Radiol. 2015, 45, 840–846. [Google Scholar] [CrossRef]
- Browne, L.P.; Malone, L.J.; Englund, E.K.; Fujiwara, T.; Fluta, C.; Lu, Q.; Grover, T.R.; Fuhr, P.G.; Barker, A.J. Free-breathing magnetic resonance imaging with radial k-space sampling for neonates and infants to reduce anesthesia. Pediatr. Radiol. 2022, 52, 1326–1337. [Google Scholar] [CrossRef]
- Wernz, M.M.; Voskrebenzev, A.; Muller, R.A.; Zubke, M.; Klimes, F.; Glandorf, J.; Czerner, C.; Wacker, F.; Olsson, K.M.; Hoeper, M.M.; et al. Feasibility, Repeatability, and Correlation to Lung Function of Phase-Resolved Functional Lung (PREFUL) MRI-derived Pulmonary Artery Pulse Wave Velocity Measurements. J. Magn. Reson. Imaging 2024, 60, 2216–2228. [Google Scholar] [CrossRef] [PubMed]
- van Beek, E.J.R.; Wild, J.M. Xenon MRI for Future Assessment of Lung Function and Treatment Response: A Commentary. J. Magn. Reson. Imaging 2021, 54, 1363–1364. [Google Scholar] [CrossRef] [PubMed]
- Pöhler, G.H.; Voskrebenzev, A.; Heinze, M.L.; Skeries, V.; Klimeš, F.; Glandorf, J.; Eckstein, J.; Babazade, N.; Wernz, M.; Pfeil, A.; et al. Phase-resolved Functional Lung MRI Reveals Distinct Lung Perfusion Phenotype in Children and Adolescents with Post-COVID-19 Condition. Radiology 2025, 314, e241596. [Google Scholar] [CrossRef]
- Newman, B. Magnetic resonance imaging for congenital lung malformations. Pediatr. Radiol. 2022, 52, 312–322. [Google Scholar] [CrossRef]
- Kellenberger, C.J.; Amaxopoulou, C.; Moehrlen, U.; Bode, P.K.; Jung, A.; Geiger, J. Structural and perfusion magnetic resonance imaging of congenital lung malformations. Pediatr. Radiol. 2020, 50, 1083–1094. [Google Scholar] [CrossRef]
- Zirpoli, S.; Munari, A.M.; Primolevo, A.; Scarabello, M.; Costanzo, S.; Farolfi, A.; Lista, G.; Zoia, E.; Zuccotti, G.V.; Riccipetitoni, G.; et al. Agreement between magnetic resonance imaging and computed tomography in the postnatal evaluation of congenital lung malformations: A pilot study. Eur. Radiol. 2019, 29, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Willers, C.C.; Frauchiger, B.S.; Stranzinger, E.; Bauman, G.; Moeller, A.; Jung, A.; Hector, A.; Regamey, N.; Zanolari, M.; Mueller-Suter, D.; et al. Feasibility of unsedated lung MRI in young children with cystic fibrosis. Eur. Respir. J. 2022, 60, 2103112. [Google Scholar] [CrossRef]
- Sileo, C.; Corvol, H.; Boelle, P.Y.; Blondiaux, E.; Clement, A.; Ducou Le Pointe, H. HRCT and MRI of the lung in children with cystic fibrosis: Comparison of different scoring systems. J. Cyst. Fibros. 2014, 13, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Munidasa, S.; Zanette, B.; Couch, M.; Grimm, R.; Seethamraju, R.; Dumas, M.P.; Wee, W.; Au, J.; Braganza, S.; Li, D.; et al. Inter- and intravisit repeatability of free-breathing MRI in pediatric cystic fibrosis lung disease. Magn. Reson. Med. 2023, 89, 2048–2061. [Google Scholar] [CrossRef]
- Wielputz, M.O.; von Stackelberg, O.; Stahl, M.; Jobst, B.J.; Eichinger, M.; Puderbach, M.U.; Nahrlich, L.; Barth, S.; Schneider, C.; Kopp, M.V.; et al. Multicentre standardisation of chest MRI as radiation-free outcome measure of lung disease in young children with cystic fibrosis. J. Cyst. Fibros. 2018, 17, 518–527. [Google Scholar] [CrossRef]
- Sodhi, K.S.; Gauba, R.; Bhatia, A.; Saxena, A.K.; Mathew, J.L. Lung MRI- Changing Paradigms in Evaluation of Chronic Granulomatous Disease in Children. J. Clin. Immunol. 2022, 42, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.S.; Khandelwal, N.; Saxena, A.K.; Singh, M.; Agarwal, R.; Bhatia, A.; Lee, E.Y. Rapid lung MRI in children with pulmonary infections: Time to change our diagnostic algorithms. J. Magn. Reson. Imaging 2016, 43, 1196–1206. [Google Scholar] [CrossRef]
- Sodhi, K.S.; Khandelwal, N.; Saxena, A.K.; Bhatia, A.; Bansal, D.; Trehan, A.; Singh, M.; Agarwal, R. Rapid lung MRI-paradigm shift in evaluation of febrile neutropenia in children with leukemia: A pilot study. Leuk. Lymphoma 2016, 57, 70–75. [Google Scholar] [CrossRef]
- Rupprecht, T.; Bowing, B.; Kuth, R.; Deimling, M.; Rascher, W.; Wagner, M. Steady-state free precession projection MRI as a potential alternative to the conventional chest X-ray in pediatric patients with suspected pneumonia. Eur. Radiol. 2002, 12, 2752–2756. [Google Scholar] [CrossRef]
- Sodhi, K.S.; Sharma, M.; Lee, E.Y.; Saxena, A.K.; Mathew, J.L.; Singh, M.; Khandelwal, N. Diagnostic Utility of 3T Lung MRI in Children with Interstitial Lung Disease: A Prospective Pilot Study. Acad. Radiol. 2018, 25, 380–386. [Google Scholar] [CrossRef]
- Biederer, J.; Wielputz, M.O.; Jobst, B.J.; Dinkel, J. [MRI of interstitial lung diseases: What is possible?]. Radiologe 2014, 54, 1204–1212. [Google Scholar] [CrossRef]
- Lonzetti, L.; Zanon, M.; Pacini, G.S.; Altmayer, S.; Martins de Oliveira, D.; Rubin, A.S.; Gazzoni, F.F.; Barros, M.C.; Hochhegger, B. Magnetic resonance imaging of interstitial lung diseases: A state-of-the-art review. Respir. Med. 2019, 155, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Navallas, M.; Inarejos Clemente, E.J.; Iglesias, E.; Rebollo-Polo, M.; Anton, J.; Navarro, O.M. Connective Tissue Disorders in Childhood: Are They All the Same? Radiographics 2019, 39, 229–250. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, C.L.; Ianniello, S.; Trinci, M.; Galluzzo, M.; Tonerini, M.; Zeccolini, M.; Guglielmi, G.; Miele, V. Diagnostic Imaging in pediatric thoracic trauma. Radiol. Med. 2017, 122, 850–865. [Google Scholar] [CrossRef] [PubMed]
- Nyilas, S.; Bauman, G.; Pusterla, O.; Ramsey, K.; Singer, F.; Stranzinger, E.; Yammine, S.; Casaulta, C.; Bieri, O.; Latzin, P. Ventilation and perfusion assessed by functional MRI in children with CF: Reproducibility in comparison to lung function. J. Cyst. Fibros. 2019, 18, 543–550. [Google Scholar] [CrossRef]
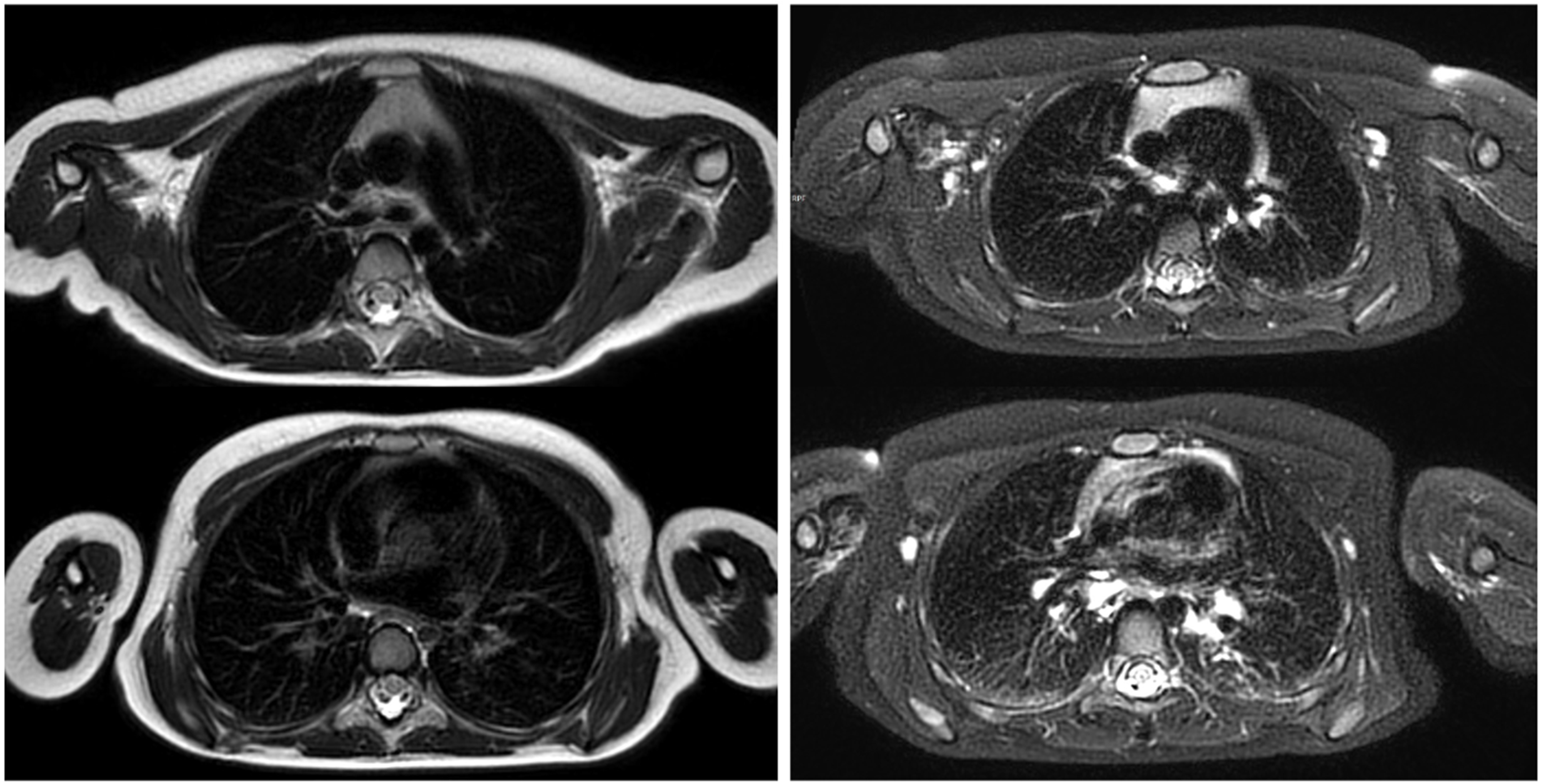

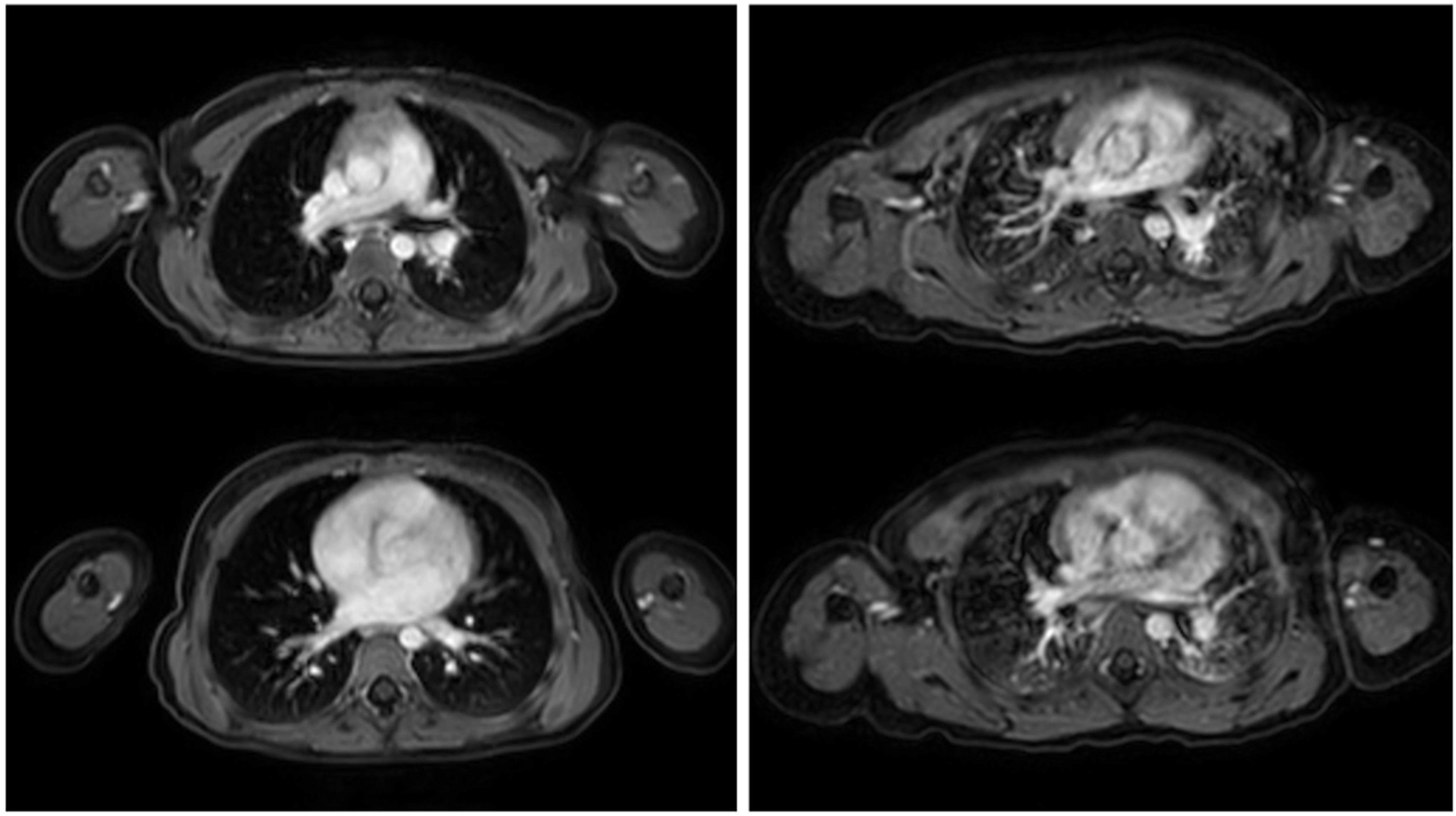


| Imaging Modality | Radiation Exposure | Morphological Resolution | Functional Assessment | Availability | Pediatric Tolerance |
|---|---|---|---|---|---|
| Conventional radiography | Yes (low) | Low | No | High | High |
| Computed tomography | Yes (high) | Very High | Limited (indirect) | High | Moderate |
| Magnetic resonance imaging | No | Moderate–High | Possible | Moderate | Moderate |
| Ultrasound | No | Low (superficial only) | Limited | High | High |
| Sequence | Primary Utility | Advantages | Pediatric Relevance | Reference |
|---|---|---|---|---|
| TSE/FSE | Anatomic detail of mediastinum & chest wall | Excellent soft tissue contrast | Clear visualization of mediastinum, chest wall | [3,19,20] |
| GRE | Dynamic imaging | Fast acquisition, cardiac imaging | Functional cardiovascular evaluation | [19,20] |
| DWI | Inflammatory/tumor detection | Microstructural imaging without contrast agent | Useful for infection and tumor staging | [25,26,27] |
| UTE | Parenchymal lung imaging | Visualizes previously “invisible” lung structures | Detect structural lung changes | [7,11,28] |
| Technique | Primary Utility | Advantages | Pediatric Relevance | References |
|---|---|---|---|---|
| Respiratory Gating/Navigator | Motion reduction | Sharper images | Reduced need for general anesthesia | [29,30,31,32] |
| Parallel Imaging | Faster acquisition | Less motion artifact | Reduced need for general anesthesia or sedation | [37,38] |
| Radial Imaging | Artifact suppression | Robust to motion, improved air–tissue interface visualization | Suitable for restless or sedated patients | [47,48] |
| Phase-Resolved Functional Lung | Perfusion & ventilation imaging | Free-breathing, functional imaging | Ideal for young/uncooperative children | [8,10,49] |
| Hyperpolarized Xenon-129 | Ventilation/perfusion & gas exchange | Exceptional functional assessment | Non-invasive functional lung assessment | [17,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tischendorf, P.; Beck, L.; Krähling, T. Pediatric Thoracic MRI: Safer, Sharper and Smarter Diagnostics. Children 2025, 12, 1576. https://doi.org/10.3390/children12111576
Tischendorf P, Beck L, Krähling T. Pediatric Thoracic MRI: Safer, Sharper and Smarter Diagnostics. Children. 2025; 12(11):1576. https://doi.org/10.3390/children12111576
Chicago/Turabian StyleTischendorf, Patricia, Laura Beck, and Tobias Krähling. 2025. "Pediatric Thoracic MRI: Safer, Sharper and Smarter Diagnostics" Children 12, no. 11: 1576. https://doi.org/10.3390/children12111576
APA StyleTischendorf, P., Beck, L., & Krähling, T. (2025). Pediatric Thoracic MRI: Safer, Sharper and Smarter Diagnostics. Children, 12(11), 1576. https://doi.org/10.3390/children12111576






